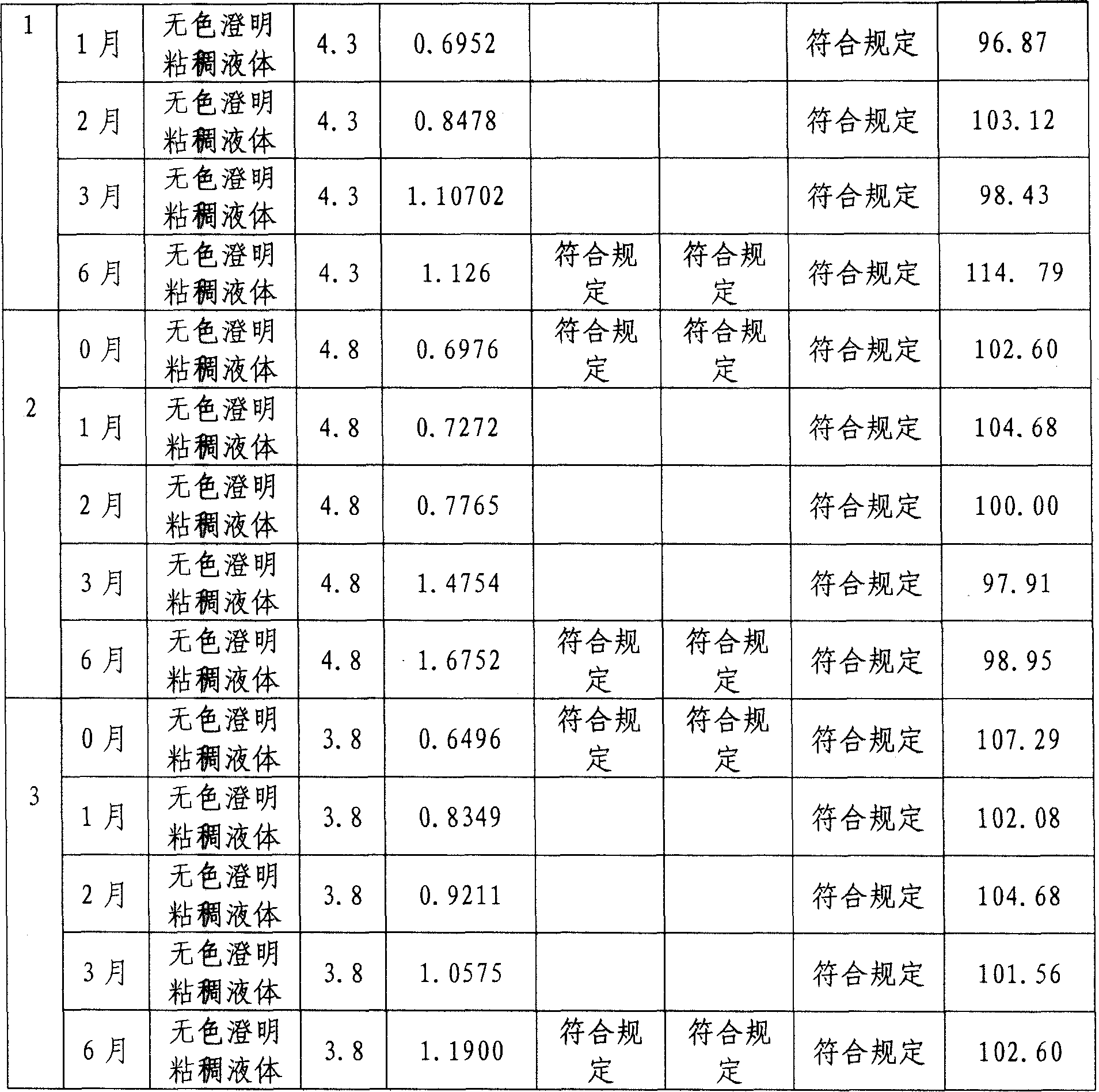
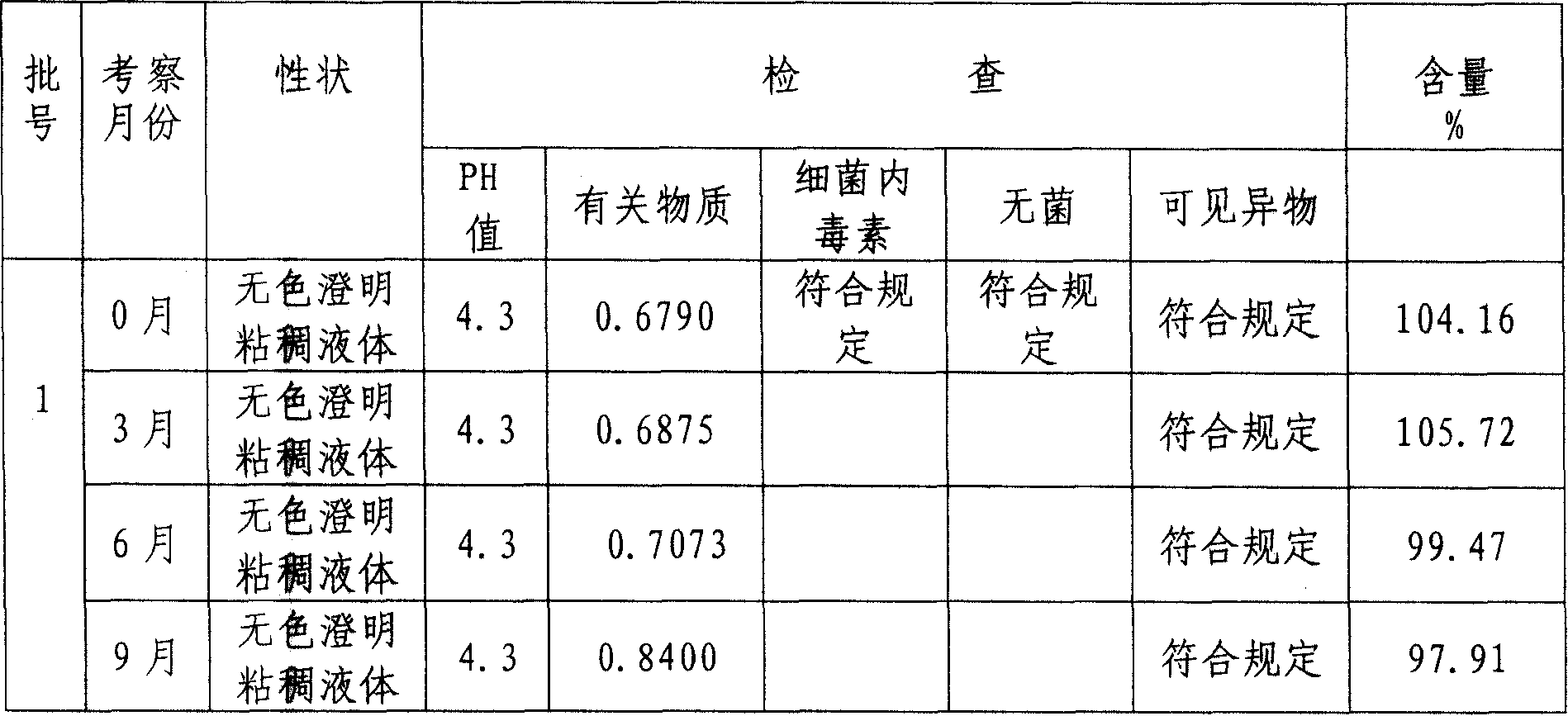
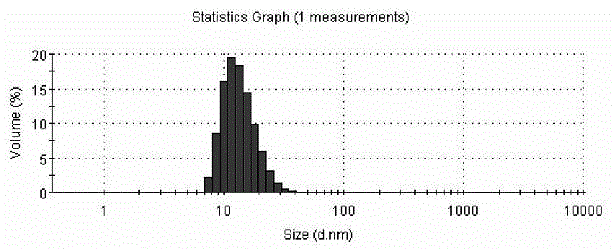
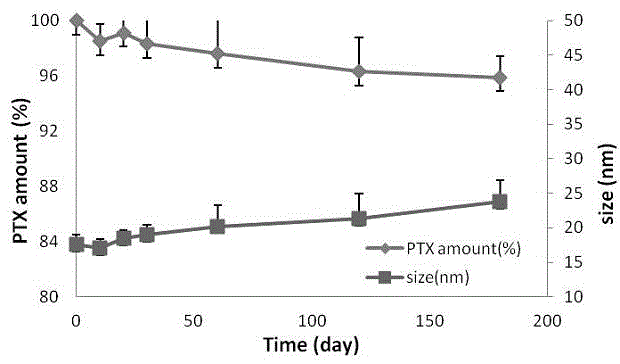
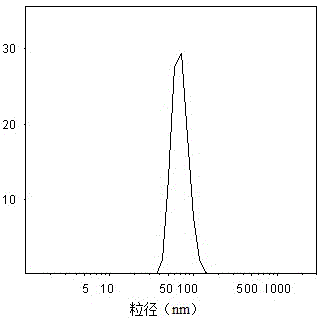
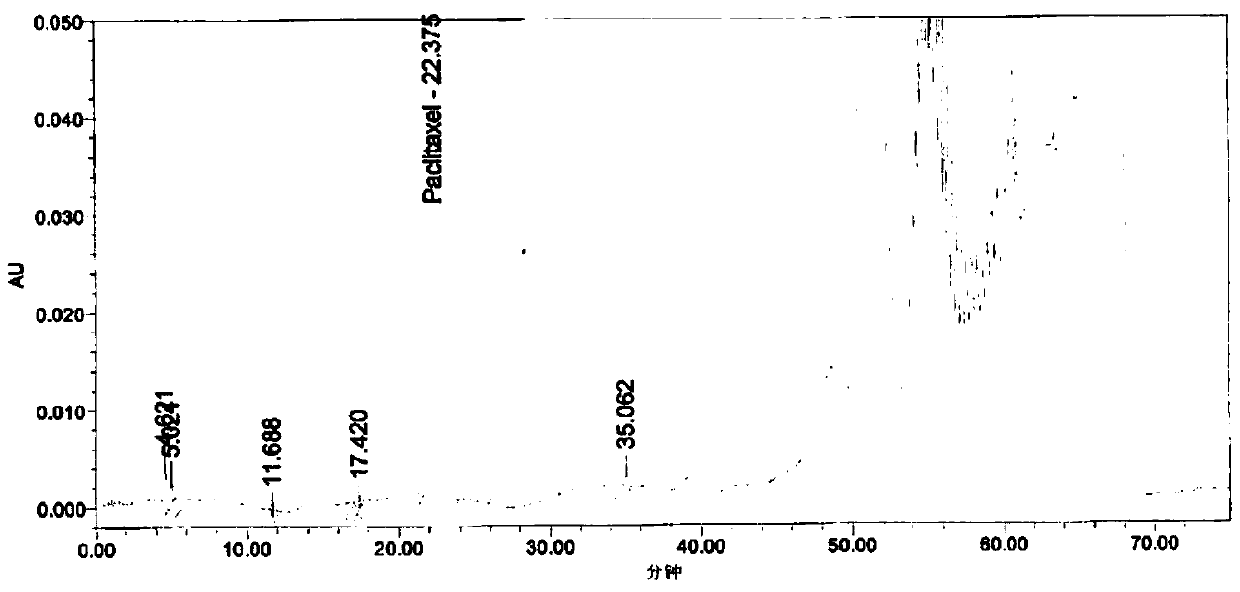
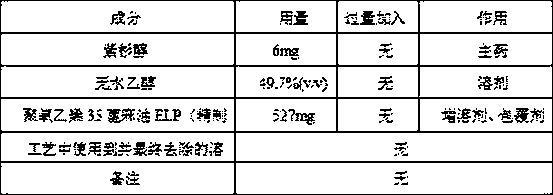
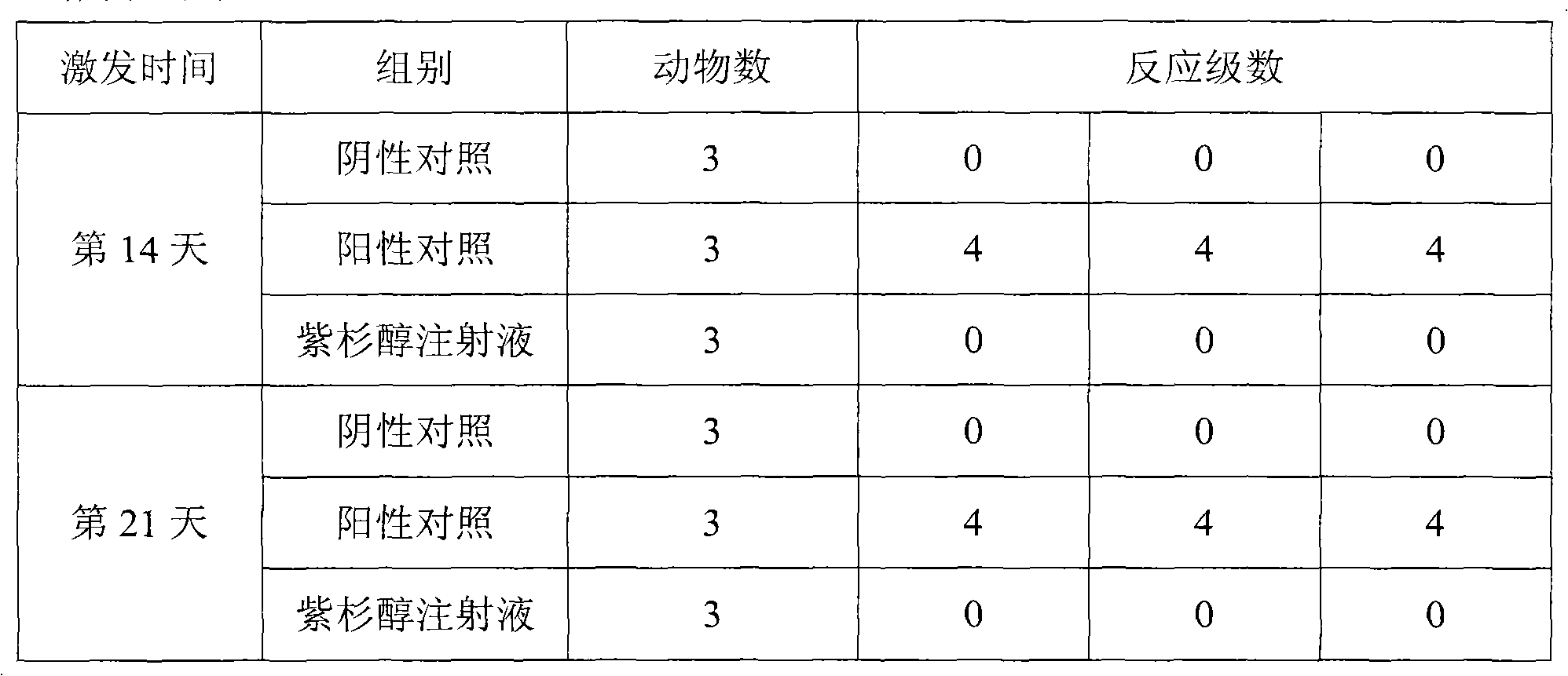
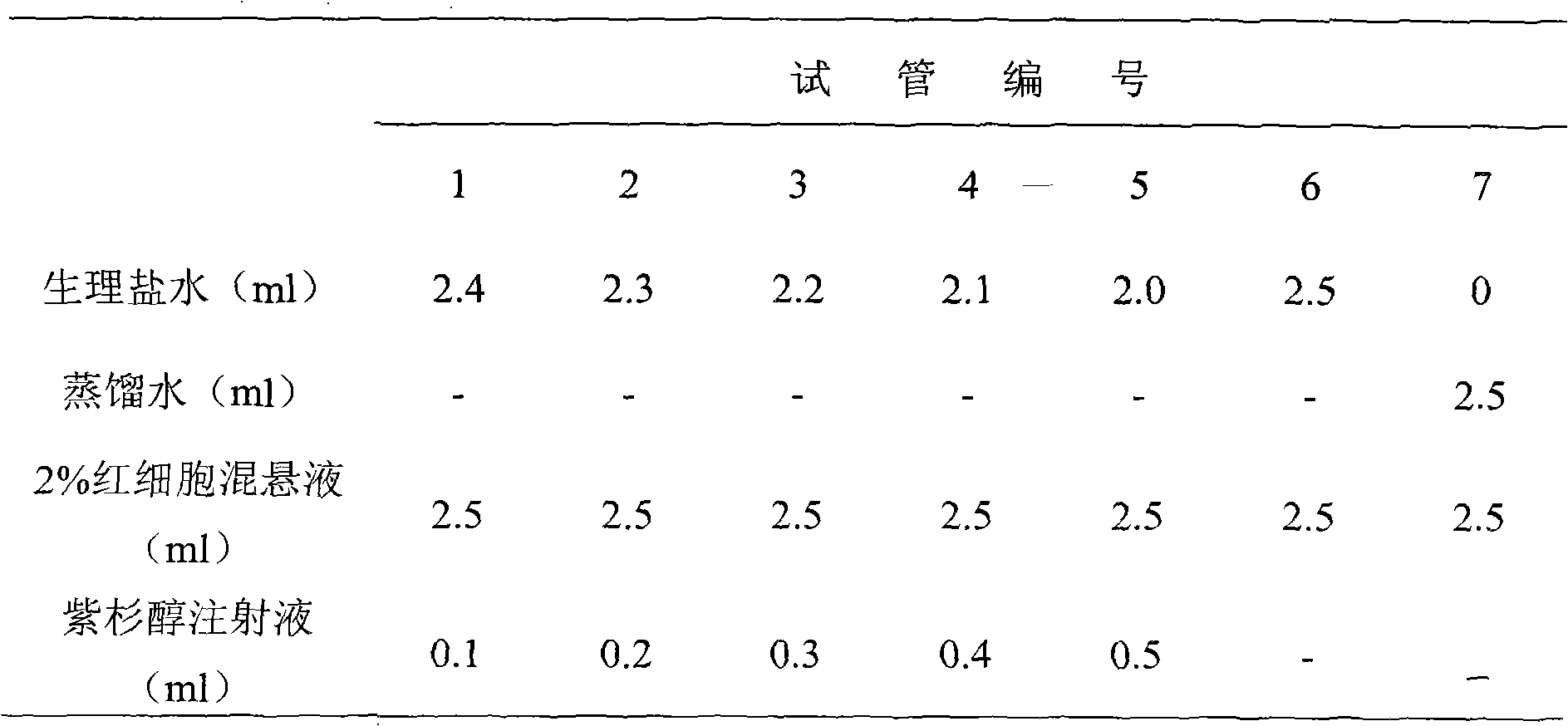
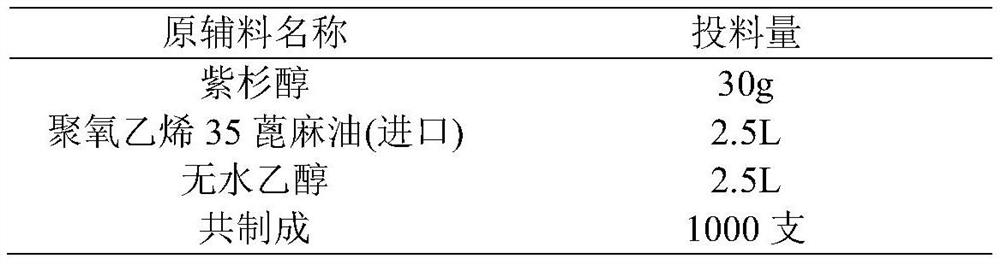
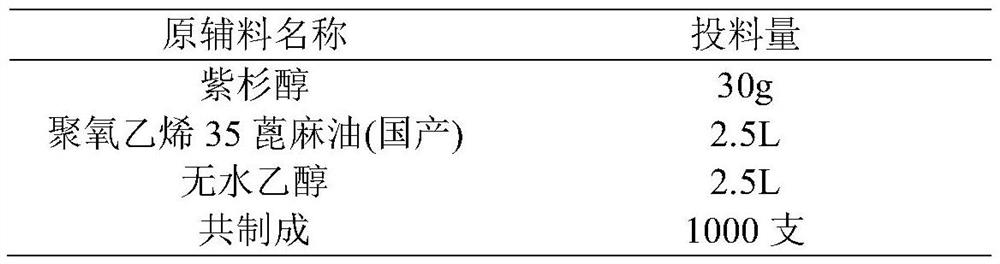
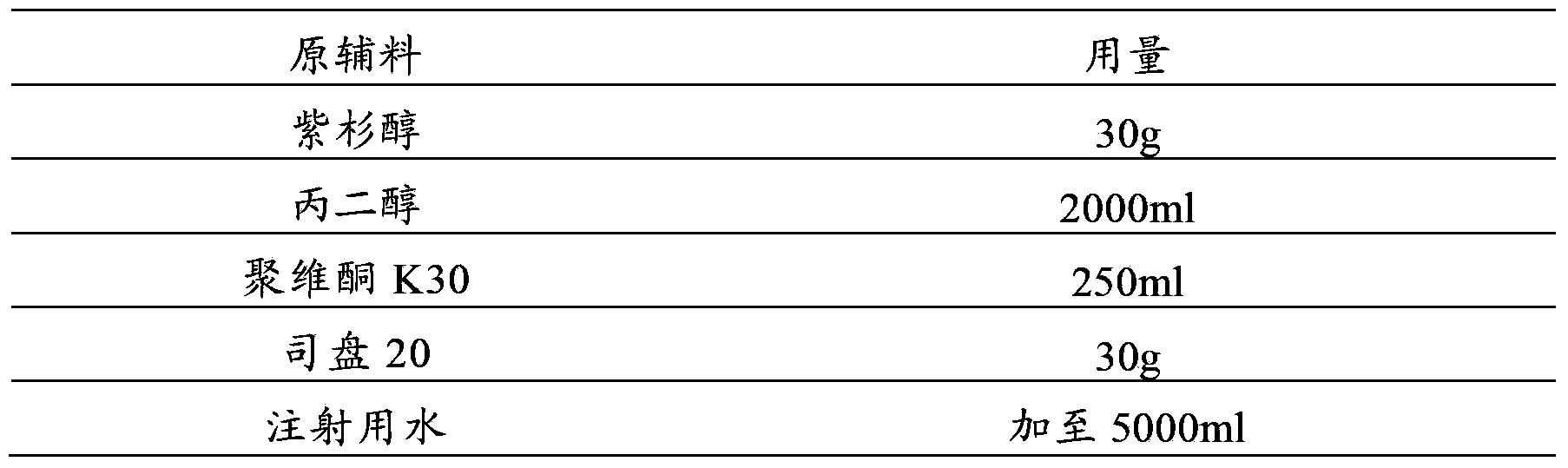
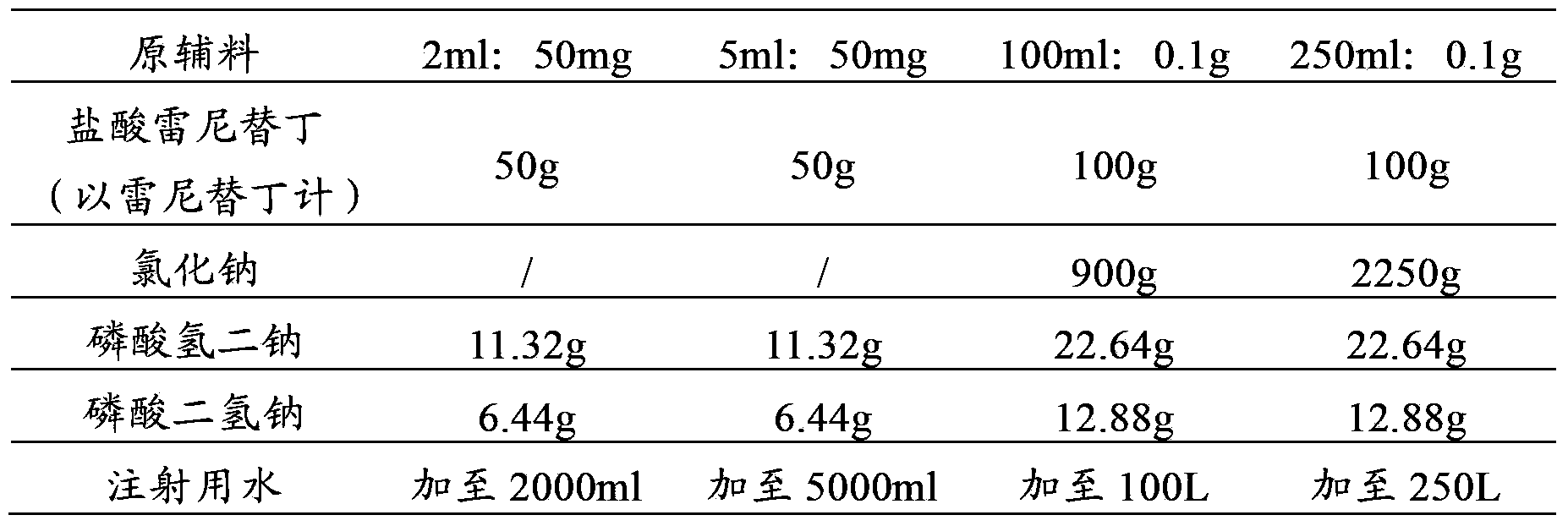
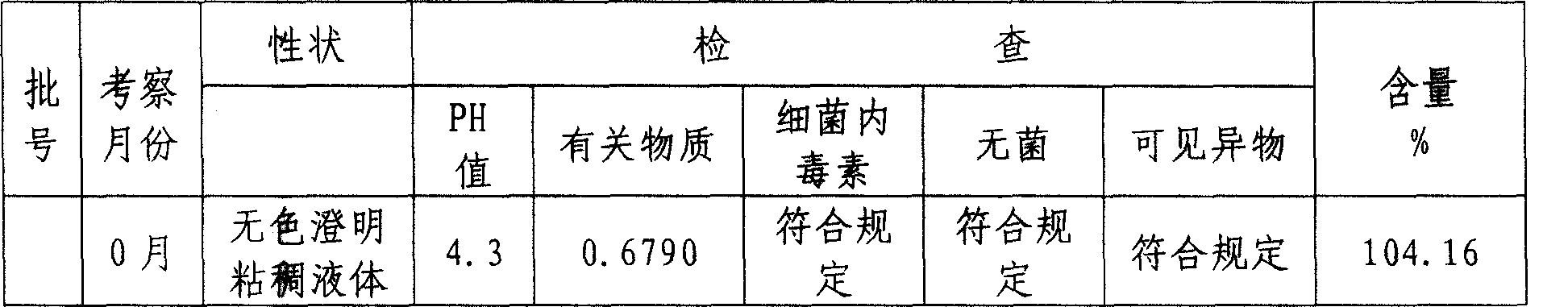
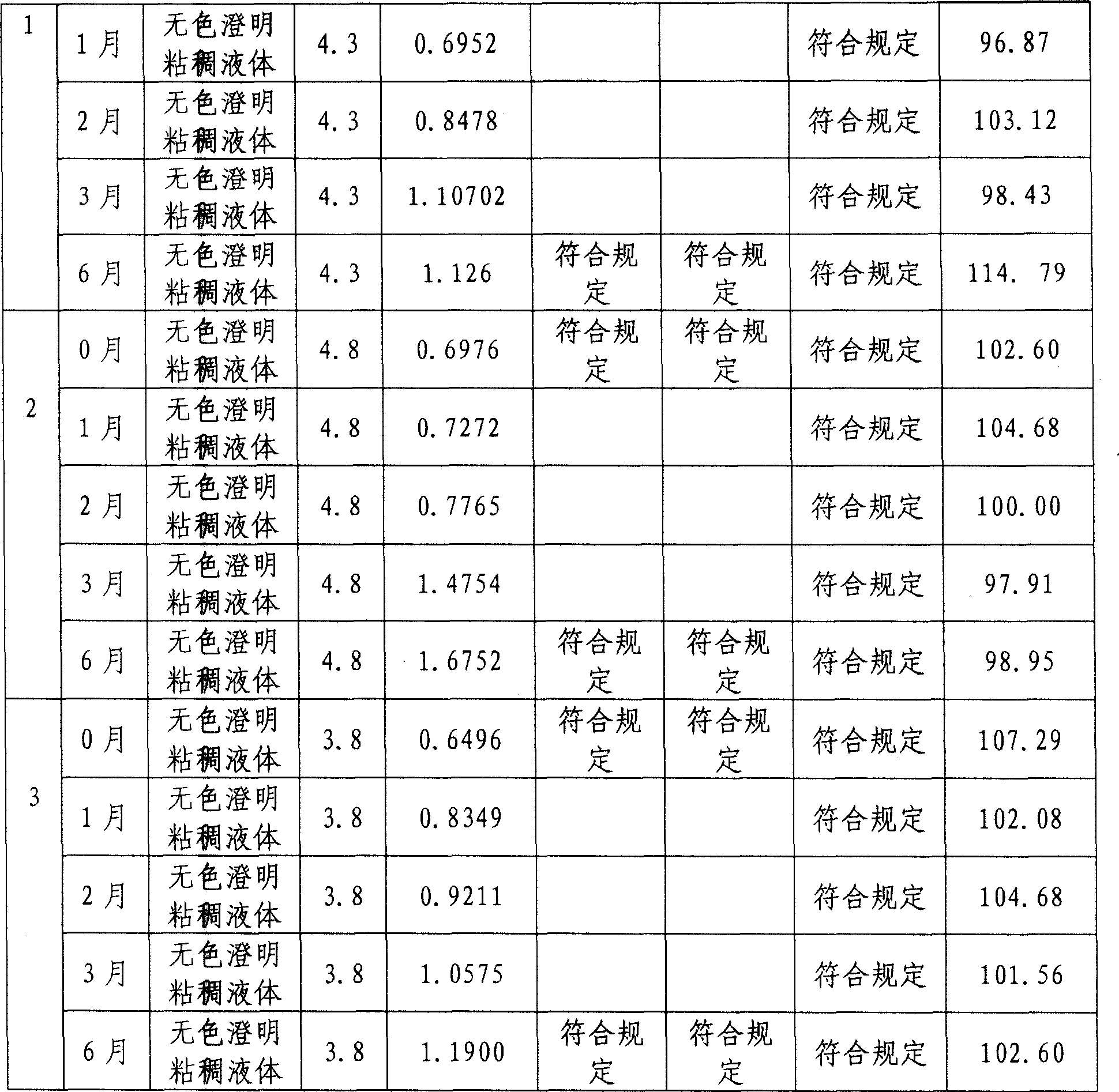
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Paclitaxel Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
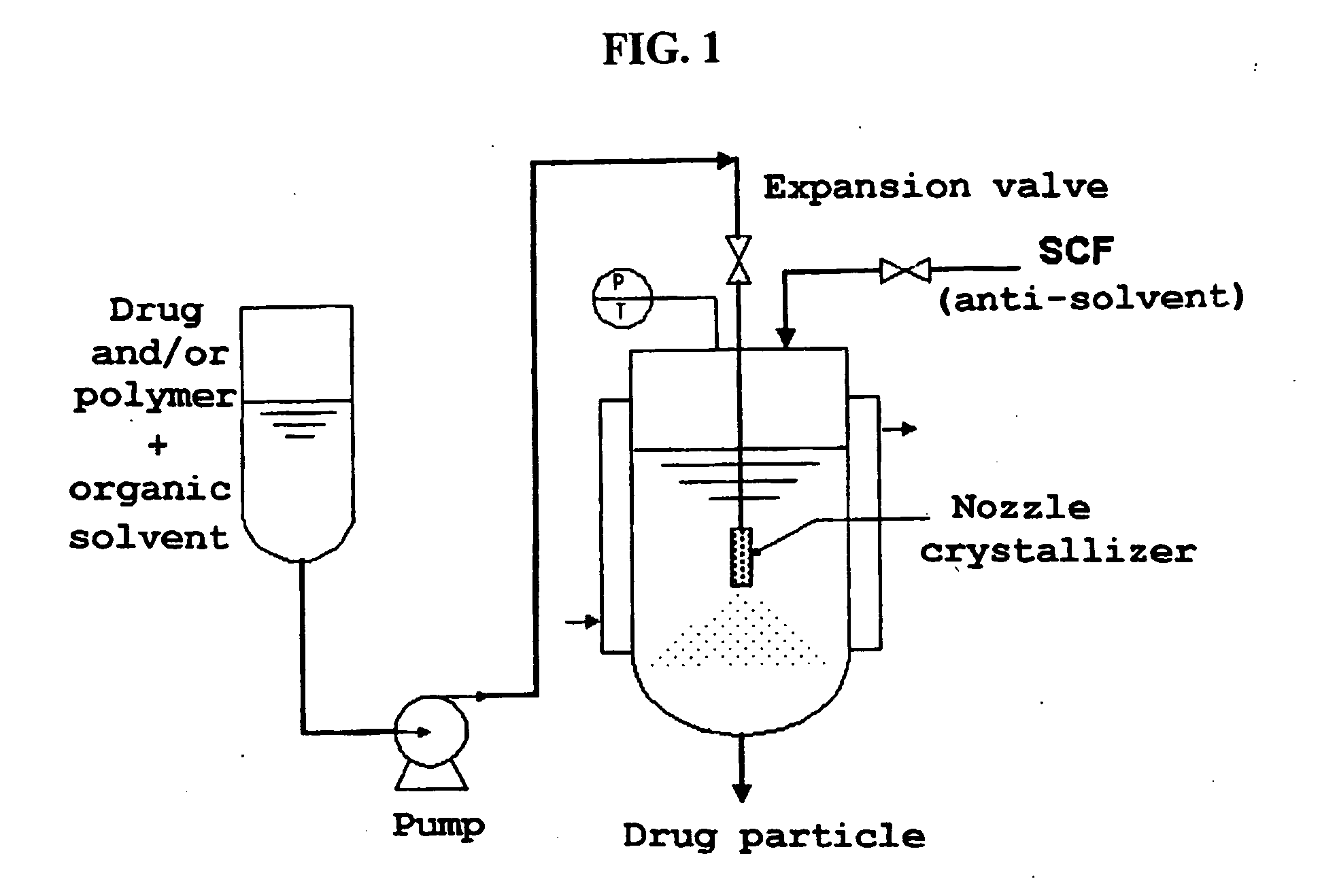
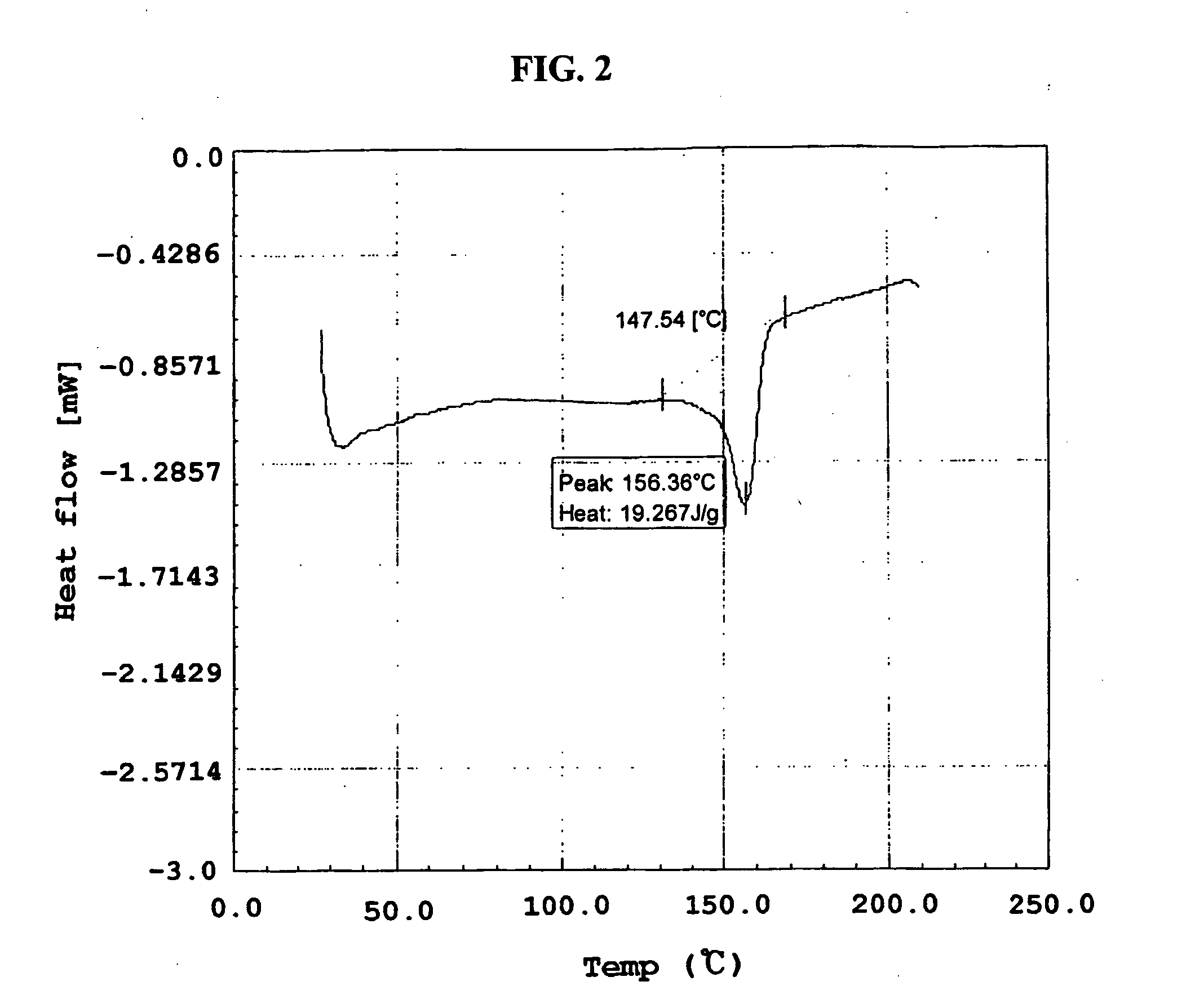
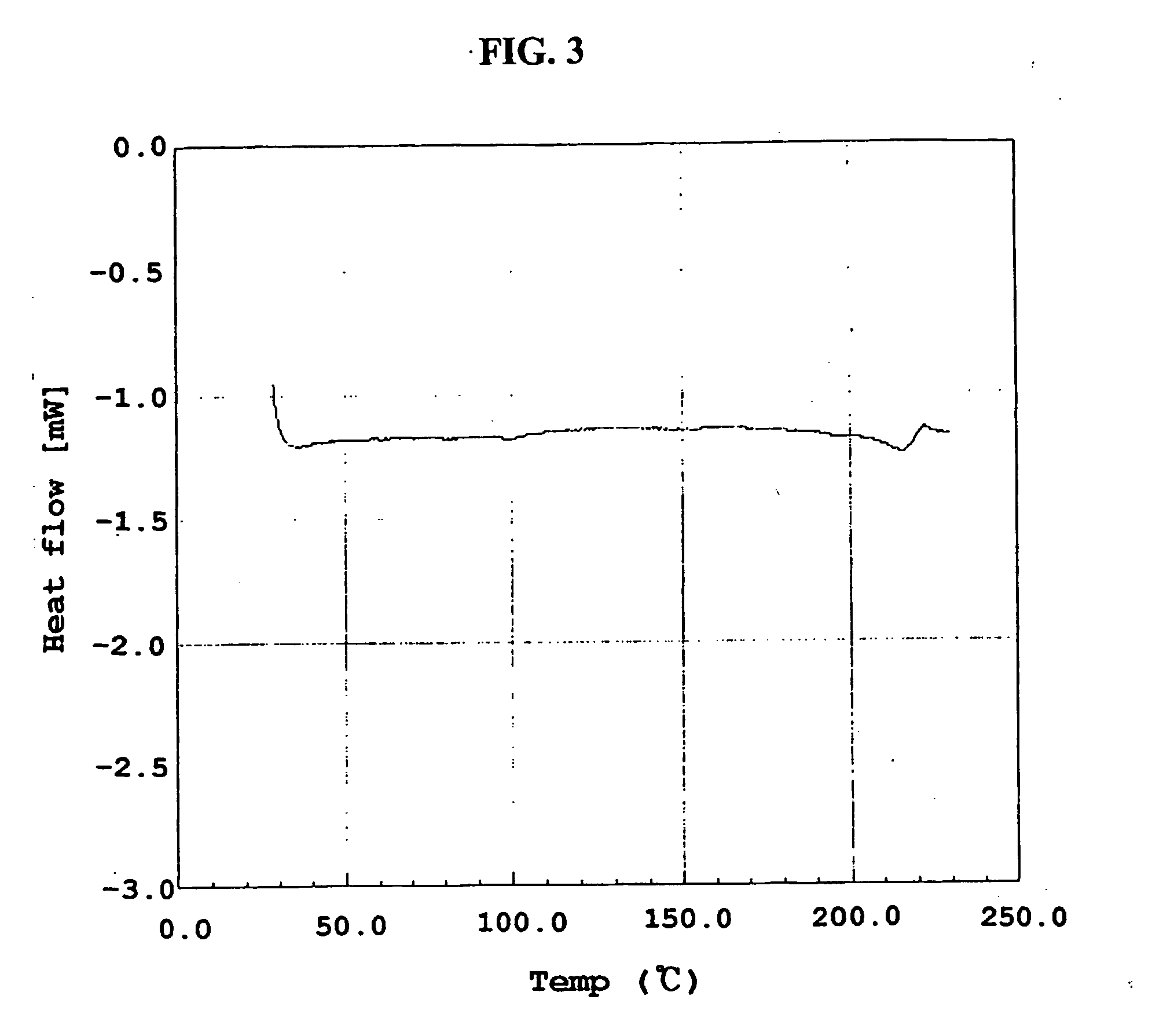
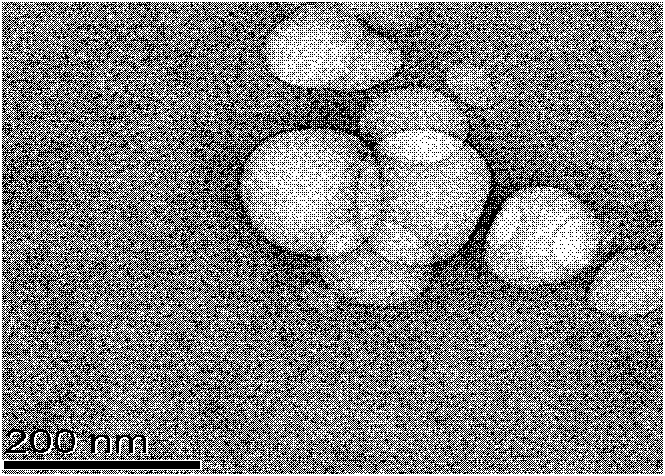
Method for the preparatin of paclitaxel solid dispersion by using the supercritical fluid process and paclitaxel solid dispersion prepared thereby
The present invention relates to a method for the preparation of paclitaxel solid dispersion by using the supercritical fluid process and paclitaxel solid dispersion prepared thereby, the paclitaxel solid dispersion being highly homogeneous and showing an improved solubility, thereby being effectively used for the preparation of paclitaxel injection and oral preparation having a high bioavailability.
Owner:HANMI PHARMA
Paclitaxel mixed micelle preparation, and preparation method thereof
InactiveCN102198084AReduce toxic and side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPolyoxyethylene castor oil
The invention discloses a paclitaxel mixed micelle preparation, comprising 100 to 300 milligram of tocopherol polyethylene glycol succinate 1000 (TPGS), 0 to 50 milligram of phosphatide, 0.5 milliliter of anhydrous ethanol and 6 milligram of paclitaxel. The preparation method is as follows: the TPGS is dissolved in the anhydrous ethanol, or the TPGS and the phosphatide are dissolved in the anhydrous ethanol; the paclitaxel is added and dissolved under stirring; the mixture is filtered with a millipore filtration of 0.22 micrometer so as to obtain the paclitaxel mixed micelle preparation. In the invention, the TPGS and the phosphatide are used to form mixed micelles which have good stability and little toxic and side effects; the preparation method is simple and practicable, having a good application prospect. Compared to the prior art, polyoxyethylene castor oil in conventional prescription is substituted in the invention, thereby reducing toxic side effects of paclitaxel injections and greatly enhancing the safety of the injections on condition that solubility is guaranteed.
Owner:SHANDONG UNIV
DSPE-PEG-FA-modified nanometer paclitaxel liposome and preparation method thereof
InactiveCN102188382AHigh encapsulation efficiencyGuaranteed purityOrganic active ingredientsPharmaceutical non-active ingredientsPaclitaxel InjectionDspe peg
The invention relates to a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. A molar ratio of the DSPE-PEG2000-FA to egg yolk lecithin is 0.05% to 0.15%, and a particle size of the liposome is less than 150 nm. A preparation method of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome comprises the following steps: first, preparing a DSPE-PEG2000-FA into a DSPE-PEG2000-FA micelle; second, performing incubation on the phosphatidyl ethanolamine-polyethylene glycol2000-folic acid micelle and a paclitaxel liposome together to obtain a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. Materials, which are forbidden to be used in clinical practice, are not used as crude materials in the present invention. According to the invention, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has a small particle size, and the content of the DSPE-PEG2000-FA is low; besides, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has good drug entrapment efficiency and good colloid stability. Moreover, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome can be absorbed effectively by an ovarian cancer cell having properties of sensitiveness to folic acid (+) and drug resistance, and the cytotoxicity of folic acid dependence is displayed; therefore, the efficacy of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome is stronger than that of a paclitaxel injection.
Owner:李红霞
Paclitaxel injection and preparation method thereof
ActiveCN101190214AQuality assuranceEasy to useOrganic active ingredientsPharmaceutical delivery mechanismSaline waterPEG 400
The invention relates to a paclitaxel injection and a preparation method of the injection: (1) 200 to 400ml of absolute alcohol and 200 to 400ml of normal saline are taken to be stirred, and 150 to 400ml of polyethylene glycol 400 is added to be evenly mixed; (2) 20 to 40g of tween-80 is added in to the mixture of (1) to be evenly mixed, and then 0.2 to 0.5g of active carbon used for injection is added to be heated by 35 to 40 DEG C, and raw filtering is carried out and the PH value is controlled to be between 3 to 5; filtering is done and filling and sealing process is carried out; sterilization is carried out for 20 to 35 minutes at the temperature of 100 to 125 DEG C; (3) a transfusion bottle is washed and then is dried for 25 to 35 minutes the temperature of 230 to 260 DEG C, and butyl rubber plug is sterilized; (4) 0.6 to 3g of paclitaxel is taken to be added into the mixture of (2) for dissolving, and then sterilization and filtering are done; and then the solution is filled into the transfusion bottle in the sterilized environment; finally, the bottle is sealed to get the finished product. The invention can prepare safe and reliable paclitaxel injection, by adopting the method of preparing menstruum firstly and paclitaxel solution secondly, the quality of the injection is guaranteed.
Owner:GUANGDONG KELUN PHARMACEUTICAL CO LTD
Paclitaxel lipid microspheres injection and preparation method thereof
InactiveCN101204373ALess irritatingNot easy to precipitateOrganic active ingredientsSolution deliveryLipid formationPolyoxyethylene castor oil
The invention relates to a paclitaxel lipid microsphere injection and a manufacturing method thereof. In the invention, a paclitaxel lipid microsphere injection that does not contain polyoxyethylene castor oil is prepared. The medicine 90-98 per cent paclitaxel is coated in the oil phase and the oil-water interfacial film of the lipid microsphere. Thus the toxicity and stimulation of paclitaxel in clinic use are largely reduced, and the adverse reaction resulted from the excipient in the existing paclitaxel injection is prevented. With low stimulation, low toxicity and high efficiency, the preparation as a antineoplastic drug is administrated by intravenous injection.
Owner:李时海 +1
Paclitaxel entrapped polymeric micelle for treating tumors, and preparation method thereof
InactiveCN104997758AHigh drug loadingReduce adverse reactionsOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention belongs to the technical field of biomedicines, and relates to a paclitaxel entrapped polymeric micelle for treating tumors, and a preparation method thereof. The preparation method comprises the following steps: preparing paclitaxel PEG-PLA / Vitamin E-TPGS mixed micelle through adopting a film dispersion technology, and entrapping paclitaxel into the hydrophobic core of the mixed micelle by using the mixed micelle composed of PEG-PLA and Vitamin E-TPGS as a carrier. The prepared paclitaxel paclitaxel PEG-PLA / Vitamin E-TPGS mixed micelle can effectively increase the solubility of paclitaxel and substantially improves the paclitaxel load of a micelle system to reach 35% (w / w), and Vitamin E-TPGS in the mixed micelle system has a P-gp efflux inhibition biologic function, and can efficiently reverse the multidrug resistance of tumors in order to improve the tumor treatment effect. The mixed micelle does not contain Tween80 or ethanol; and compared with commercial paclitaxel injections, the above preparation provided by the invention has the advantages of no allergy of commercial preparations, reduction of toxic side effects, and increase of the safety of clinic application.
Owner:FUDAN UNIV
Paclitaxel-entrapped biodegradable nanocomposite and preparation method thereof
InactiveCN102836147AImprove solubilityPlay the role of "invisibility"Organic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention belongs to the technical field of biological medicines and relates to a paclitaxel-entrapped biodegradable nanocomposite and a preparation method thereof. A paclitaxel polyethylene glycol-poly trimethylene carbonate (PEG-PTMC) nanocomposite is prepared by adopting an emulsification / solvent evaporation method, and by taking a PEG-PTMC copolymer as a carrier, paclitaxel is entrapped in a hydrophobic core of PTMC. The solubility of the paclitaxel can be effectively increased by the prepared paclitaxel PEG-PTMC nanocomposite, and a PEG long chain of a carrier material can take effect of invisibility, and thereby, the phagocytosis of an in-vivo reticulo-endothelial system is avoided. The nanocomposite has a long circulation effect, and the half-life period of the nanocomposite in blood can be prolonged. Moreover, through controlling the grain size of the nanocomposite, a passive targeting effect on a tumor tissue is realized, and thereby, a treatment effect is improved. As the nanocomposite does not contain Cremophor El or ethanol, compared with commercially available paclitaxel injections, the toxic and side effects of the nanocomposite can be reduced, and the safety of the nanocomposite in clinical application is enhanced.
Owner:FUDAN UNIV
Paclitaxel injection, and its prepn. method
InactiveCN1723887AImprove stabilityGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPaclitaxel Injection
Owner:重庆华立药业股份有限公司
TPE material for medical infusion device tube and preparation method thereof
InactiveCN104448668AGood biocompatibilityImprove antioxidant capacityPolyoxyethylene castor oilProcedure Agents
The invention discloses a TPE material for medical infusion device tube and a preparation method thereof. The TPE materials includes the following raw materials by weight: 100 portions of SEBS, 30 portions of co-polypropylene, 20 portions of oleamide and 1 portion of an antioxidant. The medical infusion device tube prepared from the TPE material has no adsorption effect on many drugs, so that drug dosage accuracy and the therapeutic effect are guaranteed; and the problem of participation of released paclitaxel injection and the polyoxyethylene castor oil in plasticizing agent in the reaction are avoided, so as to ensure the use effect of anti-cancer drug such as paclitaxel. In addition, the medical infusion device tube can avoid the problem of migration of processing aid or plasticizer into the blood or in medical liquid, and the important indexes including pH value variation, reducing substances, heavy metal content, cell toxicity, UV absorbance, sensitization response and hemolytic reaction are superior to those of the similar polyvinyl chloride products, so as to ensure the safety of patients.
Owner:SHANGHAI KANGDELAI ENTERPRISE DEV GRP CO LTD
Pharmaceutical composition of paclitaxel injection
InactiveCN103330941AImprove solubilityEasy to prepareOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPaclitaxel Injection
The invention relates to a pharmaceutical preparation composition, in particular to a pharmaceutical composition containing a paclitaxel injection. The pharmaceutical composition consists of paclitaxel, sodium acetylsalicylate, 1,2-propylene glycol and absolute ethyl alcohol.
Owner:河北三禾实创生物科技有限公司
Taxol microemulsion drug composition and preparation method thereof
ActiveCN103110581ASolve insolubleSolve the freeze-drying formabilityOrganic active ingredientsPowder deliveryFreeze-dryingPaclitaxel Injection
The invention discloses a taxol microemulsion drug composition and a preparation method thereof. The drug composition comprises the following active ingredients: taxol, an oil phase, a solubilizing agent, a stabilizing agent, an excipient, an acidity regulator and water for injection and is characterized in that the main grain size of taxol microemulsion ranges from 10nm to 40nm; and the parts by weight of taxol, an emulsifying agent, the oil phase and the water phase are respectively 30, 1500-3000, 100-350 and 5000-50000. The drug composition has the beneficial effects that taxol is taken as the main drug and different auxiliary materials are added to prepare microemulsion; the taxol microemulsion freeze-dried preparation prepared through freeze drying has good water solubility and stability; the clinical defect of severe allergic reaction of the taxol injection in the market is overcome; meanwhile, the preparation process is simple and is suitable for industrial mass production; and a new administration dosage form is provided for clinical application of taxol.
Owner:SOUTHWEST UNIVERSITY +1
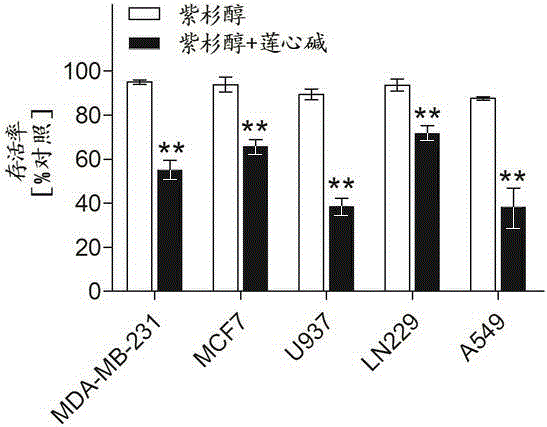
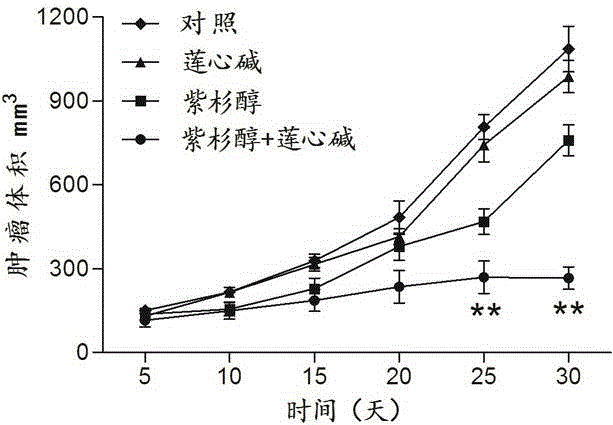
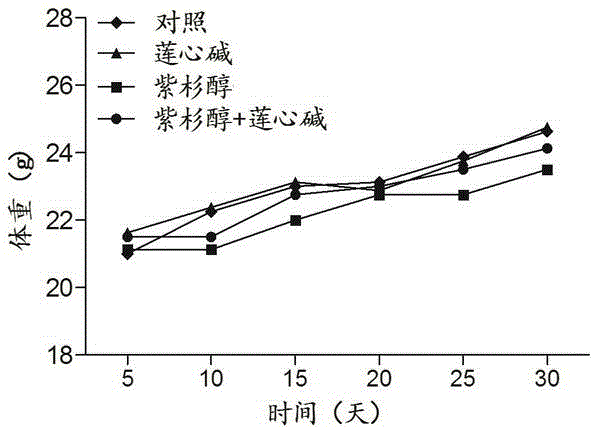
Pharmaceutical composition using liensinine to work in coordination with paclitaxel for chemotherapy and application method of pharmaceutical composition
InactiveCN104586847AGood curative effectReduce adverse reactionsOrganic active ingredientsAntineoplastic agentsTreatment effectDrug withdrawal
The invention relates to a pharmaceutical composition using liensinine to work in coordination with paclitaxel for chemotherapy and an application method of the pharmaceutical composition. The pharmaceutical composition comprises a liensinine injection solution and a chemotherapy drug paclitaxel injection solution. The application method comprises the following steps: (1) performing intravenous injection of liensinine once daily according to the dose that actual dosage in the liensinine injection solution to the body weight of a patient is equal to 4.88mg / kg; (2) performing intravenous injection of paclitaxel once according to the dose that the actual dosage in the paclitaxel injection solution to the body weight of the patient is equal to 2.562mg / kg on the day of injecting liensinine according to the step (1); (3) performing intravenous injection of the paclitaxel injection solution once again according to the dose in the step (2) on the twenty-second day; and (4) determining whether to circulate according to the method of the steps (1)-(3) or not, the number of circulation times and the intermediate drug withdrawal time from the forty-third day according to the bearing capacity and the treatment effect of a patient body. Compared with the prior art, in the aspect of inhibiting and killing various malignant tumor cells, the pharmaceutical composition provided by the invention has the advantages of reducing the dosage, shortening the treatment period and reducing the occurrence of toxic and side reactions.
Owner:ARMY MEDICAL UNIV
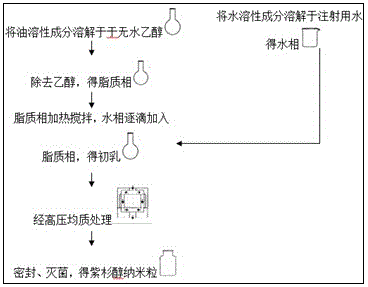
Preparation method of paclitaxel injection
ActiveCN109044969AReduce direct effectReduced stabilityOrganic active ingredientsPharmaceutical delivery mechanismAnhydrous ethanolSolubility
The invention discloses a preparation method of paclitaxel injection. A formula comprises the following components: 30 parts by weight of paclitaxel, 1000 to 1500 parts by volume of anhydrous ethanol,18 to 24 parts by volume of lactic acid, and 1000 to 1500 parts by volume of polyoxylethylene castor oil ether (35). Compared with the prior art, the prepared paclitaxel injection is high in solubility, uniform in concentration, stable in quality, and low in acute adverse reaction, and the preparation method is simple, easy to control, high in production efficiency, and low in production energy consumption.
Owner:海南卓泰制药有限公司
Paclitaxel injection and preparation method thereof
ActiveCN110882213AImprove solubilityUniform concentrationOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPaclitaxel Injection
The invention discloses a paclitaxel injection and a preparation method thereof, which belong to the technical field of biological medicines. The paclitaxel injection is composed of paclitaxel, absolute ethyl alcohol, castor oil polyalkoxy ester (35) and a pH regulator. 250ml + / -5% of absolute ethyl alcohol and 250 ml + / -5% of castor oil polyalkoxy ester (35) need to be added to every 3g + / -5% ofpaclitaxel in a matched manner. The paclitaxel injection disclosed by the invention has the advantages of good solubility, uniform concentration, low content of related substances and stable quality.The preparation method is simple, easy to control and high in production efficiency.
Owner:海南紫杉园制药有限公司
Paclitaxel lipid nanoparticle injection liquid with anti-tumor activity
InactiveCN102871963AImprove solubilityReduce eliminationOrganic active ingredientsPowder deliveryPaclitaxel InjectionActive agent
The invention provides paclitaxel lipid nanoparticle injection liquid, which comprises paclitaxel medicine, lipid materials, water for injection, surfactant, stabilizers and metal ion chelating agents. Most medicine is wrapped inside lipid, the direct contact between the medicine and blood or other liquid is avoided, and the local irritation of the medicine on the injection position is reduced, and the toxicity is reduced. No any organic solvents are contained, no solvents with toxicity and irritation on human bodies are contained, and no irritation and no sensitization are caused on the human bodies. The adverse reaction of the existing preparation caused by the recipe problem can be eliminated, and the goals of safety and efficiency are reached. Meanwhile, the paclitaxel lipid nanoparticle injection liquid has the excellent characteristic that the tumor medicine resistance is overcome, and the cure efficiency of the paclitaxel on the existing various tumors is greatly improved.
Owner:ZHEJIANG UNIV
Preparation method of paclitaxel injection resistant to terminal sterilization
ActiveCN110123745ARealize the packageAvoid material degradationOrganic active ingredientsPharmaceutical delivery mechanismMaterial DegradationDecomposition
The invention relates to a preparation method of paclitaxel injection resistant to terminal sterilization, belonging to the technical field of pharmaceutical preparations. The preparation method of the paclitaxel injection comprises the following steps: (1) adding a prescribed amount of paclitaxel into a prescribed amount of polyoxyethylene (35) castor oil, and stirring and shearing at a high speed; (2) adding a prescribed amount of absolute ethanol into the solution, stirring until the solution is mixed evenly, and deoxidizing; (3) sterilizing and filtering the solution obtained in the step (2), filling and deoxidizing, sealing, and carrying out high temperature sterilization. The preparation method of the paclitaxel injection enables the paclitaxel to be wrapped by the polyoxyethylene (35) castor oil by changing a feeding sequence, so that the material degradation and decomposition of the paclitaxel can be reduced in a terminal sterilization process, and the finally obtained paclitaxel injection is good in quality and high in stability.
Owner:SICHUAN HUIYU PHARMA
Paclitaxel injection and preparation method thereof
ActiveCN102151243AAvoid side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPaclitaxel Injection
The invention belongs to the technical field of medicine, and particularly relates to a paclitaxel injection and a preparation method thereof. The invention provides a paclitaxel injection having stable quality and low toxicity. The paclitaxel injection provided by the invention contains paclitaxel, polyglycol 15-hydroxystearate, anhydrous alcohol and one or more of citric acid, hydrochloric acid, tartaric acid and malic acid. The preferable formula of the paclitaxel injection comprises 5.4-6.6mg / ml paclitaxel, 5-80(V / V) polyglycol 15-hydroxystearate, 20-95%(V / V) anhydrous alcohol and 0.005-0.02%(mg / ml) citric acid.
Owner:CISEN PHARMA
DSPE-PEG-FA-modified nanometer paclitaxel liposome and preparation method thereof
InactiveCN102188382BSmall particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsYolkPaclitaxel Injection
The invention relates to a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. A molar ratio of the DSPE-PEG2000-FA to egg yolk lecithin is 0.05% to 0.15%, and a particle size of the liposome is less than 150 nm. A preparation method of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome comprises the following steps: first, preparing a DSPE-PEG2000-FA into a DSPE-PEG2000-FA micelle; second, performing incubation on the DSPE-PEG2000-FA micelle and a paclitaxel liposome together to obtain a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. Materials, which are forbidden to be used in clinical practice, are not used as crude materials in the present invention. According to the invention, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has a small particle size, and the content of the DSPE-PEG2000-FA is low; besides, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has good drug entrapment efficiency and good colloid stability. Moreover, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome can be absorbed effectively by an ovarian cancer cell having properties of sensitiveness to folic acid (+) and drug resistance, and the cytotoxicity of folic acid dependence is displayed; therefore, the efficacy of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome is stronger than that of a paclitaxel injection.
Owner:李红霞
Preparation method of paclitaxel injection
ActiveCN114344251AImprove complianceImprove securityOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilPaclitaxel Injection
According to the preparation method of the paclitaxel injection with the stable property, the blank auxiliary materials are pretreated, carboxylate anions in the auxiliary materials are reduced, degradation of main components is inhibited, and the stability of the paclitaxel injection in the storage period is improved; meanwhile, introduction of a pH regulator is avoided, the pH value of the obtained preparation is 4-6, in the pH value range of infusion tolerable by the human body, adverse reactions can be reduced, and the compliance and safety of the medicine in the clinical use process are improved. Besides, finished products which are prepared from domestic and imported polyoxyethylene 35 castor oil and have obvious difference in quality can achieve similar stability, are consistent with related substances of a reference preparation in level and are good in stability, and the safety of the products in the using process can be effectively guaranteed.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Anti-tumor pharmaceutical composition as well as preparation method and application thereof
ActiveCN107929750AImprove securityReduce serious adverse reactionsOrganic active ingredientsPharmaceutical delivery mechanismMedicineRetention time
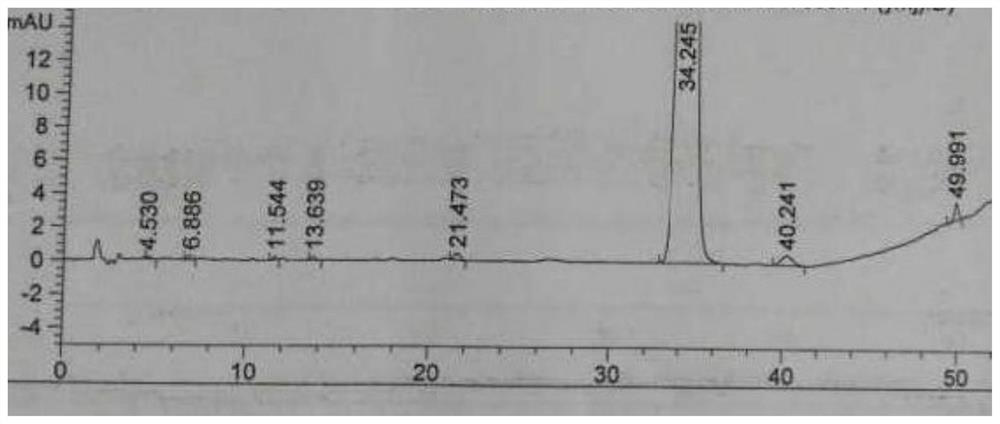
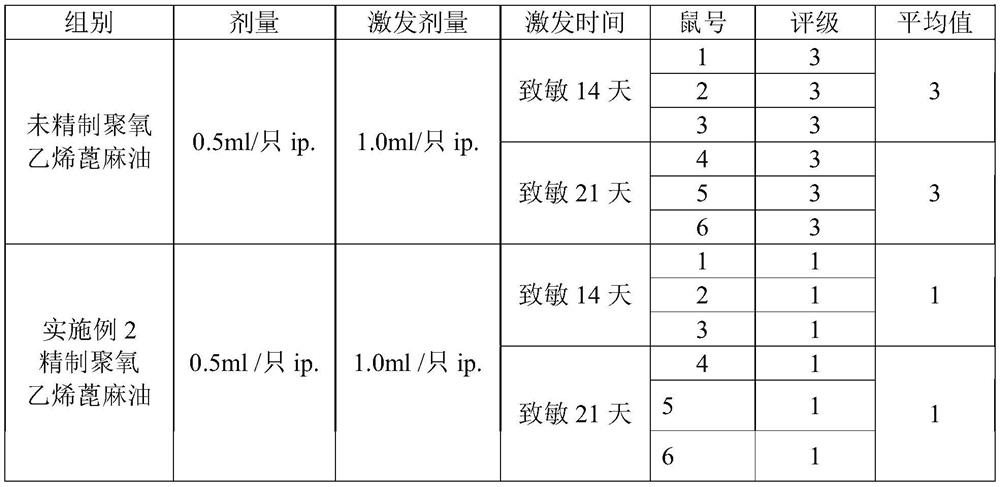
The invention relates to the technical field of anti-tumor drugs and particularly relates to an anti-tumor pharmaceutical composition as well as a preparation method and application thereof. By utilizing the anti-tumor pharmaceutical composition prepared from refined polyoxyethylated castor oil, the occurrence rate of serious untoward effects of a taxol injection is decreased, and the pharmaceutical safety and the pharmaceutical compliance of a patient are improved. The comparison of impurities before and after the polyoxyethylated castor oil is refined shows that the contents of polyoxyethylated castor oil impurities at relative retention time 0.6 and 1.2 in a related substance related substance of the taxol injection are remarkably reduced, and the safety of the refined polyoxyethylatedcastor oil is obviously improved.
Owner:JIANGSU JIUXU PHARMA +1
Pharmaceutical composition of paclitaxel and ranitidine hydrochloride
InactiveCN103393633ASolve the problem of water solubilityFix stability issuesOrganic active ingredientsPowder deliveryPaclitaxel InjectionRanitidine Hydrochloride
The invention relates to a pharmaceutical composition of paclitaxel and ranitidine hydrochloride, and especially relates to an application product of the pharmaceutical composition, wherein the application product comprises paclitaxel injection and an injection containing ranitidine hydrochloride. Administration comprises following steps, the injection containing ranitidine hydrochloride is applied firstly by intravenous injection, the paclitaxel injection is dissolved and diluted by normal saline or 5% glucose and sodium chloride solution, and then the diluted paclitaxel injection is applied by intravenous drop infusion.
Owner:HAINAN LINGKANG PHARMA CO LTD
Taxol microemulsion drug composition and preparation method thereof
ActiveCN103110581BSolve the ease of processingSolve technical problems such as ease of processingPowder deliveryOrganic active ingredientsFreeze-dryingPaclitaxel Injection
Owner:SOUTHWEST UNIV +1
Paclitaxel injection and preparation method thereof
ActiveCN101190214BEasy to useReduce pollutionOrganic active ingredientsPharmaceutical delivery mechanismSaline waterAlcohol
The invention relates to a paclitaxel injection and a preparation method of the injection: (1) 200 to 400ml of absolute alcohol and 200 to 400ml of normal saline are taken to be stirred, and 150 to 400ml of polyethylene glycol 400 is added to be evenly mixed; (2) 20 to 40g of tween-80 is added in to the mixture of (1) to be evenly mixed, and then 0.2 to 0.5g of active carbon used for injection isadded to be heated by 35 to 40 DEG C, and raw filtering is carried out and the PH value is controlled to be between 3 to 5; filtering is done and filling and sealing process is carried out; sterilization is carried out for 20 to 35 minutes at the temperature of 100 to 125 DEG C; (3) a transfusion bottle is washed and then is dried for 25 to 35 minutes the temperature of 230 to 260 DEG C, and butyl rubber plug is sterilized; (4) 0.6 to 3g of paclitaxel is taken to be added into the mixture of (2) for dissolving, and then sterilization and filtering are done; and then the solution is filled into the transfusion bottle in the sterilized environment; finally, the bottle is sealed to get the finished product. The invention can prepare safe and reliable paclitaxel injection, by adopting the method of preparing menstruum firstly and paclitaxel solution secondly, the quality of the injection is guaranteed.
Owner:GUANGDONG KELUN PHARMACEUTICAL CO LTD
Paclitaxel pharmaceutical composition and preparation method thereof
InactiveCN102670579BSolve the problem of spray bottle technologyEasy to shapeOrganic active ingredientsPharmaceutical non-active ingredientsPolyoxyethylene castor oilHemolysis
The invention discloses a paclitaxel pharmaceutical composition and a preparation method thereof. The paclitaxel pharmaceutical composition consists of paclitaxel, a solubilizing agent, a stabilizing agent, an excipient, an acidity regulator and water for injection. A paclitaxel injection is prepared by adopting a freeze drying method. Compared with the prior art, the paclitaxel pharmaceutical composition disclosed by the invention is different in solubilizing process, solubilizing effect and stability. The composition does not contain an organic solvent, thus, the paclitaxel injection has the characteristics of simpler production process, more perfect appearance, shorter freeze drying time and lower equipment requirement. The composition does not contain polyoxyethylenated castor oil, so that the paclitaxel composition has no anaphylactic reaction or hemolysis or irritation. By detection, the paclitaxel injection prepared by adopting the composition and the preparation method disclosed by the invention is high in dissolvability and stability and is suitable for large-scale industrial production.
Owner:姚定全
A kind of antitumor pharmaceutical composition and its preparation method and application
ActiveCN107929750BLow impurity contentPrevent precipitationOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilDrug utilisation
The invention relates to the technical field of anti-tumor drugs, in particular to an anti-tumor pharmaceutical composition and its preparation method and application. In order to reduce the incidence of serious adverse reactions of paclitaxel injection, improve drug safety and drug compliance of patients, the invention adopts an anti-tumor pharmaceutical composition made of refined polyoxyethylene castor oil. The comparison of impurities before and after refining polyoxyethylene castor oil showed that the content of impurities in polyoxyethylene castor oil at the retention time 0.6 and 1.2 relative to paclitaxel in the chromatogram of related substances in paclitaxel injection was significantly reduced, and the safety of polyoxyethylene castor oil was significantly improved after refining.
Owner:JIANGSU JIUXU PHARMA +1
Paclitaxel injection and preparation method thereof
ActiveCN102151243BAvoid side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholPaclitaxel Injection
The invention belongs to the technical field of medicine, and particularly relates to a paclitaxel injection and a preparation method thereof. The invention provides a paclitaxel injection having stable quality and low toxicity. The paclitaxel injection provided by the invention contains paclitaxel, polyglycol 15-hydroxystearate, anhydrous alcohol and one or more of citric acid, hydrochloric acid, tartaric acid and malic acid. The preferable formula of the paclitaxel injection comprises 5.4-6.6mg / ml paclitaxel, 5-80(V / V) polyglycol 15-hydroxystearate, 20-95%(V / V) anhydrous alcohol and 0.005-0.02%(mg / ml) citric acid.
Owner:CISEN PHARMA
Paclitaxel mixed micelle preparation, and preparation method thereof
InactiveCN102198084BReduce toxic and side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPolyoxyethylene castor oil
The invention discloses a paclitaxel mixed micelle preparation, comprising 100 to 300 milligram of tocopherol polyethylene glycol succinate 1000 (TPGS), 0 to 50 milligram of phosphatide, 0.5 milliliter of anhydrous ethanol and 6 milligram of paclitaxel. The preparation method is as follows: the TPGS is dissolved in the anhydrous ethanol, or the TPGS and the phosphatide are dissolved in the anhydrous ethanol; the paclitaxel is added and dissolved under stirring; the mixture is filtered with a millipore filtration of 0.22 micrometer so as to obtain the paclitaxel mixed micelle preparation. In the invention, the TPGS and the phosphatide are used to form mixed micelles which have good stability and little toxic and side effects; the preparation method is simple and practicable, having a good application prospect. Compared to the prior art, polyoxyethylene castor oil in conventional prescription is substituted in the invention, thereby reducing toxic side effects of paclitaxel injections and greatly enhancing the safety of the injections on condition that solubility is guaranteed.
Owner:SHANDONG UNIV
A kind of paclitaxel injection and preparation method thereof
ActiveCN106880589BImprove stabilityOvercome the defects that patients are prone to side effects such as allergiesOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilFormulary
The invention discloses a paclitaxel injection, which, in proportion by weight, comprises: paclitaxel, polyoxyethylene 35 castor oil, vitamin E, Tween 80, absolute ethanol and a pH regulator; the pH regulator injects paclitaxel into The pH of the solution was adjusted to 3.0 to 5.0. At the same time, a preparation method of paclitaxel injection is also disclosed: adding polyoxyethylene 35 castor oil and Tween 80 into part of absolute ethanol to dissolve to obtain mixed solution A; dissolving vitamin E in the remaining absolute ethanol, and then adding Add paclitaxel to the ethanol solution of vitamin E to obtain mixed solution B; mix mixed solution A and mixed solution B, adjust the pH value, and filter to obtain. The paclitaxel injection prepared by the invention has good stability, and the usage amount of polyoxyethylene 35 castor oil in the formula is small, which overcomes the problem that the traditional paclitaxel injection easily causes allergies to patients, has high clinical drug safety, and is suitable for industrial production and popularization and application. .
Owner:NORTH CHINA PHARMA COMPANY
Preparation method of paclitaxel dressing bin and prepared paclitaxel dressing bin
ActiveCN103768040AAvoid side effectsLow costOrganic active ingredientsAntineoplastic agentsPaclitaxel InjectionOncology
The invention provides a preparation method of a paclitaxel dressing bin. The preparation method comprises the steps: after uniformly mixing paclitaxel, lecithin and various accessories, coating on bottom cotton and medicine cotton, finally preparing the bin, and applying to a focus position by using an ultrasonic 3 MHZ guide instrument during use. The preparation method is low in cost, and simple in process; the prepared paclitaxel dressing bin is used for preventing and controlling breast cancer, and applied to the focus position by arranging the ultrasonic 3 MHZ guide instrument. The toxic and side effects of an existing paclitaxel injection are overcome.
Owner:安徽爱喆生物医药科技有限公司
Paclitaxel composition for injection and preparation method thereof
ActiveCN105534903BHigh drug loadingLow in lipidsOrganic active ingredientsEmulsion deliveryLipid formationYolk
The invention provides a taxol composition for injection, which comprises taxol, oil for injection, an emulsifying agent and a stabilizing agent, wherein the oil for injection is selected from at least one of soybean oil, olive oil, coix seed oil, medium chain triglyceride as well as vitamin E and derivatives thereof, and the emulsifying agent is selected from at least one of egg yolk lecithin, soybean lecithin and synthetic lipid as well as amino acid. The invention also provides a preparation method of the taxol composition for injection, wherein an oil phase is prepared by the following steps: mixing and stirring taxol, the emulsifying agent and absolute ethyl alcohol until the solution is clear, then adding the oil for injection and the stabilizing agent, further stirring until the solution is clear, and volatilizing ethyl alcohol in vacuum so as to obtain the oil phase. The taxol composition for injection, provided by the invention, is high in drug loading capacity, low in lipid content and good in stability and is capable of reducing the medication risk of taxol for injection and increasing the compliance of patients.
Owner:HUIYU HEALTH HANGZHOU PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com