Paclitaxel composition for injection and preparation method thereof
A technology for paclitaxel and injection, which is applied in the field of paclitaxel composition for injection and its preparation, which can solve the problems of inability to withstand high pressure sterilization, risk of drug safety, high production cost, etc., and achieve reduced drug risk, low lipid content, The effect of high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
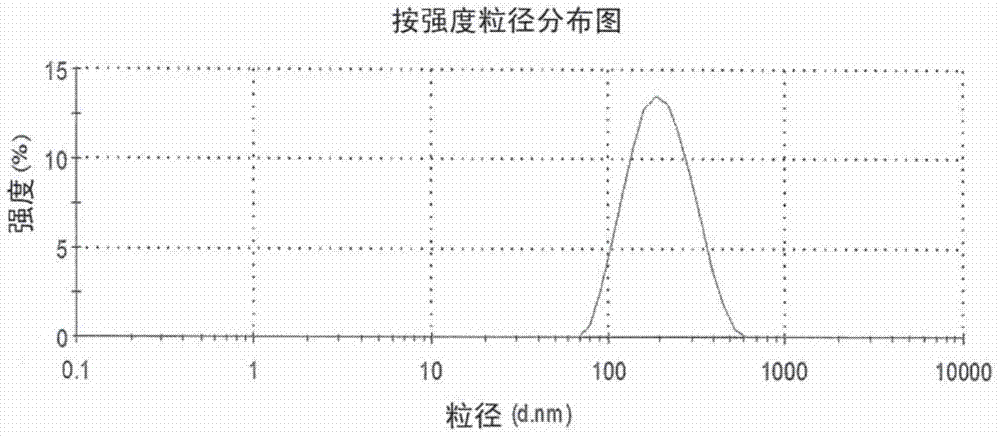
Image
Examples
preparation example Construction
[0075] [Preparation of paclitaxel composition for injection]
[0076] The present invention provides a method for preparing a paclitaxel composition for injection, which is characterized in that when preparing an oil phase, paclitaxel, emulsifier and absolute ethanol are mixed and stirred until clear, and then injection oil and stabilizer are added, and further stirred When it is clear, the ethanol is evaporated under vacuum to obtain the oil phase.
[0077] Specifically, when preparing the paclitaxel composition for injection of the present invention, the amount of anhydrous ethanol used is much lower than the prescription amount described in the prior art, and it is finally removed under vacuum conditions. As a result, the risk of ethanol irritation can be reduced, and compared with other organic solvents such as acetone, the medication safety is better.
[0078] Hereinafter, the case where the paclitaxel composition for injection of the present invention is a paclitaxel microemul...
Embodiment 1
[0100] Example 1 (Paclitaxel freeze-dried microemulsion for injection)
[0101] prescription
[0102]
[0103] Preparation:
[0104] (1) Oil phase: Mix paclitaxel, egg yolk lecithin, soybean lecithin, and absolute ethanol at 40-70°C and stir until clear, then add vitamin E and oleic acid, stir further until clear, and evaporate the ethanol in a vacuum , The oil phase is obtained;
[0105] (2) Aqueous phase: Add arginine and sucrose to the water for injection, stir until completely dissolved, and filter with a 0.22μm microporous membrane to obtain the aqueous phase;
[0106] (3) Colostrum: firstly preheat the hot water phase and the oil phase to 50-70°C, then use a high-speed disperser to slowly add the water phase to the oil phase under shearing and stirring at 5000 to 20000 rpm. , Continuously shearing and stirring at 10000rpm for 30 minutes to obtain colostrum;
[0107] (4) Final milk: Cool the colostrum to room temperature, then transfer it to a high-pressure homogenizer, and homoge...
Embodiment 2
[0132] Example 2 (Paclitaxel Microemulsion for Injection)
[0133] prescription
[0134]
[0135] Preparation:
[0136] (1) Oil phase: Mix paclitaxel, egg yolk lecithin and absolute ethanol at 40-70°C and stir until clear, then add vitamin E, dl-α-tocopherol and oleic acid, and further stir until clear. Ethanol is evaporated in vacuum to obtain oil phase;
[0137] (2) Aqueous phase: Add lysine and sucrose to the water for injection, stir until completely dissolved, and filter with a 0.22μm microporous membrane to obtain the aqueous phase;
[0138] (3) Colostrum: first preheat the hot water phase and the oil phase to 50-70°C, then slowly add the water phase to the oil phase using a high-speed disperser under shearing and stirring at 5000 to 20000 rpm. Stir continuously at 8000rpm for 20 minutes to obtain colostrum;
[0139] (4) Final milk: Cool the colostrum to room temperature, then transfer the colostrum to a high-pressure homogenizer, and homogenize 6 times at 25~45℃ under 1000bar co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com