Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
171 results about "Egg phospholipids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Egg Phospholipids are a very bioavailable way of delivering phosphorus, choline and polyunsaturated fatty acids to the human organism.
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
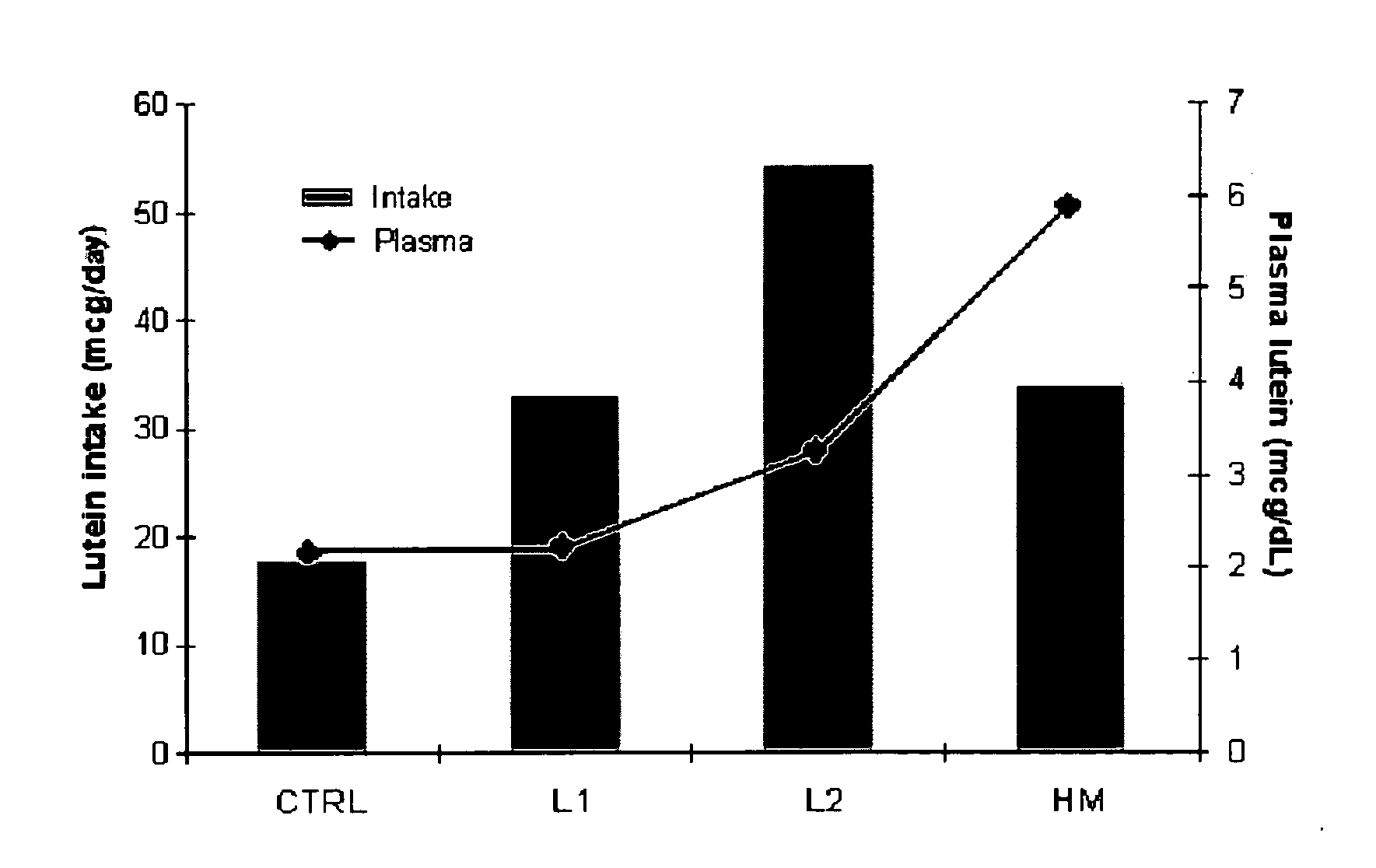
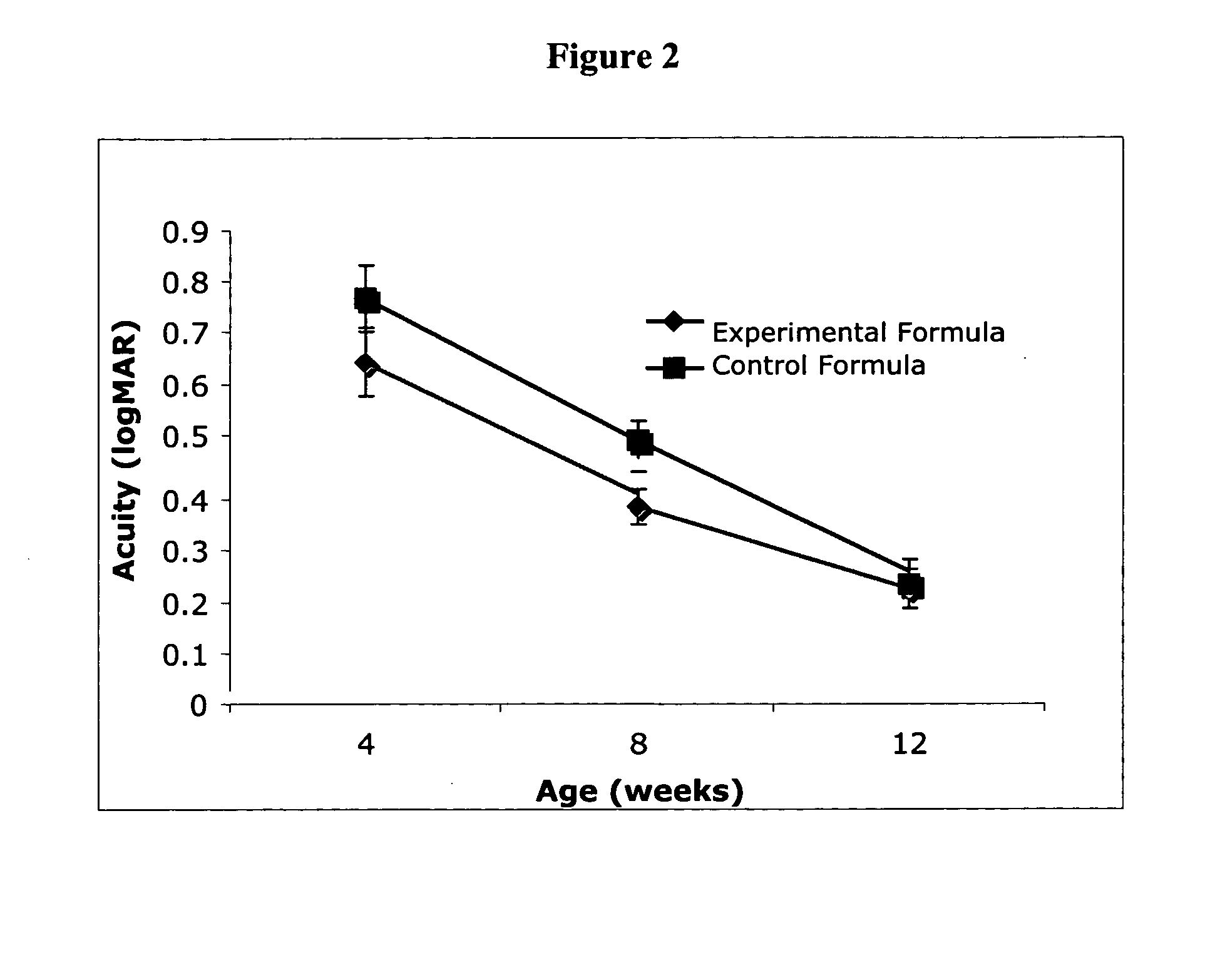
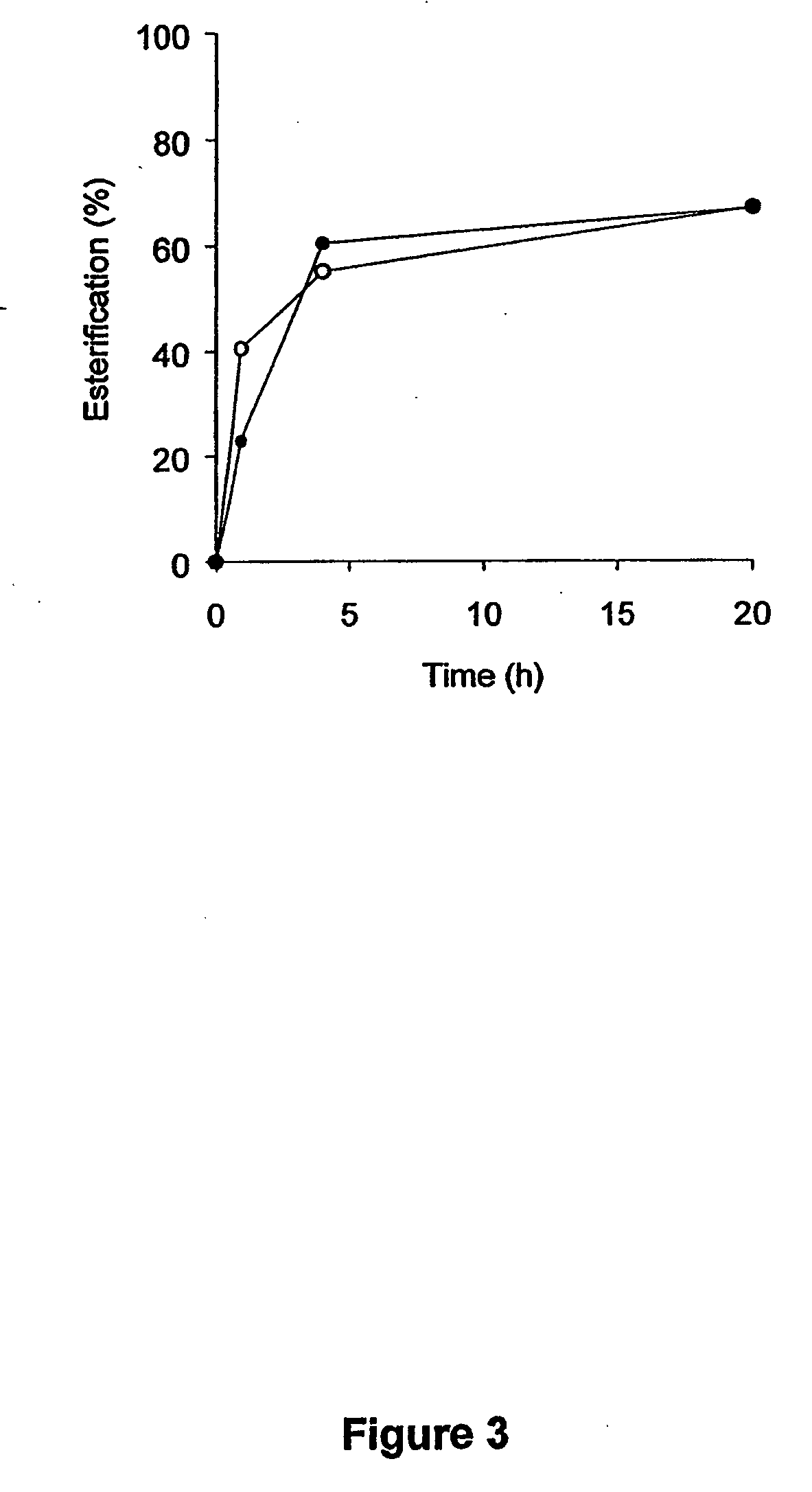
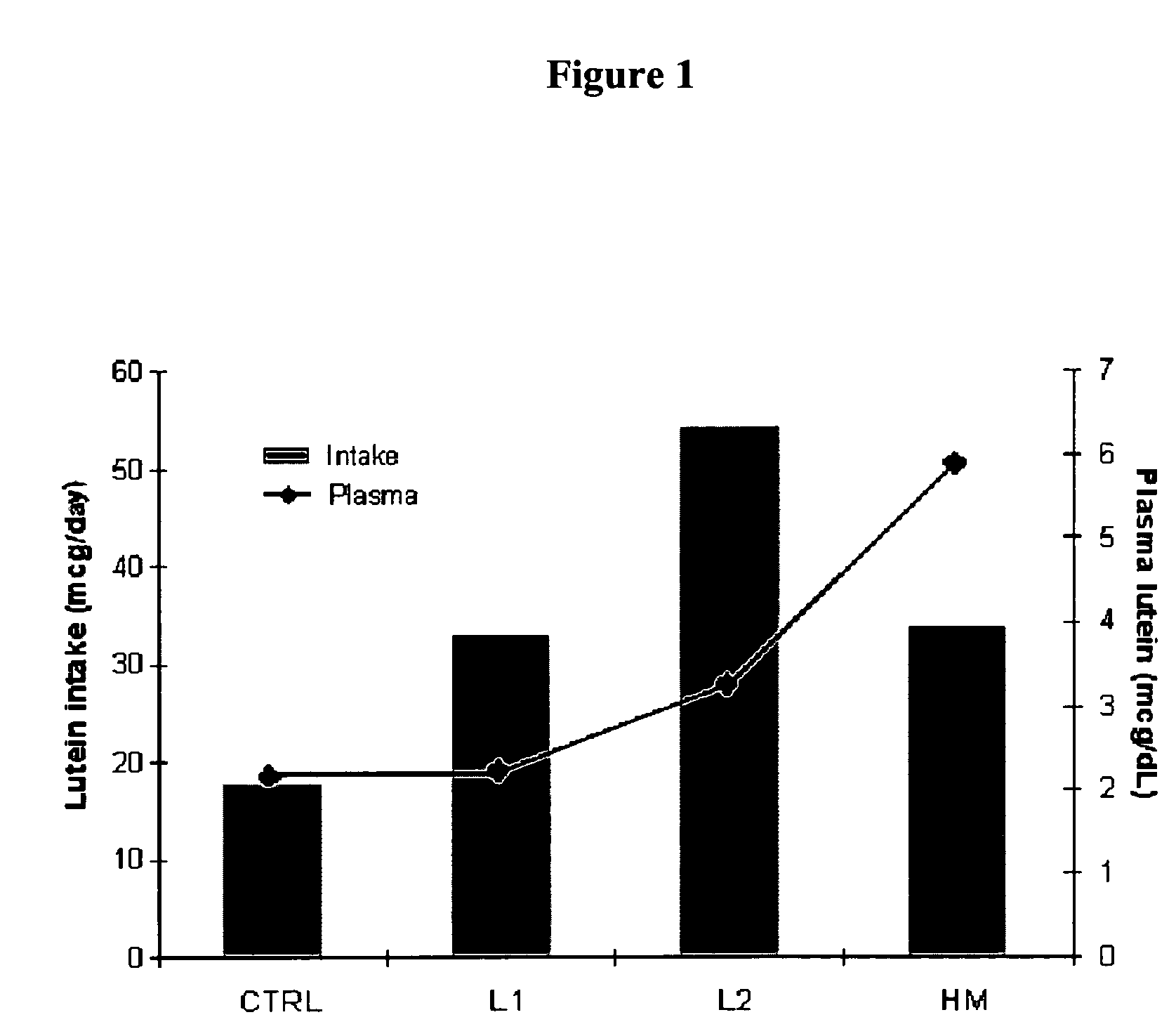
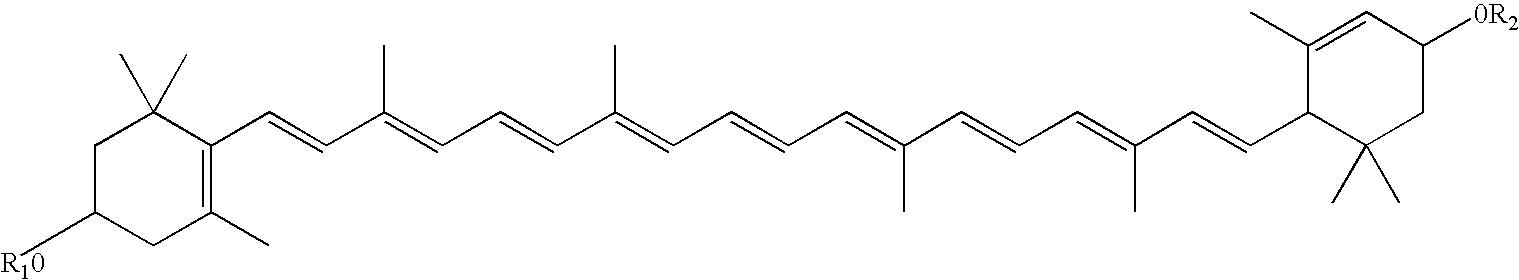
Infant formulas containing docosahexaenoic acid and lutein
ActiveUS20070098849A1Promote retinal healthPromote vision developmentBiocideHeavy metal active ingredientsLuteinDHA - Docosahexaenoic acid
Disclosed are infant formulas and corresponding methods of using them to promote retinal health and vision development in infants. The formulas, which are free of egg phospholipids and comprise fat, protein, carbohydrate, vitamins, and minerals, including docosahexaenoic acid and, on a ready-to-feed basis, at least about 50 mcg / liter of lutein, wherein the weight ratio of lutein (mcg) to docosahexaenoic acid (mg) is from about 1:2 to about 10:1. The formulas are also believed to be especially useful in reducing the risk of retinopathy of prematurity in preterm infants.
Owner:ABBOTT LAB INC
Acyltransferase
InactiveUS20060141457A1Easily calculateReduce needSugar derivativesPeptide/protein ingredientsAcyltransferaseNucleotide sequenc
The invention relates to at least one nucleotide sequence, derived from a nucleotide sequence encoding an acyltransferase polypeptide comprising at least one membrane-spanning region, encoding an improved active membrane independent acyltransferase polypeptide in which at least one amino acid residue of the membrane-spanning region has been deleted and / or substituted as compared to the original acyltransferase polypeptide, wherein the encoded active membrane independent acyltransferase polypeptide can produce fatty acid esters and / or fatty acid thioesters such as triacylglycerols, diacylglycerols, monoacylglycerols, phospholipids, glycolipids, waxesters, acylated carbohydrates, acylated amino acids, and lysolipids, e.g. lysophosphospholipid, lysolecithin. Thereby one single acyltransferase can be used for the production of a huge number of products. The invention also relates to means and methods for the production of such an improved active membrane independent acyltransferase and the use of such a membrane independent acyltransferase in industry.
Owner:GENENCOR INT INC
Infant formulas containing docosahexaenoic acid and lutein
ActiveUS7829126B2Promote retinal health and vision developmentReduce riskBiocideHeavy metal active ingredientsDocosahexaenoic acidLutein
Disclosed are infant formulas and corresponding methods of using them to promote retinal health and vision development in infants. The formulas, which are free of egg phospholipids and comprise fat, protein, carbohydrate, vitamins, and minerals, including docosahexaenoic acid and, on a ready-to-feed basis, at least about 50 mcg / liter of lutein, wherein the weight ratio of lutein (mcg) to docosahexaenoic acid (mg) is from about 1:2 to about 10:1. The formulas are also believed to be especially useful in reducing the risk of retinopathy of prematurity in preterm infants.
Owner:ABBOTT LAB INC
Acyltransferase
InactiveUS7498026B2Fatty acid esters) is increasedEasy to calculateSugar derivativesPeptide/protein ingredientsNucleotideNucleotide sequencing
Owner:GENENCOR INT INC
DSPE-PEG-FA-modified nanometer paclitaxel liposome and preparation method thereof
InactiveCN102188382AHigh encapsulation efficiencyGuaranteed purityOrganic active ingredientsPharmaceutical non-active ingredientsPaclitaxel InjectionDspe peg
The invention relates to a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. A molar ratio of the DSPE-PEG2000-FA to egg yolk lecithin is 0.05% to 0.15%, and a particle size of the liposome is less than 150 nm. A preparation method of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome comprises the following steps: first, preparing a DSPE-PEG2000-FA into a DSPE-PEG2000-FA micelle; second, performing incubation on the phosphatidyl ethanolamine-polyethylene glycol2000-folic acid micelle and a paclitaxel liposome together to obtain a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. Materials, which are forbidden to be used in clinical practice, are not used as crude materials in the present invention. According to the invention, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has a small particle size, and the content of the DSPE-PEG2000-FA is low; besides, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has good drug entrapment efficiency and good colloid stability. Moreover, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome can be absorbed effectively by an ovarian cancer cell having properties of sensitiveness to folic acid (+) and drug resistance, and the cytotoxicity of folic acid dependence is displayed; therefore, the efficacy of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome is stronger than that of a paclitaxel injection.
Owner:李红霞
Glycyrrhetic acid and phospholipid composites of glycyrrhetate and process for preparing same
ActiveCN1594332APromote absorptionOrganic active ingredientsDigestive systemPhysical chemistrySolvent
The invention provides a glycyrrhetic acid and phospholipid composites of glycyrrhetate and process for preparing same by forming phospholipid compound in suitable dissolvent from glycyrrhizinic acid and its salts with a definite quantity of lecithin, thus converting the physical and chemical property, and improving the bioavailability of the glycyrrhizic acid and its salts.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A nano liposome preparation of snake oil and preparation process thereof
InactiveCN1875919AComply with hygienic requirementsCosmetic preparationsToilet preparationsNitrogenFree cooling
The invention relates to a snake oil nano liposome preparation used for cosmetic products and process for preparing same, wherein the preparation is made from snake oil, phosphatidy icholine, cholesterin, anti-oxidant, solubilizing agent, thickening agent and glycerin as isotonic agent through the steps of proportioning raw materials according to formulation quantity, preparing oily phase and aqueous phase, mixing the oily phase and aqueous phase, venting nitrogen at 60-85 deg. C to obtain coarse dispersible article, then carrying out high pressure homogenization circulating treatment at 60-150MPa till transparency, finally cooling naturally.
Owner:苏州国纳生物技术有限公司
Targeting cisplatin sodium nano-alginate liposome
InactiveCN103520207AGood antitumor activityHeavy metal active ingredientsEmulsion deliveryLiposomeCisplatin
The invention relates to a targeting cisplatin sodium nano-alginate liposome. The targeting performance of the targeting cisplatin sodium nano-alginate liposome is embodied by that the liposome is modified by targeting molecules, and the liposome has the grain diameter of about 100nm and is composed of phosphatidylcholine, cholesterol, sodium alginate, an anti-tumor drug cisplatin and targeting molecules. The liposome has a high systematic cisplatin carrying rate (15.24%) and a high encapsulation rate (89.92%), can be subjected to specific combination with targeting molecules on surfaces of tumor cells so as to achieve toxicity attenuation and synergism, and is locally and specifically applied to the preparation of drugs for tumor treatment. Particularly, the liposome has more excellent anti-tumor activity to oophoroma resisting.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
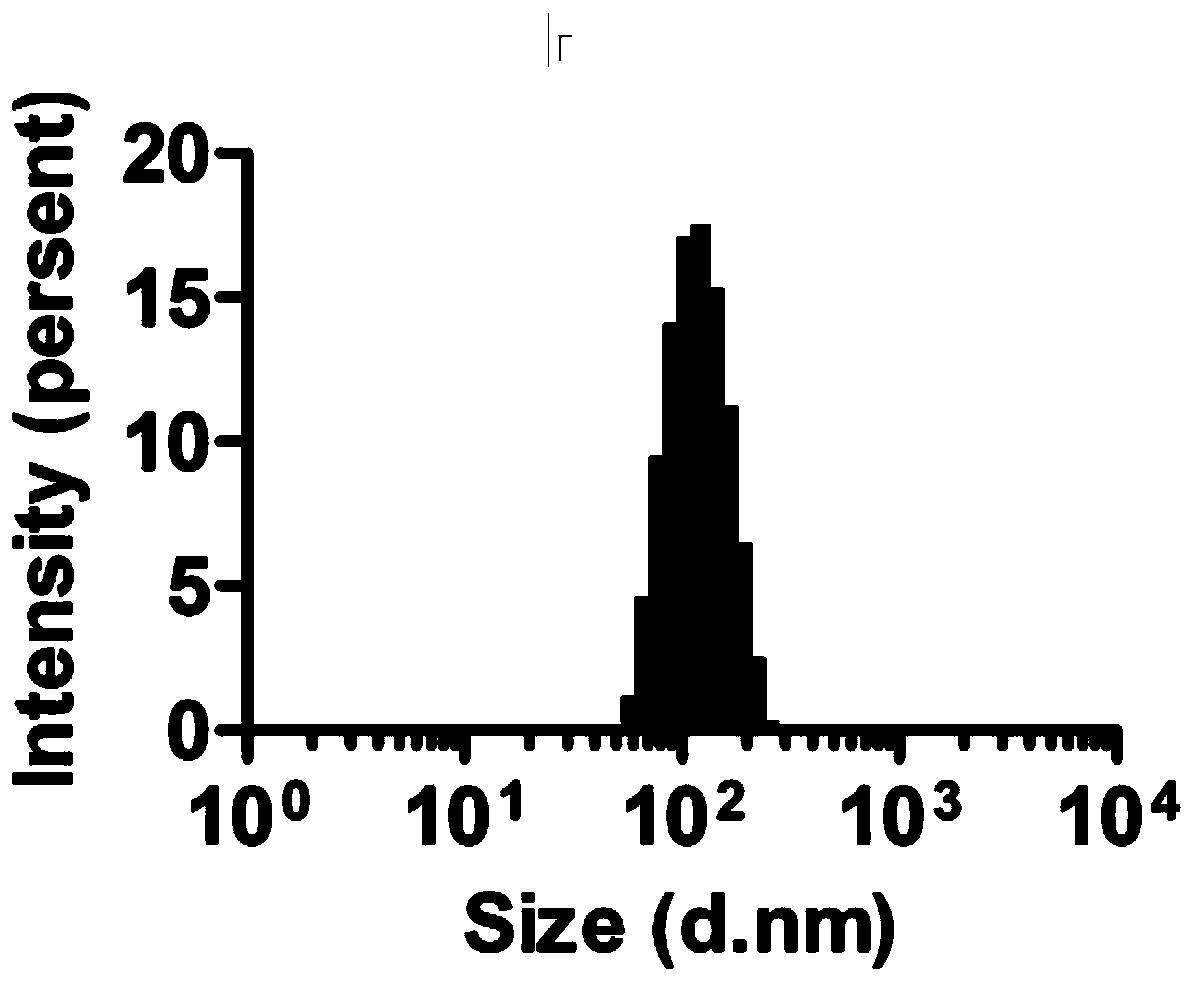
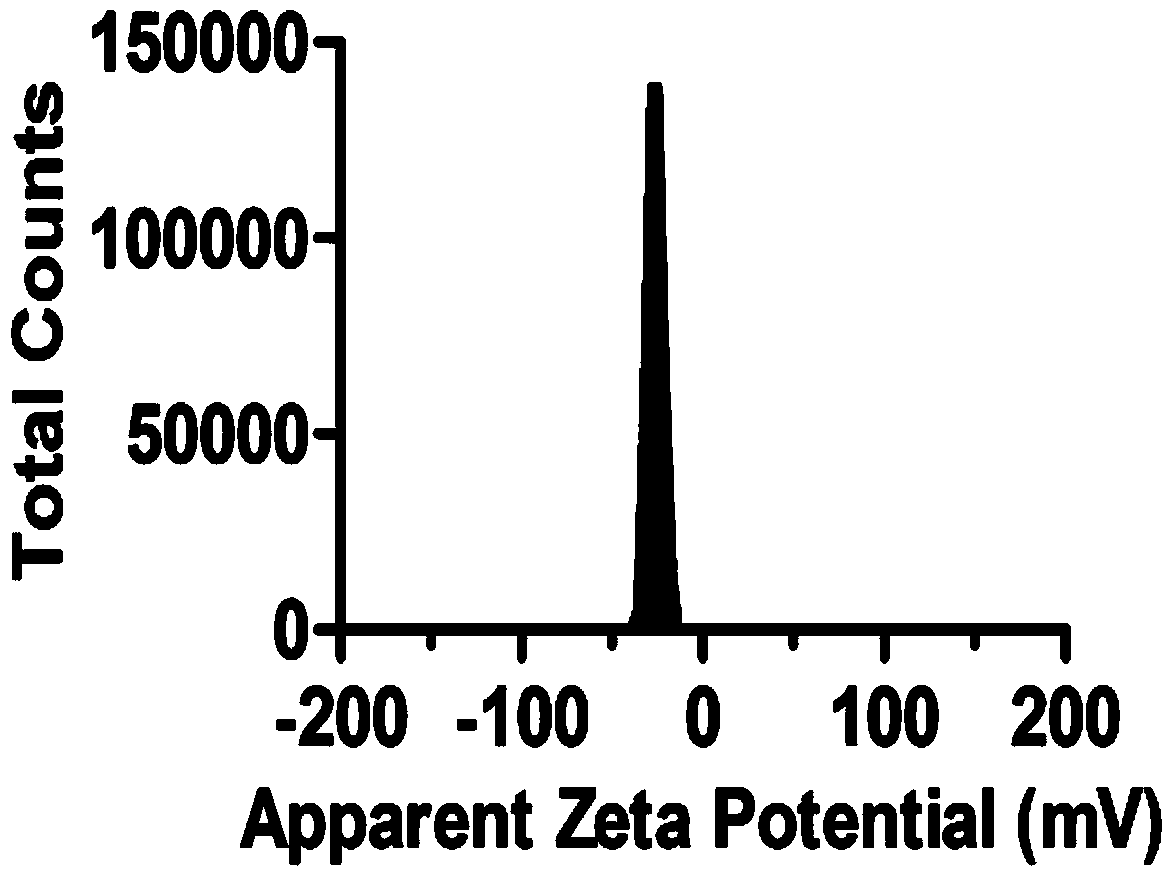
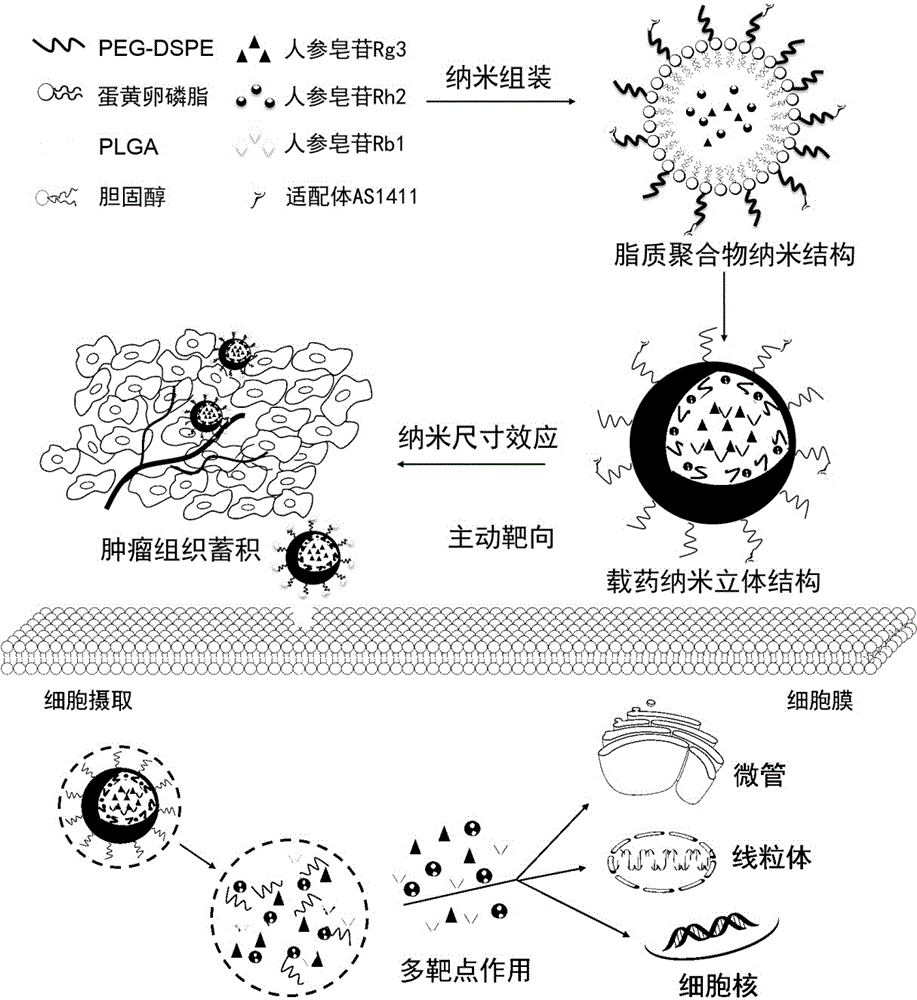
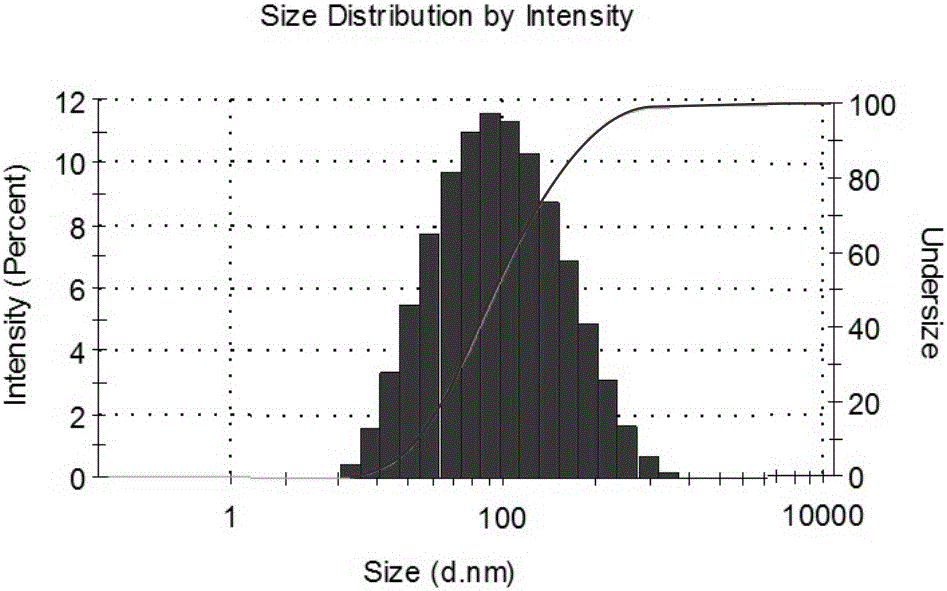
Preparing method and application of ginsenoside-multi-component jointly-loading targeting nanometer system
ActiveCN105708847AOvercoming differences in metabolic behaviorGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsGinsenoside Rb1Solvent
The invention discloses a preparing method of a ginsenoside-multi-component jointly-loading targeting nanometer system.The preparing method includes the steps that after egg yolk lecithin, cholesterol and polyethylene glycol-dipalmitoyl phosphatidyl choline are dissolved, warm water is slowly and dropwise added for stirring, and a lipid water solution is obtained; the multi-component active ingredients containing ginsenoside Rg3, ginsenoside Rh2 and ginsenoside Rb1 and a copolymer of polylactic acid-hydroxyacetic acid are dissolved to be slowly and dropwise added into the lipid water solution to be stirred to be even, a solvent is removed, and lipidosome nanometer particles are obtained; aptamer is dissolved to be modified to the surfaces of the lipidosome nanometer particles, and an even and opalescence-flooding nanometer system solution is obtained by filtering and sterilizing.According to the preparing method, the anti-tumor effect of medicine is improved with the nanocrystallization technology, joint transmission of a multi-component tumor tissue can be achieved in the mode that the three ginsenoside ingredients are jointly loaded to the nanometer system, and therefore the problems that the metabolic behaviors and the tumor-cell entering capacity of different ingredients are different are solved.
Owner:CHENGDU UNIV
Low-phospholipid medium-growing freshwater shrimp feed
The invention discloses a low-phospholipid medium-growing freshwater shrimp feed. The feed consists of the following components in percentage by mass: 15 to 16 percent of fish meal, 12 to 13 percent of fermented bean pulp, 15 to 16 percent of bean pulp, 13.95 to 25 percent of wheat flour, 10 to 11 percent of rapeseed meal, 10 to 12 percent of cottonseed meal, 3 to 4 percent of rice bran, 3 to 4 percent of squid meal, 2 to 3 percent of shrimp shell meal, 2 to 3 percent of zeolite powder, 1.4 to 2 percent of calcium dihydrogen phosphate, 0.5 to 1.0 percent of soybean phospholipid oil, 0.05 to 0.1 percent of lysolecithin and 1.0 percent of premix. The feed is used for medium-growing freshwater shrimps with body lengths of over 2.0 centimeters; and the consumption of phospholipid is reduced in the feed, and the consumption of cheap raw materials such as the rapeseed meal, the cottonseed meal, the wheat flour and the like is increased, so the cost of the feed is reduced and the benefits ofan enterprise are increased under the condition that survival and growth are not affected.
Owner:HUAIYIN TEACHERS COLLEGE
Recombination strain for expressing phospholipase D and application
ActiveCN108949721AGood transphosphatidyl transfer abilityShort fermentation cycleFungiBacteriaPhospholipaseNucleotide
The invention relates to phospholipase D having an amino acid sequence shown as SEQ ID NO.1. The invention further relates to a gene sequence coding the phospholipase D. The nucleotide sequence of thegene is shown as SEQ ID NO.2. The invention provides a method for improving the expression level of the phospholipase D by virtue of a system modification expression component. The method comprises the following steps: screening and replacing signal peptides, ribosome binding sites and promoters, and transforming the constructed recombinant plasmid into host bacteria, wherein the recombination strain is capable of successfully expressing the phospholipase D. The phospholipase D disclosed by the invention has excellent phosphatidyl transformation ability, and the product phosphatidylserine canbe synthesized by taking lecithin and L-serine as the substrate. The recombinant bacteria have excellent enzyme activity stability, the fermentation cycle is short, and a foundation is laid for large-scale industrial production.
Owner:JIANGNAN UNIV
Hypocrellin cationic liposome preparation and preparation method and application thereof
InactiveCN105477633AGood biocompatibilityEnhanced biophotodynamic activitySenses disorderPhotodynamic therapyCholesterolPhospholipid
The invention discloses a hypocrellin cationic liposome preparation and a preparation method and application thereof. The hypocrellin cationic liposome preparation is prepared from hypocrellin, cationic phospholipid, 1-2-dioleoyl phosphatidylcholine, cholesterol and octadecyl triphenylphosphonium bromide. The liposome is a novel hypocrellin liposome designed aiming at macular degeneration photodynamic therapy, the cationic liposome is prepared according to the film-ultrasonic wave dissolving technique, and due to wrapping of octadecyl triphenylphosphonium bromide molecules containing TPP (triphenylphosphine), targeting at new vessel endothelium of a lesion and selectively gathering at a target of endothelial cell mitochondria are realized. In addition, the liposome is technically simple and convenient in preparation and high in stability, biological photodynamic activity of the liposome is more than 2 times of that of parent hypocrellin, and the hypocrellin cationic liposome preparation is a highly potential medicine for macular degeneration photodynamic therapy.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA +1
Efficient Gd-loaded liposome preparation and preparation method thereof
InactiveCN102078624AHigh gadolinium loadingSmall particle sizeNMR/MRI constrast preparationsEmulsion deliveryGadoliniumLiposome
The invention relates to a composite for diagnosing a human body or an animal body, namely, an efficient Gd-loaded liposome preparation and a preparation method thereof. The liposome preparation is prepared from the following components in parts by weight: 30-80 parts of paramagnetic Gd contrast agent, 250-500 parts of lecithin, 50-200 parts of liposome stabilizer and 200-600 parts of buffer solution, based on the weight of metal Gd. The Gd-loaded liposome is prepared by using a phospholipid gel method, and the Gd-loaded amount of the liposome prepared by the method is high, thereby effectively delaying the retention period of the contrast agent in the body; and the liposome is good in storage stability.
Owner:EAST CHINA UNIV OF SCI & TECH
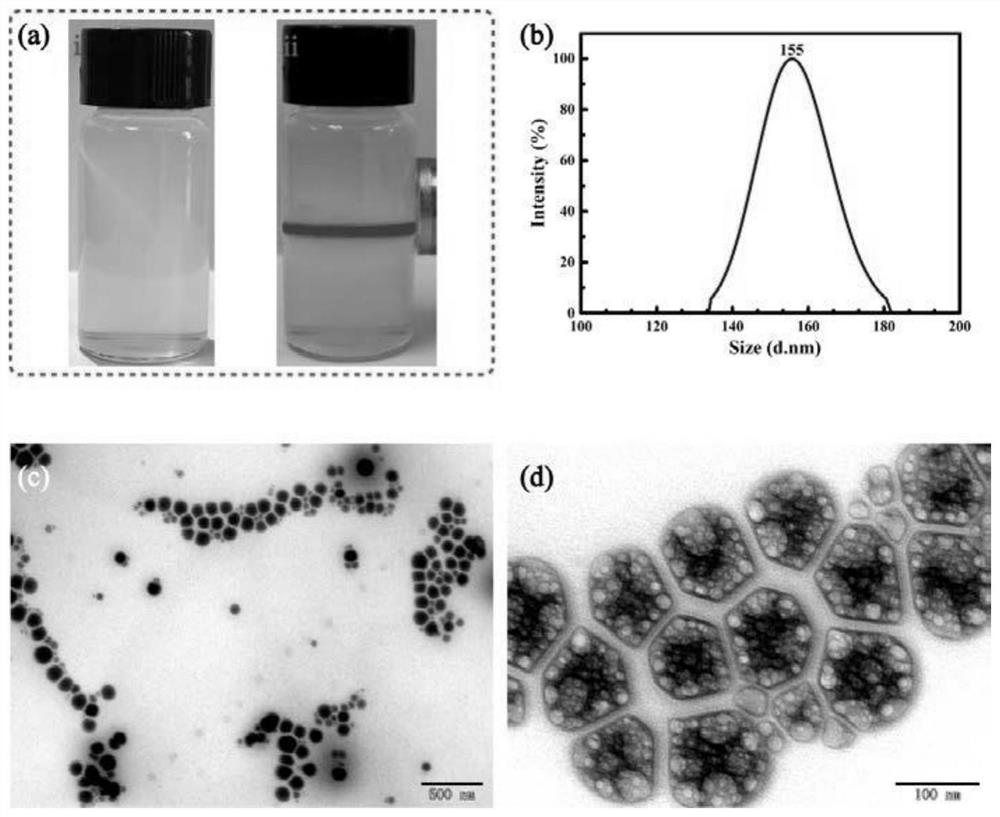
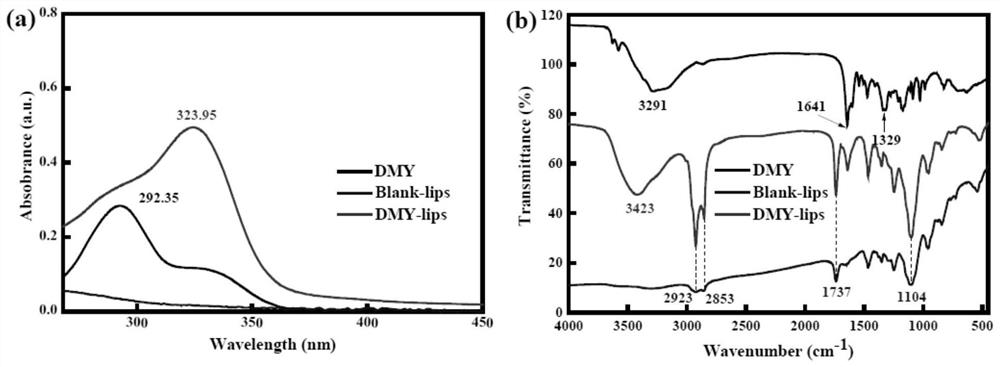
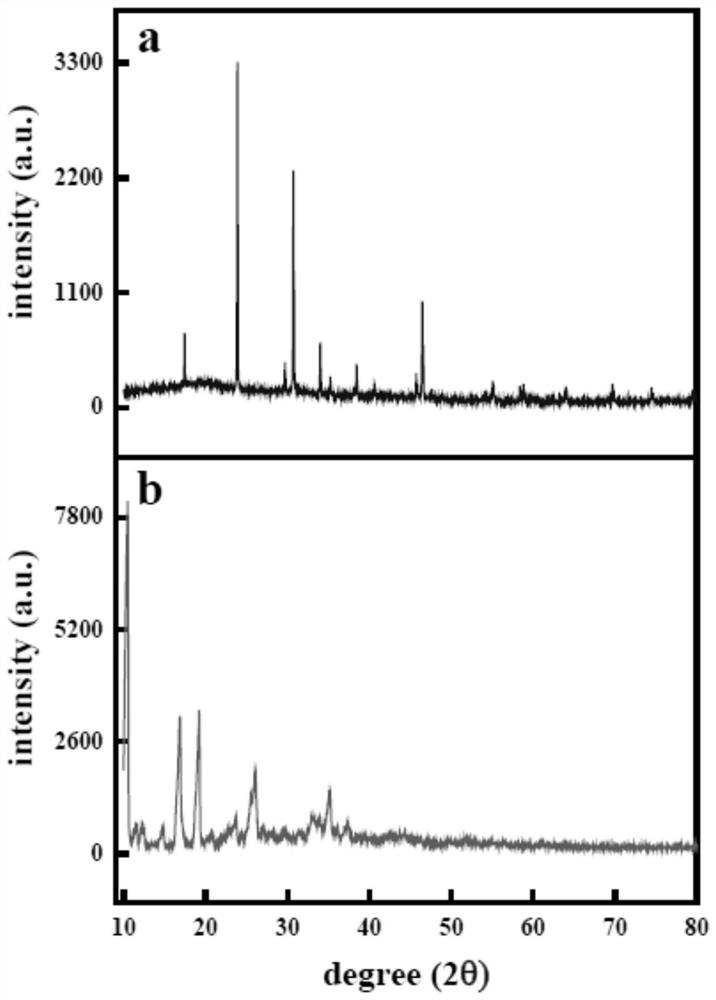
Preparation method and an application of multi-vesicle type dihydromyricetin liposome
PendingCN111991353ASolution to low degree of hydrolysisSolve the short biological half-lifeAntibacterial agentsOrganic active ingredientsCholesterolCentrifugation
The invention relates to the technical field of dihydromyricetin, in particular to a preparation method and an application of a multi-vesicle type dihydromyricetin liposome. The preparation method comprises the following steps of (1) collecting vine tea stems and leaves to ampelopsis grossedentata powder, and performing water bath treatment, concentrating, filtering and cooling to dihydromyricetinextracted ingredients; (2) weighing the extracted ingredients, performing dissolving to obtain a dihydromyricetin solution, and detecting purity; (3) preparing an aqueous phase solution from Tween 80and PEG-4000, preparing an oil phase solution from the dihydromyricetin, cholesterol and egg yolk lecithin, and dropwise adding the oil phase solution to the aqueous phase solution, to prepare the multi-vesicle type dihydromyricetin liposome; and (4) performing centrifugation on the taken multi-vesicle type dihydromyricetin liposome solution, taking supernatant, and detecting absorbance. For extraction of the dihydromyricetin, the egg yolk lecithin is used as a liposome formwork, and macrogol 4000 is used as a modification wall material to prepare the multi-vesicle type dihydromyricetin liposome, so that the problems that the degree of hydrolysis of the dihydromyricetin is low, the biological half-life is short, and the film penetrating force is poor, are solved.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Capsule containing cefixime liposome and preparation method thereof
InactiveCN101966166AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefixime liposome and a preparation method thereof as well as a capsule containing the cefixime liposome. The capsule comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.1-2.5 parts of polysorbate 80. The capsule not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime pharmaceutical composition at a room temperature.
Owner:王丽燕
Oral administration system capable of promoting trans-mucus penetration of protein drug and preparation of oral administration system
ActiveCN111450258AImprove permeabilityIncrease intakePeptide/protein ingredientsMetabolism disorderCholic acidSilicic acid
The invention belongs to the field of biomedicine, and relates to an oral administration system capable of promoting trans-mucus penetration of a protein drug and preparation of the oral administration system, in particular to preparation of a drug carrying system capable of promoting trans-gastrointestinal tract mucus layer penetration of the protein medicine and application of the drug carryingsystem in oral administration of the protein drug. The preparation of the drug carrying system comprises the following steps: taking cetyl trimethyl ammonium bromide as a template, tetraethoxysilane as a silicon source and styrene as a pore-enlarging agent, and removing the temperature through high-temperature calcination to prepare a mesoporous silica carrier; and after the carrier adsorbs and carries the protein drug, carrying out hydrophobization modification on the surface of the drug-carrying silicon dioxide with stearic acid or cholic acid, and further using hydrophobic acting force anda zwitterionic surfactant dodecyl dimethyl betaine or dilauroyl phosphatidylcholine to form self-assembly nanoparticles. The oral administration system can promote penetration of the protein drug in the gastrointestinal tract mucus layer and improve transmembrane absorption of the drug, has the advantages of low toxicity, high drug loading capacity and the like, and has broad application prospectsin oral administration of the protein drug.
Owner:SHENYANG PHARMA UNIVERSITY
Procationic/ cationic liposome curcumin preparation for interventional treatment of hepatic carcinoma and preparation method of preparation
InactiveCN103054802ASolve the unstable "bottleneck" problemEmbody innovationKetone active ingredientsAntineoplastic agentsYolkLiposome
The invention relates to a procationic / cationic liposome curcumin preparation for interventional treatment of hepatic carcinoma and a preparation method of the preparation. The preparation consists of curcumin and multiple components which can be pharmaceutically prepared into a procationic / cationic liposome carrier. The preparation is mainly prepared from the following raw materials in percentage by weight: 0.001 to 5 percent of curcumin, and 95 to 99.999 percent of procationic / cationic liposome carrier. The cationic liposome curcumin preparation is prepared from egg yolk lecithin, cholesterol, octadecyl amine, vitamin E, ether, anhydrous ethanol, curcumin and the like by adopting a film dispersion method. The procationic liposome curcumin preparation is prepared from egg yolk lecithin, cholesterol, [2-[[4-[(carboxymethyl) dithio]-1-imidobutyl] amino] ethyl] carbamic acid cholesteryl ester (CHETA), vitamin E, ether, anhydrous ethanol, curcumin and the like by adopting the film dispersion method. The preparation for the interventional treatment of the hepatic carcinoma is efficient and safe.
Owner:湖北省中医院
Novel composite nano preparation based on sonodynamic/immune combined therapy and preparation method and application thereof
The invention discloses a novel composite nano preparation based on sonodynamic / immune combined therapy and a preparation method and application thereof, the novel composite nano preparation comprises a sound-sensitive agent, an immune activator, an active oxygen enhancing drug and liposome, the sound-sensitive agent and the active oxygen enhancing drug are entrapped in a liposome phospholipid bimolecular layer, and the immune activator is embedded on the lipidosome phospholipid bimolecular layer. The method comprises the following steps: (1) preparing a sound-sensitive agent, a cinnamyl aldehyde derivative, MPLA, a lecithin and DSPE-PEG5k into a solution; (2) evaporating out the solvent in the solution obtained in the step (1), and dispersing the drug-loaded lipid film in a buffer solution to obtain a drug-loaded liposome suspension; and (3) enabling the solution obtained in the step (2) to pass through a polycarbonate film to obtain the composite nano-liposome. The preparation can induce anti-tumor reaction, not only can prevent the development of in-situ solid tumors, but also can prevent the tumors from metastasis to far-end tissues. The composition is especially suitable for a combined treatment preparation of sonodynamic therapy and immunotherapy.
Owner:DALIAN UNIV OF TECH
Preparation method of multifunctional hyaluronic acid lipidosome and quality control standard
The invention provides a preparation method of a multifunctional hyaluronic acid lipidosome and a quality control standard. The method comprises the following steps: (1) weighing phosphatidylcholine (PC), cholesterol (CH) and hyaluronic acid (HA) according to a proportion of 0.1 to 0.03 to 1, dissolving the PC and CH in a mixed solution prepared from petroleum ether / methanol in a volume ratio of 2to 1, dissolving the HA in phosphate buffered saline (PBS), and performing ultrasonic treatment for later use; (2) combining the products obtained in the step (1) to form emulsion, and performing reduced-pressure rotary evaporation for removing an organic solvent to obtain a semi-solid gel substance; (3) adding the PBS into the semi-solid gel substance obtained in the step (2), continually performing rotary evaporation to assist the falling of gel on the wall of a device, and performing ultrasonic treatment to obtain a lipidosome suspension; (4) calculating the encapsulation efficiency of thelipidosome to serve as a target value for evaluating the index of the lipidosome quality. By adopting the method, the hyaluronic acid is conveyed to the keratoderma better, and can act stably.
Owner:TIANJIN POLYTECHNIC UNIV
Glycyrrhetinic acid mediated curcumin long-circulating nanostructured lipid carrier and preparation method thereof
InactiveCN104689321AImprove hydrophilicityExtend cycle timeAntipyreticAnalgesicsLipid formationWater baths
The invention provides a glycyrrhetinic acid mediated curcumin long-circulating nanostructured lipid carrier and a preparation method thereof. The nanostructured lipid carrier is prepared from curcumin, glyceryl monostearate, caprylic / capric triglyceride, a glycyrrhetinic acid-phospholipid derivative, injection soybean lecithin and Polyoxyethylene 40 Strearate. The preparation method includes: firstly conducting water bath heating on lipid and curcumin to 75DEG C, adding anhydrous ethanol to conduct dissolution, performing rotary evaporation to eliminate anhydrous ethanol to serve as the oil phase; adding water for injection into the Polyoxyethylene 40 Strearate, carrying out ultrasonic stirring evenly, then conducting water bath heating to 75+ / - 2DEG C to serve as the water phase; under magnetic stirring, dripping the water phase into the oil phase at the same temperature, carrying out constant temperature stirring for 5min to obtain colostrum, conducting ultrasonic dispersion by an ultrasonic cell crusher for 5min while hot, performing sieving by a 0.22 micrometer microporous filtration membrane, and conducting ice-water bath cooling and curing at room temperature, thus obtaining the glycyrrhetinic acid mediated curcumin long-circulating nanostructured lipid carrier dispersion liquid.
Owner:沈阳医学院附属中心医院
Hair washing and conditioning wet tissue
The invention discloses a wet tissue for shampooing and protecting hair. It includes shampoo wet wipes and hair care wipes. The shampoo and hair care wipes are composed of absorbing shampoo and hair care lotion on the fiber towel respectively. The formula of the shampoo is: AES, APG, amine oxide, egg Phospholipids, tea saponin, non-foaming surfactant 8550, non-foaming surfactant 8551, amino silicone oil, alkyl glycosides, phytosterols, glycerin, ethylene glycol stearate, etc. The formula of hair care wipes is: cationic surfactant (quaternary ammonium salt), cetyl alcohol, stearyl alcohol, tea saponin, ginseng, angelica, Polygonum multiflorum, vitamin E, vitamin 8, silk peptide, hydrolyzed protein, hops, Seabuckthorn and other preparations. Compared with the existing hair shampoo and conditioner, the hair shampoo and conditioner wet tissue provided by the invention is a convenient and easy-to-clean hair-washing tool with no residues on hair.
Owner:HEBEI SHISHIMEI SANITARY PRODS
Konjac glucomannan-liposome composite nano food delivery system and preparation method and application thereof
The invention discloses a konjac glucomannan-liposome composite nano food delivery system and a preparation method and an application thereof, which belong to the technical fields of a biomaterial andslow release. According to the invention, reduced alicyclic amine-grafted konjac glucomannan, cholesterol, egg yolk lecithin and food functional factors are subjected to composite assembly to the nano food delivery system through an ethanol injection method and a film hydration method. The special amphipathicity of system provides simultaneous loading of a plurality of hydrophilic and hydrophobiccomponents, realizes collaborative loading, protection, activity promoting and solubilization of a plurality of food functional factors, can obviously increase the physical and chemical stability andbioavailability, and endows the colon positioning performance and passive targeting. The product has the advantages of good biological compatibility and high entrapment rate, realizes positioning controlled release, stability augmentation and synergy for a plurality of food components, the preparation method is simple, and the method has wide application prospect in the fields of food functionalfactor delivery and synergism.
Owner:HUBEI UNIV OF TECH
Antiaging nutrient composition and application
PendingCN107982280AIncrease the number ofRaise the potentialHeavy metal active ingredientsHydroxy compound active ingredientsHydroxytyrosolArginine
The invention discloses an antiaging nutrient composition. The antiaging nutrient composition is prepared from nucleotide, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, glycine, aspartic acid, leucine, isoleucine, valine, serine, glutamine, glutamic acid, proline, tyrosine, cysteine, gamma-aminobutyric acid, taurine, orotic acid, granulesten, egg yolk lecithin, cephalin, alpha-linolenic acid, gamma-linolenic acid, inositol, hydroxytyrosol, vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, niacin, folic acid, iron, zinc, manganese, copper, selenium, chromium, potassium, calcium, magnesium, choline, L-carnitine, pentose, sodium carbonate and freeze-dried powder of chick embryos hatched for three days. The antiaging nutrient composition has the advantages that neural stem cell proliferation is accelerated, amount of autophagosome is increased and activity of mesenchymal stem cells telomerase is improved.
Owner:吴文国 +2
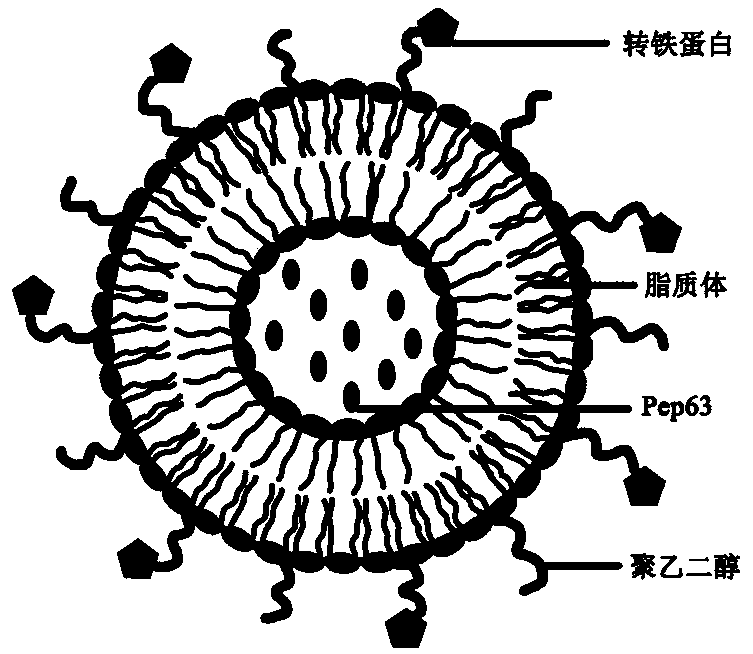
A nanocomposite with therapeutic effect on Alzheimer's disease, and preparation method and application thereof
ActiveCN109200292AInhibition of neurotoxic effectsDelay the pathological processOrganic active ingredientsNervous disorderSide effectCholesterol
The invention discloses a nanocomposite with therapeutic effect on Alzheimer's disease and a preparation method thereof. The nanocomposite comprises a liposome synthesized from lecithin, cholesterol,distearoyl phosphatidylethanolamine and phosphatidic acid and a medicine with the effect of treating Alzheimer's disease contained in the liposome, wherein the liposome is modified with polyethylene glycol and transferrin. The invention utilizes Pep63 to inhibit the neurotoxic effect of ADDLs in the brain of early Alzheimer's disease patients, and simultaneously combines the affinity effect of phosphatidic acid on A beta to play a double coordinating therapeutic effect according to different pathological processes of Alzheimer's disease; At that same time, the surface of the liposome is modifywith transferrin, so that the multifunctional nanocomposite has the brain targeting ability, and can enter the brain through the blood-brain barrier or play a therapeutic role in the periphery; And has the advantages of high safety, biocompatibility and small side effects.
Owner:XUZHOU MEDICAL UNIV
Injection containing nervonic acid and phospholipid compound as well as preparation method and application of injection
ActiveCN104523715ASmall particle sizeNarrow particle size distributionOrganic active ingredientsNervous disorderDiseaseNervonic acid
The invention provides a fat milk injection containing phospholipid, nervonic acid and water for injection. In the injection, the content of the water for injection is larger than 70% in weight ratio; the particle size of fat globules in the injection is smaller than 1 micrometer; the phospholipid comprises lecithin, phosphatidyl ethanolamine and phosphatidyl inositol, wherein the phospholipid and the nervonic acid are combined by a weight ratio of 1:(0.005-1). The invention also provides a preparation method of the fat milk injection and application of the fat milk injection in injection and replenishment of the nervonic acid, the phospholipid, unsaturated fatty acid and medicines for preventing and treating diseases such as cranial nerve degenerative changes, cerebral injuries and disordered brain function.
Owner:哈尔滨医大药业股份有限公司
MOF material grafted with phospholipid bilayer on surface as well as preparation method and application of MOF material
ActiveCN113321815AGood penetration rateGood choiceSemi-permeable membranesDispersed particle separationPolymer scienceCholesterol
The invention discloses an MOF material grafted with a phosphine ester bilayer on the surface as well as a preparation method and application thereof, and belongs to the technical field of membrane separation. The MOF material with the surface grafted with the phosphine ester bimolecular layer is characterized in that the phosphine ester bimolecular layer formed by n-octadecyl phosphonic acid-cholesterol-lecithin is grafted on the surface of a parent MOF material; and the matrix MOF material is ZIF-8, ZIF-67, ZIF-71, ZIF-90, MOF-808 or CuBTC. The preparation method comprises the steps of preparation of OPA-MOF, preparation of OLC-MOF and the like to obtain the OLC-MOF material. The mixed matrix membrane which is used for C3H6 / C3H8 separation and has good C3H6 permeation rate and C3H6 / C3H8 selectivity is obtained by taking the OLC-MOF material as a filler and a polymer and performing ultraviolet irradiation under the action of a photoinitiator.
Owner:中恒新材料科技(山东)有限责任公司
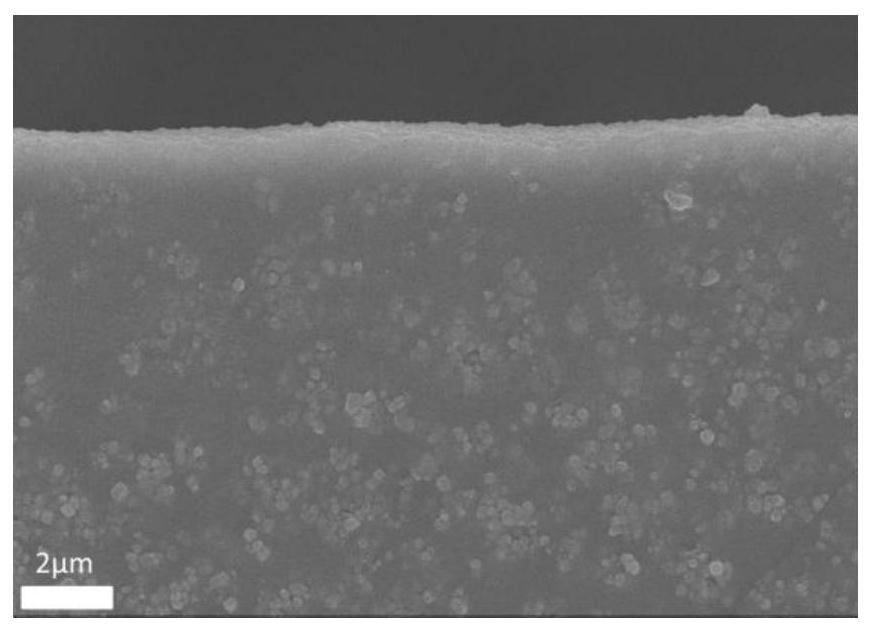
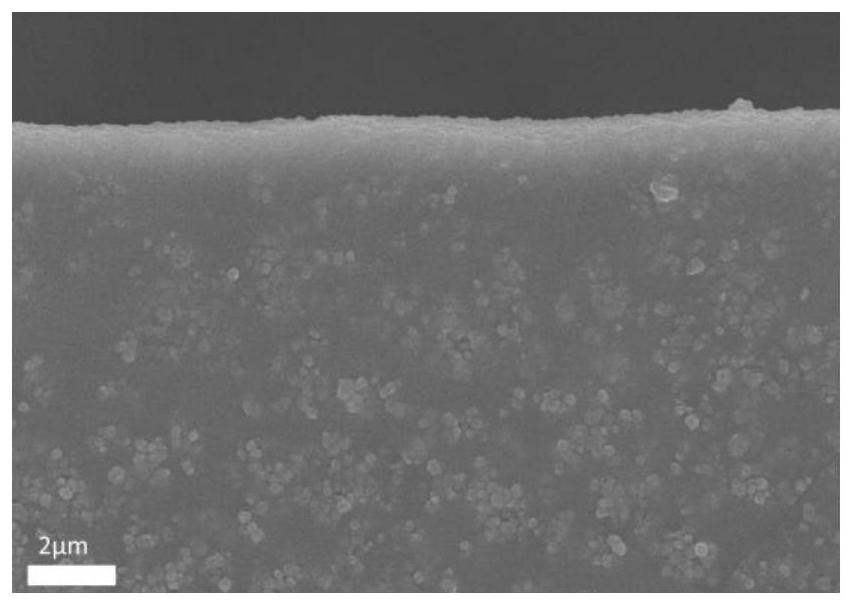
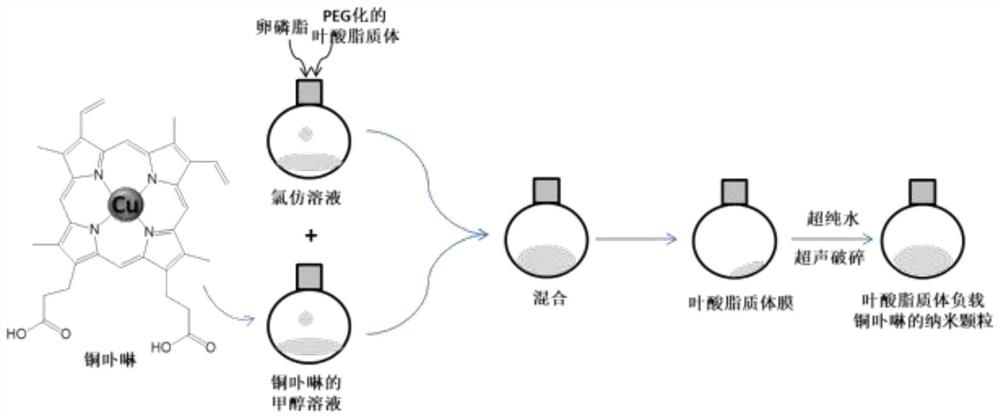
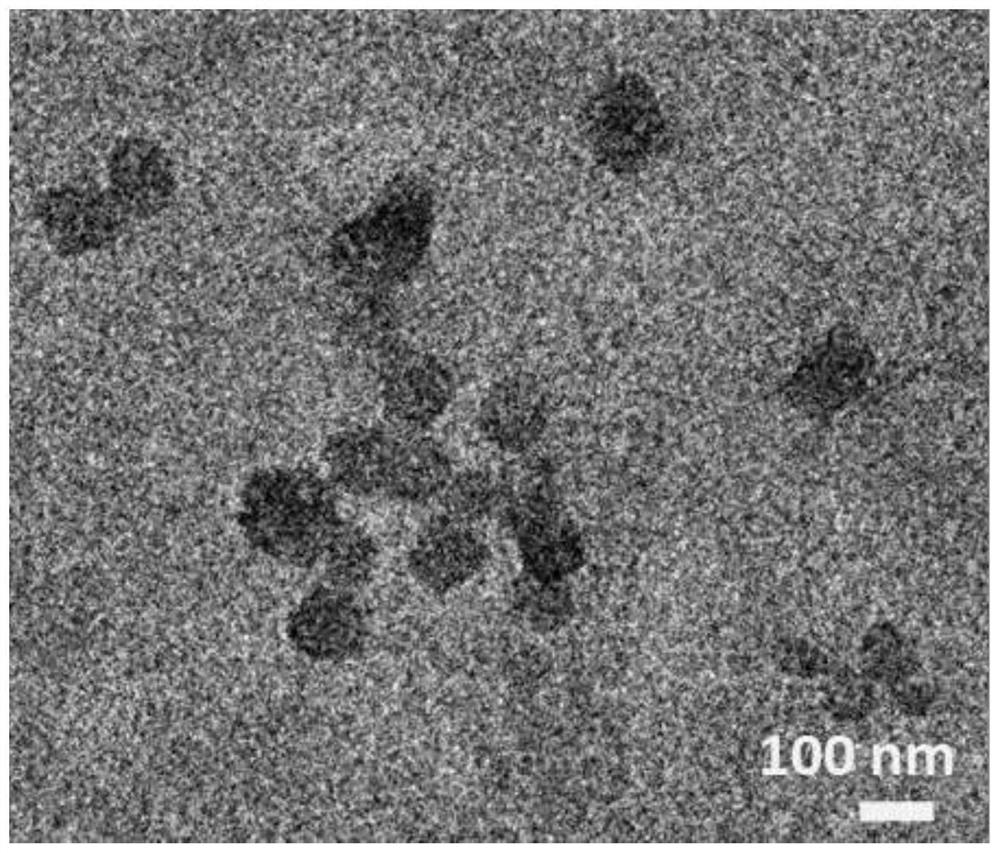
Preparation method of copper porphyrin-folate liposome nanoparticles and application thereof as sound-sensitive agent
PendingCN112451667ASolve solubilitySolve the shortcomings of poor targetingPharmaceutical non-active ingredientsAntineoplastic agentsLipid filmSonodynamic therapy
The invention discloses a preparation method of copper porphyrin-folate liposome nanoparticles and application thereof as a sound-sensitive agent. The preparation method comprises the following steps:dissolving copper porphyrin with methanol, dissolving lecithin and folate liposome in chloroform, mixing the two solutions, preparing a lipid film by a rotary evaporation method, and carrying out ultrasonic hydration with ultrapure water to synthesize the copper porphyrin-folate liposome nanoparticles. The nanoparticles have excellent targeting property in tumor cells with high expression of folate receptors, and are beneficial to enrichment at tumor sites so as to improve the anti-tumor effect; and under ultrasonic excitation, the copper porphyrin absorbs sound energy to generate transitionand converts surrounding oxygen into singlet oxygen to kill tumor cells. The invention provides the sound-sensitive agent capable of generating singlet oxygen through ultrasonic excitation to kill thetumor cells, and the folate targeted liposome is used as a carrier to carry the sound-sensitive agent, so that the water solubility and the targeting property are improved, and the sonodynamic therapy (SDT) effect is further improved.
Owner:GUANGDONG MEDICAL UNIV
Essential oil liposome as well as preparation method and application thereof
The invention relates to an essential oil liposome as well as a preparation method and application thereof. The essential oil liposome is prepared from the following materials in parts by weight: 100parts of phospholipid, 3-25 parts of cholesterol, 3-20 parts of pummelo exocarp essential oil, 3-30 parts of emulsifier and 100-500 parts of water. The phospholipid is selected from at least one of the soybean lecithin and the egg yolk lecithin; and the emulsifier is selected from at least one of the span, the tween, the propylene glycol and the glycerol. It can effectively improve stability of the essential oil and has a slow-release performance. In addition, the essential oil liposome has the functions of reducing phlegm, relieving cough, anti-inflammatory and the like, and has a fragrant smell, mild and low irritation. When being used for preparing an essential oil liposome product, it has good effects in reducing phlegm, relieving cough and anti-inflammatory, also has good stability and good experience.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Ginkgo-damole lipidosome preparation and preparation method thereof
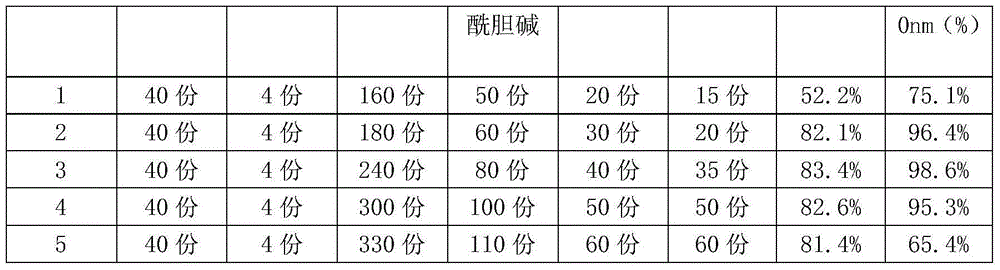
ActiveCN104971043APoor water solubilityGood fat solubilityPharmaceutical non-active ingredientsGinkgophyta medical ingredientsLong actingChemistry
The invention discloses a ginkgo-damole lipidosome preparation and a preparation method thereof. The ginkgo-damole lipidosome preparation is prepared from the following ingredients in parts by weight: 40 parts of ginkgo leaf extract, 4 parts of dipyridamole, 180-300 parts of dimyristyllecithin, 60-100 parts of phosphatidylcholine distearate, 30-50 parts of cholesterol and 20-50 parts of sucrise ester as well as other preparation auxiliary materials. The ginkgo-damole lipidosome preparation disclosed by the invention is capable of improving the storage stability of the medicine preparation, continuously releasing the medicine in lipidosome, prolonging the detention time of the medicine in blood circulation and improving the bioavailability of the medicine, thereby achieving efficient and long-acting treatment purpose.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com