Pharmaceutical composition of paclitaxel and ranitidine hydrochloride
A technology of ranitidine hydrochloride and paclitaxel, which is applied in the field of medicine and can solve problems such as unresolved side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of paclitaxel injection
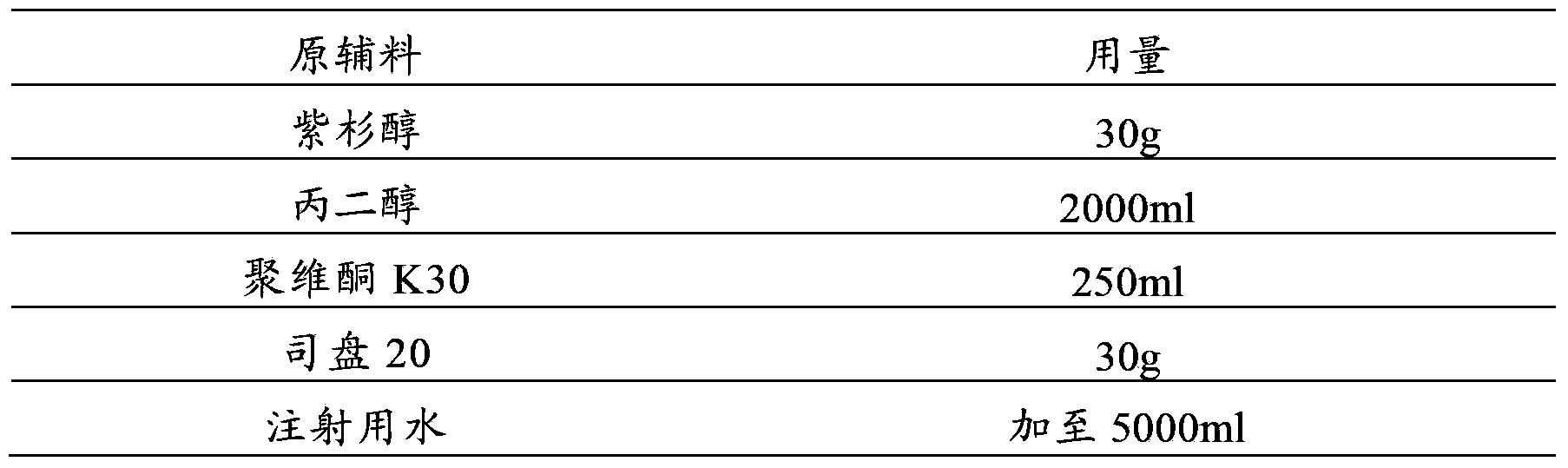
[0045] prescription:
[0046]
[0047] making process
[0048] (1) First add 2000ml propylene glycol, 250ml povidone K30 and 30g Span 20 into the container, stir and mix well;
[0049] (2) Add 30g paclitaxel, stir to dissolve completely, add water for injection to 4500ml, adjust pH to 4.6 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0050] (3) Add 2.5g of activated carbon for injection, add the remaining water for injection, set the volume to 5000ml, stir and absorb for 30 minutes;
[0051] (4) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0052] (5) Fill, 5ml / bottle, seal, sterilize, and get paclitaxel injection
Embodiment 3
[0062] Example 3 Preparation of Ranitidine Hydrochloride Injection
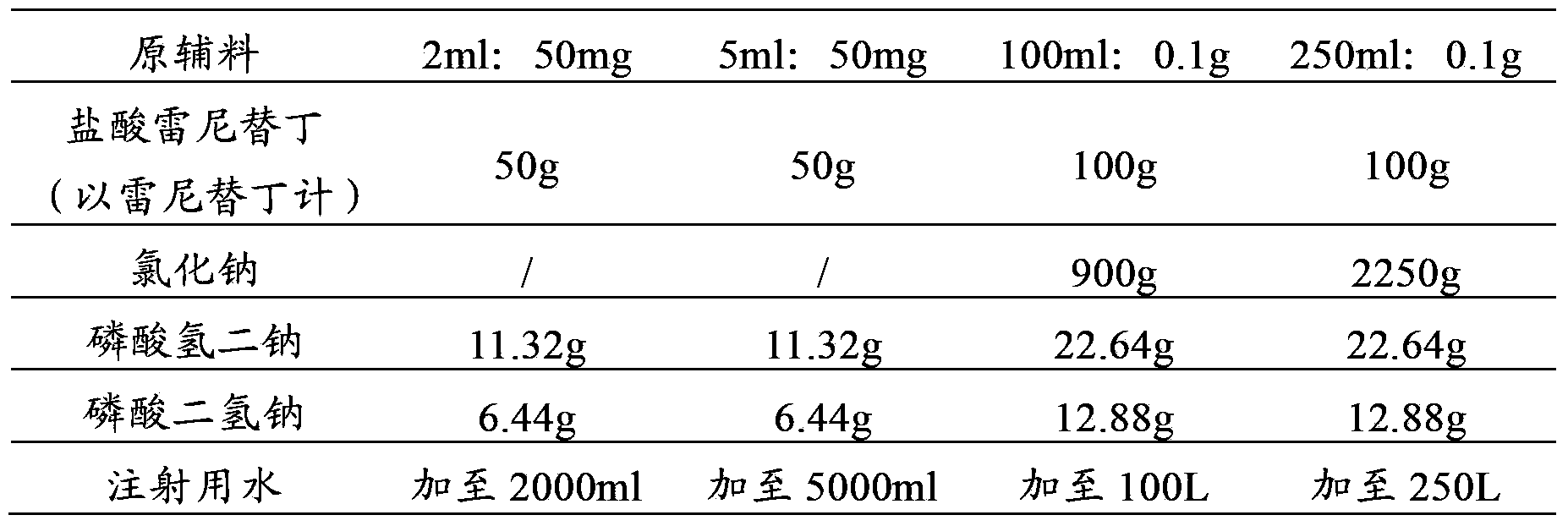
[0063] prescription:
[0064]
[0065] Preparation Process
[0066] (1) First add 1600ml water for injection into the container; add 50g ranitidine hydrochloride, 11.32g disodium hydrogen phosphate and 6.44g sodium dihydrogen phosphate, stir to dissolve completely, and use 1mol / L sodium hydroxide solution or 1mol / L Phosphoric acid solution to adjust the pH to 6.9; add 1.0g of activated carbon for injection, add the remaining water for injection, set the volume to 2000ml, stir and absorb for 30 minutes; decarbonize the solution through a 0.45μm cartridge filter, and then use a 0.22μm Sterilize and filter with a microporous membrane until visible foreign matter is qualified; fill, 2ml / bottle, seal, and sterilize to obtain ranitidine hydrochloride injection.
[0067] (2) First add 4000ml water for injection into the container; add 50g ranitidine hydrochloride, 11.32g disodium hydrogen phosphate and 6.44g ...
Embodiment 4
[0070] Example 4 Preparation of ranitidine hydrochloride for injection
[0071] prescription:
[0072]
[0073]
[0074] Preparation Process
[0075] (1) First add 4800ml of water for injection into the container;
[0076] (2) Add 150g ranitidine hydrochloride, 450g mannitol, 33.96g disodium hydrogen phosphate and 19.32g sodium dihydrogen phosphate, stir to dissolve completely, adjust the pH with 1mol / L sodium hydroxide solution or 1mol / L phosphoric acid solution to 7.0;
[0077] (3) Add 3g of activated carbon for injection, add the remaining water for injection, set the volume to 6000ml, stir and absorb for 30 minutes;
[0078] (4) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0079] (5) Filling, 2ml / bottle or 4ml / bottle, freeze-dried to obtain ranitidine hydrochloride for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com