Glycyrrhetinic acid mediated curcumin long-circulating nanostructured lipid carrier and preparation method thereof
A nano-lipid carrier, glycyrrhetic acid technology, applied in the field of medicine, can solve the problems of cytotoxicity, low drug solubility, easy drug leakage, etc., and achieve the effects of increasing hydrophilicity, prolonging circulation time, and reducing phagocytosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
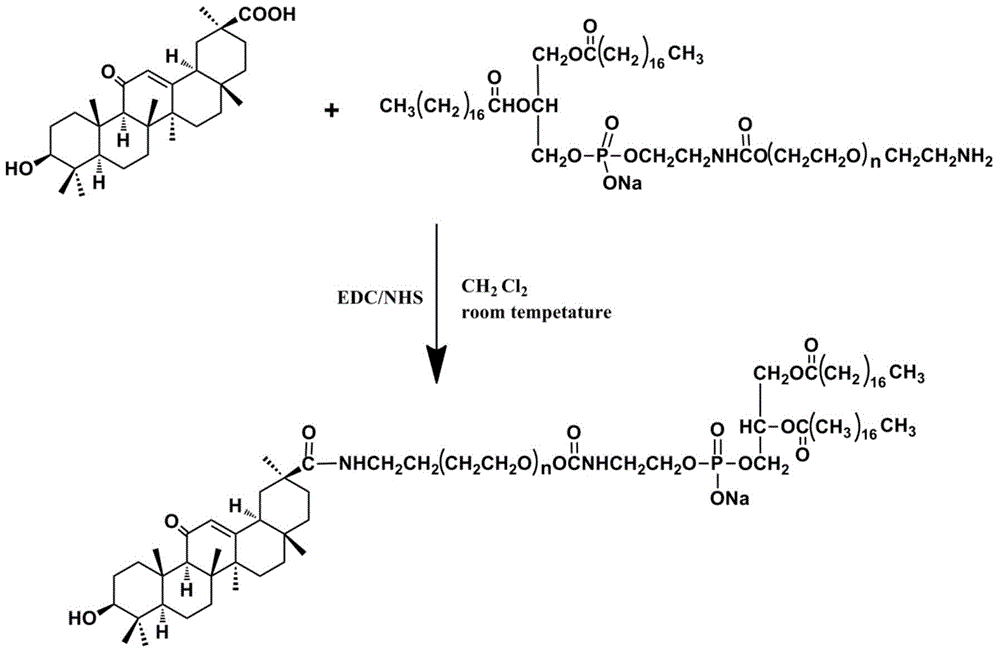
[0039] Glycyrrhetinic acid-phospholipid derivatives (GA-PEG 2000 -DSPE) synthesis:
[0040] Dissolve GA, DCC, and NHS in 10 mL of dehydrated dichloromethane, in which GA is 1.5 mmol, and the molar ratio of the three is 1.5:1.2:1.2, and the reaction is activated at room temperature for 3 h. Another 1mmol DSPE-PEG 2000 -NH 2 Dissolve in 5mL of dichloromethane solution, add dropwise to the above activator, and react for 48h at room temperature under the protection of nitrogen. Remove the reaction by-products by filtration, extract with glacial ether, and remove the unreacted GA in the supernatant. The precipitated substance is the target product. After reconstitution in DMF, put it into a dialysis bag for dialysis for 72 hours, and freeze-dry to obtain the product. Process route see figure 1 .
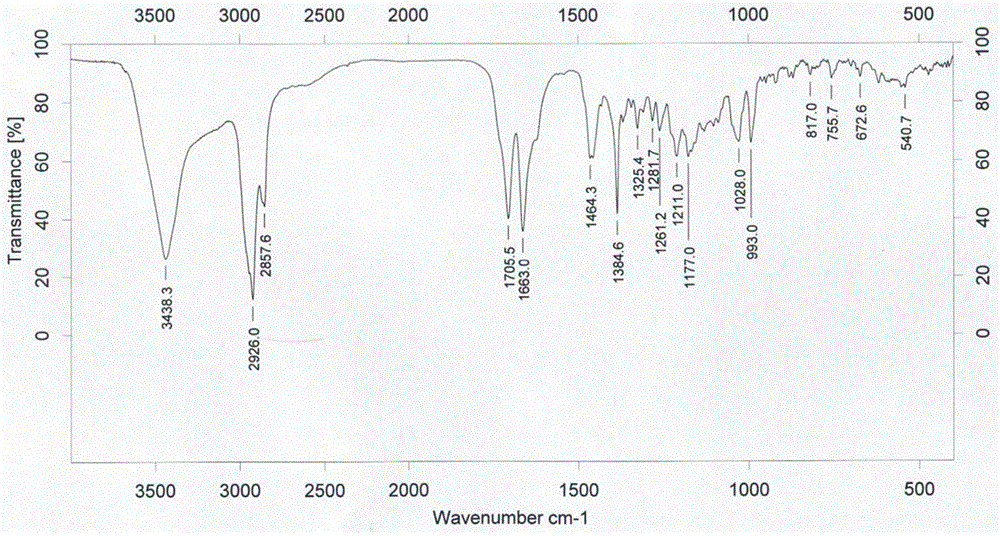
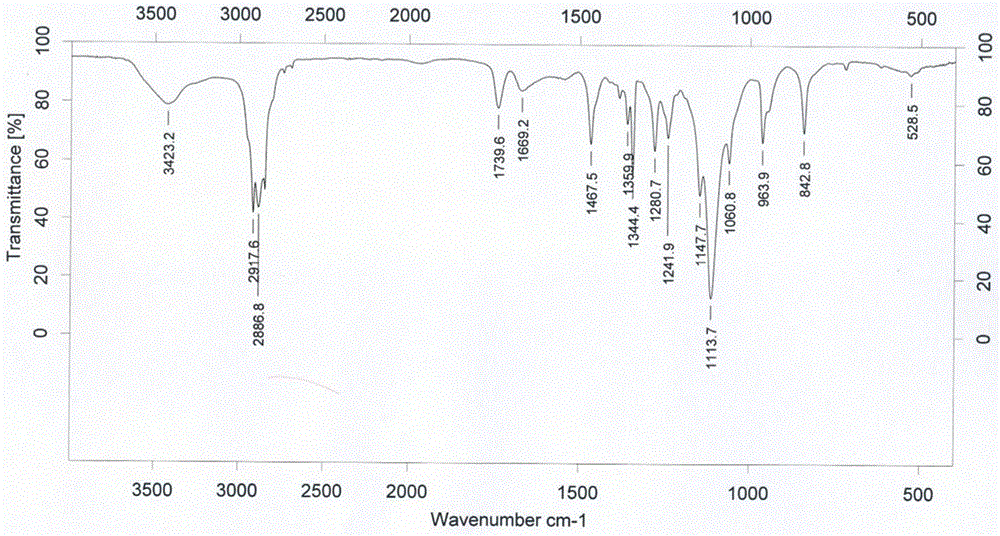
[0041] FTIR characterization: Bruker vector 22 infrared spectrometer was used to determine the reactants GA and DSPE-PEG respectively 2000 -NH 2 and the polymerization product DSPE...
Embodiment 2
[0046] (1), preparation of curcumin nano-lipid carrier (Cur-NLC):
[0047] raw material:
[0048]
[0049]
[0050] Weigh the prescribed amount of glyceryl monostearate, caprylic acid / capric triglyceride, soybean lecithin for injection and curcumin in a water bath and heat to 75°C, add 5ml of absolute ethanol to dissolve it, and remove the absolute ethanol by rotary evaporation to obtain Mix the molten phase evenly as the oil phase; weigh the prescribed amount of Polyoxyethylene 40 Strearate, add water for injection, stir it evenly with ultrasonic waves, and heat it in a water bath to 75°C as the water phase; at 3000r min -1 Add the water phase dropwise to the oil phase at the same temperature under magnetic stirring, and the drop rate is controlled at 10mL min -1 Stir at a constant temperature for 5 minutes to prepare colostrum, redisperse the colostrum with an ultrasonic cell pulverizer while it is hot, ultrasonic power 400W, ultrasonic 1s, interval 1s, time 5min, fil...
Embodiment 3
[0074] In vitro cytological evaluation of glycyrrhetinic acid-mediated long-circulating nanolipid carriers of curcumin:
[0075] Reagents and materials:
[0076]
[0077] Preparation of culture medium:
[0078] Phosphate buffered saline (PBS): Weigh 1.44 g of disodium hydrogen phosphate, 0.24 g of potassium dihydrogen phosphate, 8.0 g of sodium chloride, and 0.2 g of potassium chloride, dissolve them in 700 mL of double distilled water, mix and stir evenly. Use 1mol L -1 HCl to adjust the pH to 7.4, distilled water to 1000mL, and the concentration is 0.01mol L -1 Phosphate buffer (pH 7.4). Autoclave for 30 minutes, aliquot and store at 4°C for later use.
[0079] DMEM culture solution: Take 10.4g of DMEM dry powder, dissolve it in a small amount of double distilled water, add 3.7g of sodium bicarbonate, 2.0g of HEPES, 100units / mL penicillin, 100units / mL streptomycin, mix and stir to dissolve, and distill the volume to 1000mL. Use 1mol L -1 The pH was adjusted to 7.0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com