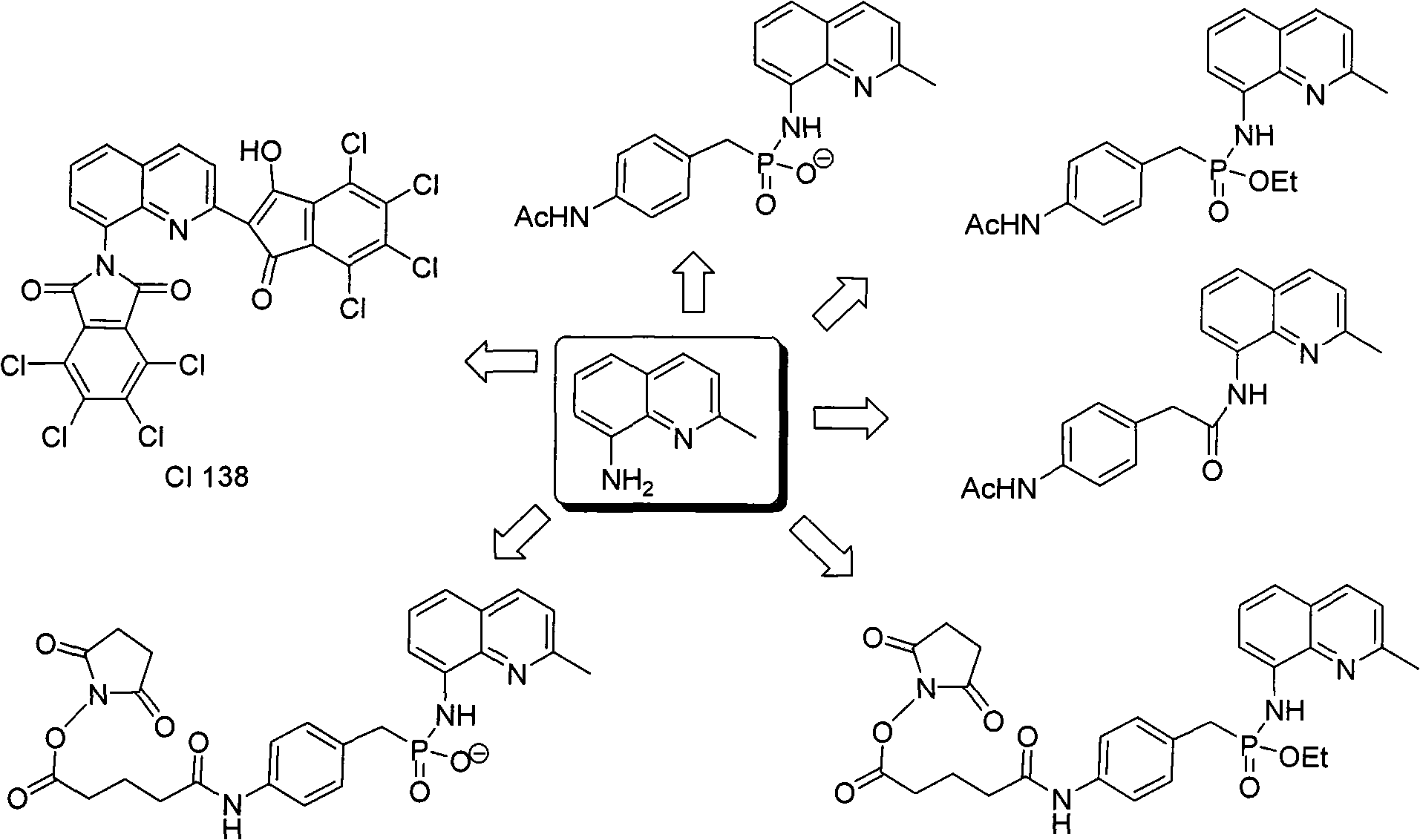
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "Caesium carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Caesium carbonate or cesium carbonate is a white crystalline solid compound. Caesium carbonate has a high solubility in polar solvents such as water, alcohol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium and sodium carbonates, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.
Preparation method of cesium-tungsten bronze powder and function film
InactiveCN104528829AReduce manufacturing costSimple manufacturing processTungsten compoundsInfraredTungstate
The invention discloses a preparation method of cesium-tungsten bronze powder and a function film. The preparation method of the cesium-tungsten bronze powder comprises the following steps: carrying out an exchange treatment on tungstate solution and cationic resin to obtain tungstic acid sol; adding citric acid solution and cesium carbonate solution in the tungstic acid sol and then carrying out a mixing treatment to obtain hydrothermal reaction precursor solution; carrying out a hydrothermal reaction on the hydrothermal reaction precursor solution; after the reaction is finished, carrying out a washing treatment and a drying treatment to obtain the cesium-tungsten bronze powder. The function film disclosed by the invention contains the cesium-tungsten bronze powder prepared by the preparation method of the cesium-tungsten bronze powder disclosed by the invention; according to the preparation method of the cesium-tungsten bronze powder disclosed by the invention, by controlling reactants and carrying out hydrothermal method, the preparation process is effectively simplified and the production cost of the cesium-tungsten bronze powder is reduced; moreover, the cesium-tungsten bronze produced under a specific hydrothermal reaction condition has excellent shielding performance for infrared ray, particularly for near-infrared ray.
Owner:SHENZHEN JIADA HIGH TECH IND DEV
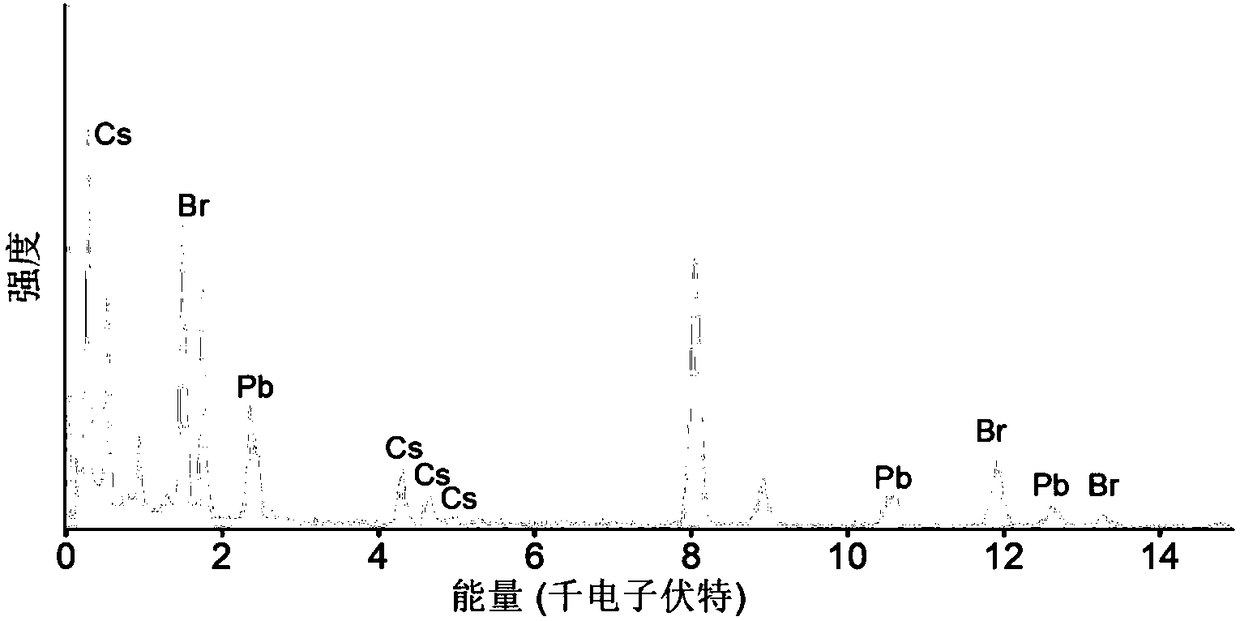
Method for preparing CsPbBr3 nanosheets with quantum size effects
InactiveCN106809872APreserve quantum size effectsAchieving controllable equipmentMaterial nanotechnologyLead compoundsOleic Acid TriglycerideQuantum size
The invention discloses a method for preparing CsPbBr3 nanosheets with quantum size effects. The method includes dissolving cesium carbonate in oleic acid under argon filling conditions, and stirring the cesium carbonate and the oleic acid under heating conditions until the cesium carbonate is dissolved to obtain cesium oleate precursors; adding lead bromide, long-chain ligands and short-chain ligands into octadecene under argon filling conditions, carrying out reaction at the temperature of 100-150 DEG C until the lead bromide is dissolved, heating first reaction products until the temperature of the first reaction products reaches 120-150 DEG C, then injecting the cesium oleate precursors into the first reaction products, carrying out reaction for 5-15 s, then continuing to carry out reaction at the temperature of 100-130 DEG C for 1-5 min to obtain second reaction products and centrifuging the second reaction products. The method has the advantages that the transverse sizes of the CsPbBr3 nanosheets can be assuredly regulated and controlled in the range from 100 nm to 1 micrometer while the thicknesses which are equal to the thicknesses of a few atomic layers can be guaranteed; the thicknesses which are lower than the diameters of Bohr excitons can be kept, and accordingly the quantum size effects of the CsPbBr3 nanosheets can be reserved.
Owner:XI AN JIAOTONG UNIV
Preparation method of total-inorganic perovskite quantum dots
InactiveCN108192606AOutstanding FeaturesHighlight significant progressNanoopticsLuminescent compositionsHigh heatHalide
The invention provides a preparation method of total-inorganic perovskite quantum dots, and relates to inorganic luminescent materials. The one-pot method is used, a precursor of a caesium source doesnot need to be prepared in advance, a required quantity of lead source and caesium source are dissolved in an organic solvent by using ligand, after vacuum drying, a halogen source is injected undera set temperature, at this moment, the three elements of Pb, Cs and halogen X can interact, and the CsPbX3 total-inorganic perovskite quantum dots are deposited. The three disadvantages of complex steps in the prior art are overcome that reagent sources are limited, wherein the reagents of the room temperature preparation method are caesium halide and lead halide, the reagents of the high temperature preparation method are caesium carbonate and lead halide, and it cannot be anything but the two substances to react; the amount ratio of the elements cannot be adjusted, that is, the amount ratioof Pb and halogen X is limited by a lead halide reactant; the precursor needs to be prepared and preheated in advance.
Owner:HEBEI UNIV OF TECH
Oligomeric hydroxy arylether phthalonitiles and synthesis thereof
An aromatic ether oligomer or polyaromatic ether comprising the formula: wherein Ar is an independently selected divalent aromatic radical; formed by reacting a dihydroxyaromatic with a dihaloaromatic; and wherein the reaction is performed in the presence of a copper compound and cesium carbonate. The polyaromatic ether is formed when neither the dihydroxyaromatic nor the dihaloaromatic is present in an excess amount. The aromatic ether oligomer is formed by using an excess of either dihydroxyaromatic or dihaloaromatic. A phthalonitrile monomer comprising the formula: formed by reacting a 3- or 4-nitrophthalonitrile with a hydroxy-terminated aromatic ether oligomer. A thermoset formed by curing the phthalonitrile monomer. Processes for forming all the above.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Perovskite nanosheet material as well as preparation method and application thereof
InactiveCN109810704AUniform thicknessShort emission wavelengthMaterial nanotechnologyLuminescent compositionsThermal insulationOleic Acid Triglyceride
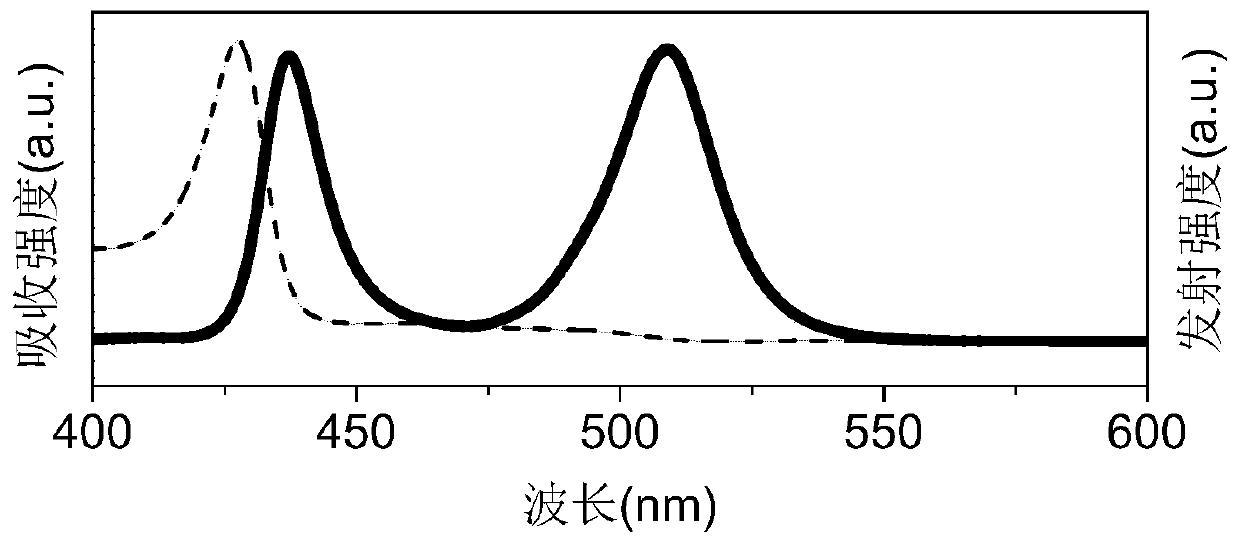
The invention belongs to the field of materials, and specifically relates to a perovskite nanosheet material as well as a preparation method and application thereof. The preparation method of the perovskite nanosheet material comprises the following steps: (1) mixing cesium carbonate powder with octadecene and oleic acid, dissolving the cesium carbonate powder by heating to 100-130 DEG C under aninert atmosphere, rising the temperature of a system to 140-160 DEG C and reacting for 10-60 min to obtain a cesium oleic acid precursor; mixing iron bromide power with octadecene, oleic acid and oleylamine, dissolving, then adding lead bromide power into an obtained mixed solution, and dissolving the lead bromide powder by heating to 100-120 DEG C under an inert atmosphere so as to obtain an ironand lead mixed solution; and (2) heating the cesium oleic acid precursor to 80-100 DEG C, uniformly mixing with the iron and lead mixed solution, performing a thermal insulation reaction for 5 s-5 min at 120-140 DEG C, cooling and then performing centrifugal separation. Therefore, the obtained perovskite nanosheet material has relatively short light-emitting wavelength; the light-emitting peak position is at about 436 nm; the half peak width is only 14 nm; the light-emitting peak is fully symmetric; and the two-dimensional perovskite nanosheet material is very uniform in size and thickness, and is easy to control.
Owner:XIAMEN UNIV
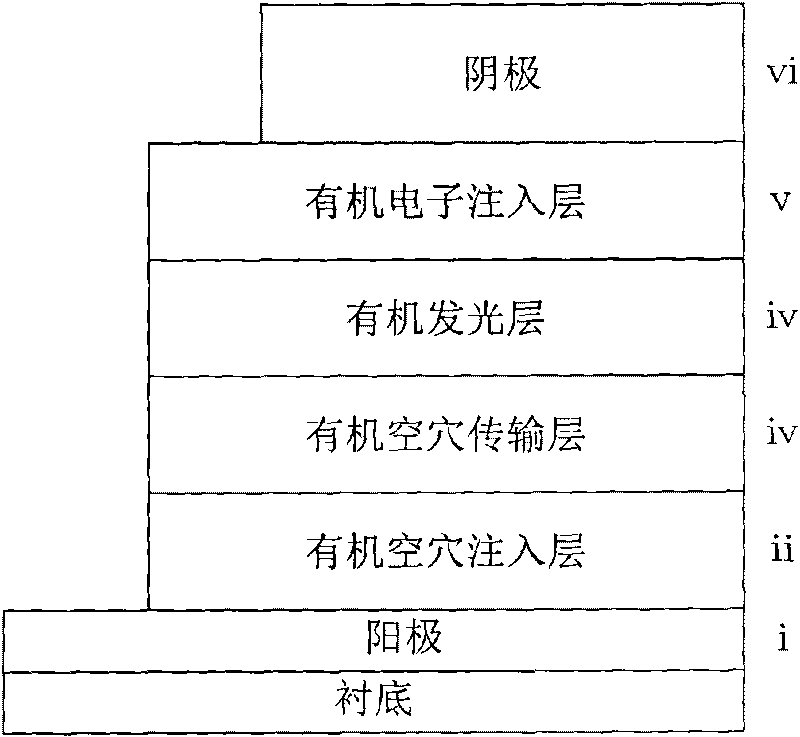
Low-voltage and high-efficiency organic LED and preparation method thereof
InactiveCN104409649ALower injection barrierImprove efficiencySolid-state devicesSemiconductor/solid-state device manufacturingFluorescenceMaterials science
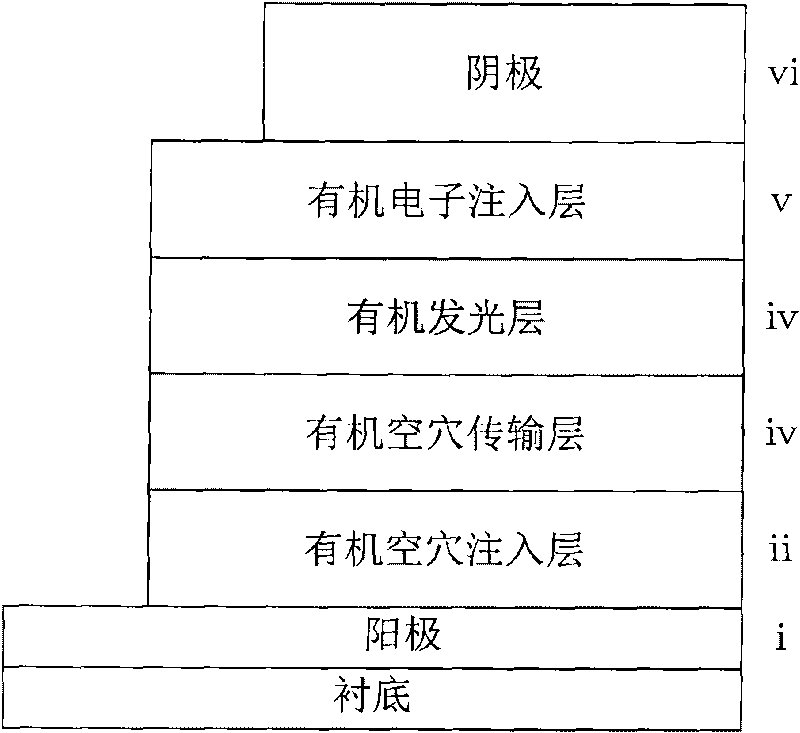
The invention provides a low-voltage and high-efficiency organic LED. The low-voltage and high-efficiency organic LED comprises a glass substrate with an ITO, a hole-transmission layer, a p type doped luminescence layer, an i type intrinsic luminescence layer, an n type doped luminescence layer, an electronic transmission layer, a composite electron injection layer and a cathode which are sequentially overlapped to form an overlapping layer; the p type doped luminescence layer is prepared through hole transmission material in which blue fluorescent dye is doped; the i type intrinsic luminescence is prepared through blue fluorescent dye; the n type doped luminescence layer is prepared through the electron transmission material in which blue fluorescent dye is doped; the three luminescence layers are named p-i-n type luminescence layer; and the composite electronic injection layer is prepared through cesium carbonate in which a thin aluminum layer is inserted. The low-voltage and high-efficiency organic LED has the advantages that the composite electron injection layer with a thin aluminum layer inserted into the cesium carbonate layer and the p-i-n type luminescence layer are arranged, so that the luminescence efficiency is improved, and the reduction of the efficiency is delayed; the driving voltage is low, the luminance is high, the efficiency is high, the stability is improved, and the preparation process is simple.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Organic light emitting diode of alkali metal carbonate-doped organic electron injecting layer
InactiveCN101710610AReduce manufacturing costGood microcavity effectSolid-state devicesSemiconductor/solid-state device manufacturingLight-emitting diodeCarbonate
The invention relates to an organic light emitting diode of an alkali metal carbonate-doped organic electron injecting layer, comprising a transparent positive electrode, an organic hole injecting layer, an organic hole transport layer, an organic luminous layer, an organic electron injecting layer and a cathode. The organic electron injecting layer is doped with lithium carbonate or caesium carbonate, and the doping weight is 1:2-20, wherein 1 is the doping weight fraction of alkali metal carbonate. An organic electron acceptor material doped with alkali metal carbonate has very high electrical conductivity and meanwhile has very strong capability of injecting electrons to the organic luminous layer, and therefore, the invention is the organic light emitting diode of an N-type doping material with high performance and low price. The organic light emitting diode is used for emitting visible light and near infrared and is applied to the fields of organic panel display and solid illumination.
Owner:HEBEI UNIV OF TECH
CsPbX3 quantum dot room temperature synthetic method
The invention discloses a CsPbX3 quantum dot room temperature synthetic method. The CsPbX3 quantum dot room temperature synthetic method comprises the following steps: dissolving cesium carbonate in alkyl chain organic acid to prepare a cesium precursor; dissolving lead bromide and branched-chain organic ammonium bromide in toluene, and carrying out stirring and dissolution in room-temperature airto obtain a lead precursor; dissolving DDAB (Didodecyl Dimethyl Ammonium Bromide) in the toluene to obtain a DDAB solution; injecting the cesium precursor into the lead precursor and carrying out stirring reaction to obtain an intermediate; adding the DDAB solution into the obtained intermediate, and carrying out stirring reaction to obtain a CsPbX3 quantum dot stock solution; adding the obtainedCsPbX3 quantum dot stock solution into a purifying solvent for centrifugation; then dispersing in an organic solvent to obtain CsPbX3 quantum dots. According to the CsPbX3 quantum dot room temperature synthetic method disclosed by the invention, the CsPbX3 quantum dot with excellent luminescence property is synthesized under room temperature by utilizing the synergism matching of three ligands, the quantum yield of an ink dispersion solution exceeds 90 percent, and meanwhile, the CsPbX3 quantum dot room temperature synthetic method also has radiative recombination of single channels.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of silica-coated all-inorganic perovskite core-shell structure quantum dots
ActiveCN111205853ALow priceLoose conditionsNanotechnologyLuminescent compositionsFluorescenceSilanes
The invention relates to a preparation method of silica-coated all-inorganic perovskite core-shell structure quantum dots. The preparation method comprises the following steps: completely dissolving cesium carbonate in n-caprylic acid to obtain a cesium precursor solution, dissolving lead bromide and tetra-n-octylammonium bromide in toluene to obtain a lead bromide / toluene solution; and then, sequentially adding an aminosilane coupling agent and the cesium precursor solution into the lead bromide / toluene solution, stirring for reaction, and separating and drying an obtained product to obtain the target product. Compared with the prior art, the preparation method disclosed by the invention is simple and loose in condition, the fluorescence characteristic of the prepared product is not reduced, the stability is greatly improved compared with that of pure CsPbBr3, and the product can be used as fluorescent powder to be packaged into a photoelectric device for application.
Owner:SHANGHAI INST OF TECH
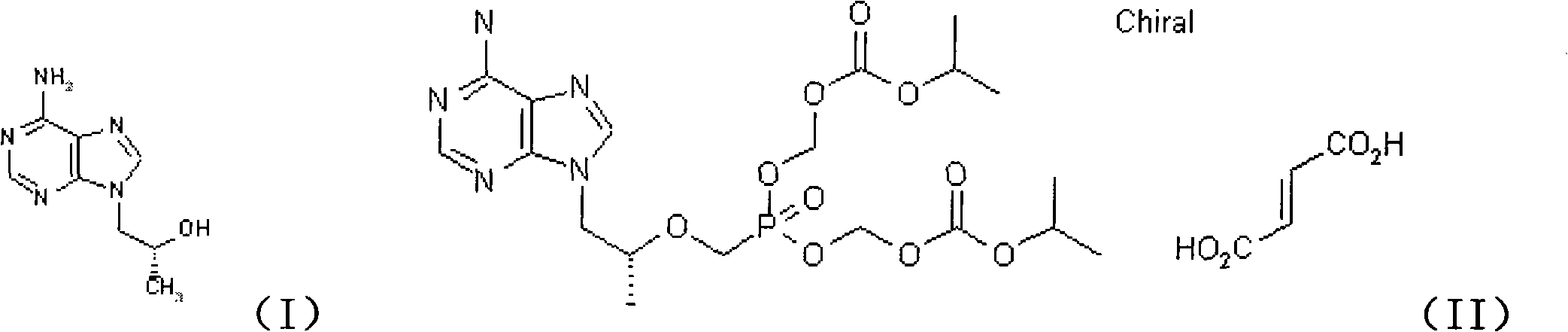
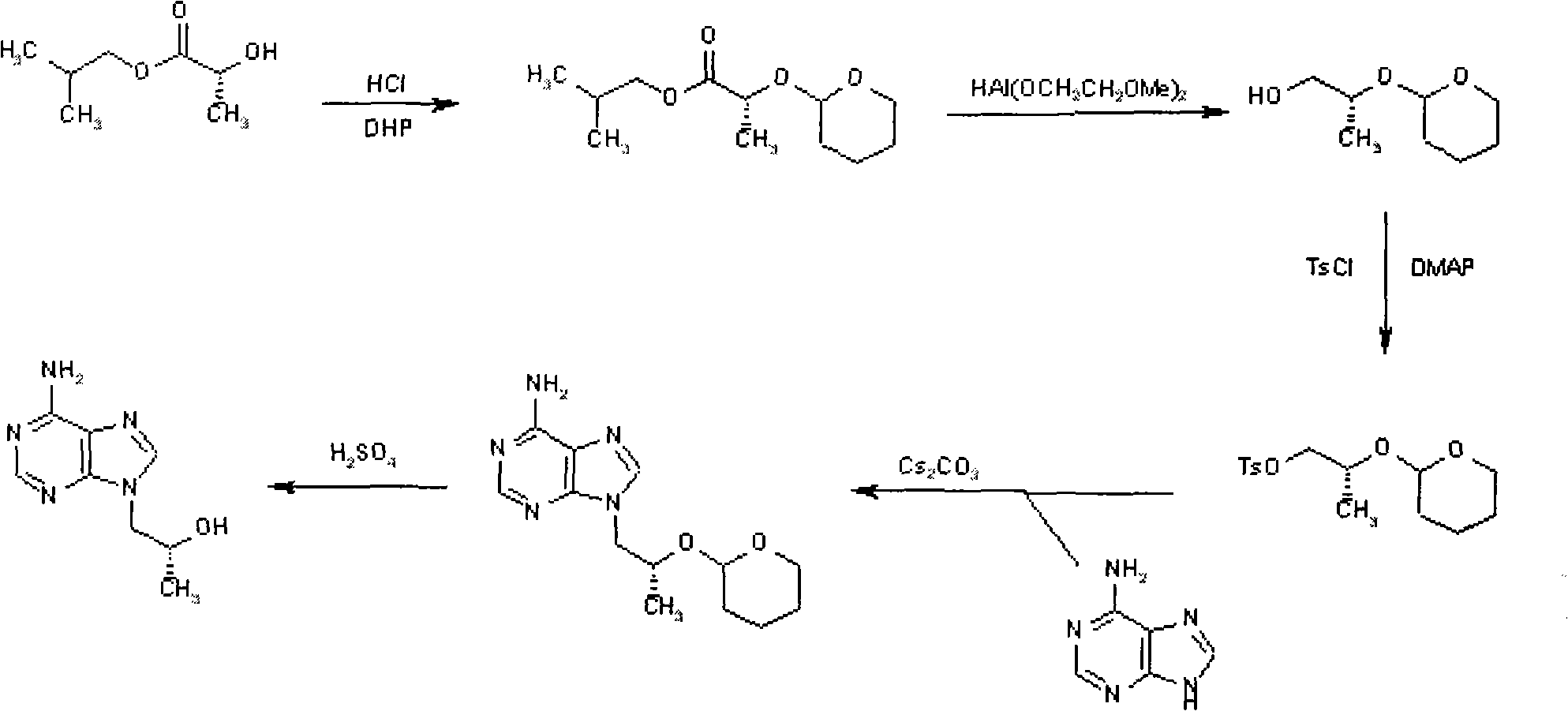
Novel method for synthesizing 9-substituted adenine compound
InactiveCN102863445ASave raw materialsMild reaction conditionsOrganic chemistrySulfonyl chlorideToluene
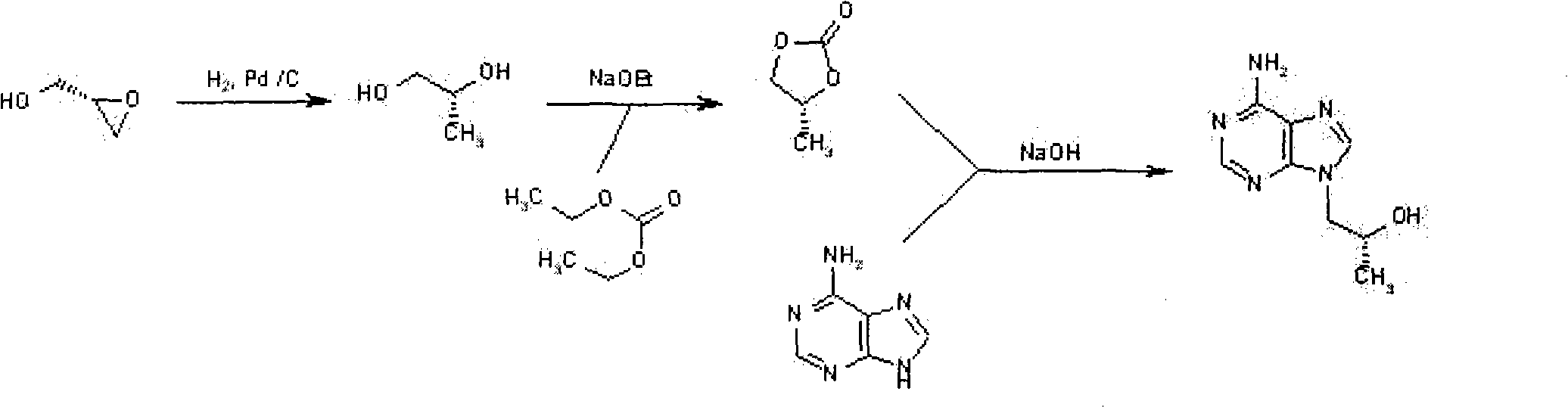
The invention provides a novel method for synthesizing tenofovir disoproxil fumarate key intermediate (R)-9-(2-hydroxide-propyl)adenine. According to the invention, (R)-epoxypropane is taken as an initial raw material and reacted to benzylalcohol to obtain (R)-2-benzyloxy propanol under the catalysis of ZrO2 / SO4, (R)-2-benzyloxy propanol is reacted to p-toluene sulfonyl chloride to obtain sulfonate, under the effect of cesium carbonate, the sulfonate is reacted to adenine to obtain (R)-9-(2-benzyloxy-propyl)adenine, and benzyl is removed under the catalysis of palladium-carbon to obtain (R)-9-(2-hydroxide-propyl)adenine. The method of the invention is characterized in that the step is short, the reaction condition of each step is mild, the yield is high, and the industrialization value is provided.
Owner:北京六盛合医药科技有限公司
Preparation method and application of modified cobalt manganese binary metal oxide catalyst
InactiveCN103877989ANo harmSimple compositionNitrous oxide captureDispersed particle separationPtru catalystManganese
The invention relates to a modified cobalt manganese binary metal oxide catalyst. The modified cobalt manganese binary metal oxide catalyst can be prepared by the following steps: synthesizing a cobalt manganese binary metal hydroxide by a weak base precipitation method, wherein the mass ratio of cobalt to manganese is (4.0-14.0):1; roasting at the temperature of 350 DEG C-550 DEG C so as to prepare a cobalt manganese binary metal oxide; impregnating the cobalt manganese binary metal oxide by auxiliary solutions of potassium carbonate, potassium oxalate or cesium carbonate and the like in equal volume; drying the cobalt manganese binary metal oxide which is impregnated by the auxiliaries at the temperature of 70 DEG C-90 DEG C; roasting the dried cobalt manganese binary metal oxide at the temperature of 350 DEG C-550 DEG C so as to obtain the modified cobalt manganese binary metal oxide catalyst. The raw materials for preparing the catalyst are all inorganic compounds and are harmless to human bodies and environments; moreover, the catalyst is simple in formation, can control preparation technological parameters, is high in activity of the catalyst, and has high cost performance.
Owner:YANTAI UNIV
Mn-CsPbCl3 nano-rod preparation and product thereof, and applications of Mn-CsPbCl3 nano-rods
ActiveCN106947477AHigh fluorescence efficiencyHigh crystallinityNanoopticsLuminescent compositionsCentrifugationToluene
The present invention relates to a Mn-CsPbCl3 nano-rod preparation method, which comprises: adding cesium carbonate into the mixture of octadecene and oleic acid, and heating to obtain a cesium oleate solution; adding PbCl2 and MnCl2.4H2O to octadecene, oleic acid and oleylamine, heating to a temperature of 120 DEG C, maintaining for 30 min, raising the temperature to a temperature of 165 DEG C, maintain for 10 min, heating to a temperature of 200 DEG C, and adding oleic acid and oleylamine until the solution is clear so as to form a Pb / Mn precursor solution; and injecting the cesium oleate solution into the Pb / Mn precursor solution, cooling with a 0 DEG C ice bath, carrying out centrifugation, washing with toluene, and repeatedly performing the steps three times to obtain the Mn-CsPbCl3 nano-rods. According to the present invention, the operation of the preparation method of the technical scheme is simple, and the obtained nano-rods have characteristics of high fluorescence efficiency, good crystallinity, large Stokes displacement.
Owner:重庆纳鼎光电科技有限公司 +1
Inorganic lead halide perovskite quantum dot, preparation method of quantum dot, nanowire and preparation method of nanowire
InactiveCN108753289AIncrease productionEasy to makeNanoopticsLuminescent compositionsNanowireQuantum dot
The invention provides an inorganic lead halide perovskite quantum dot, a preparation method of the quantum dot, a self-assembly nanowire and a preparation method of the nanowire. The preparation method of the inorganic lead halide perovskite quantum dot comprises the steps of adding cesium carbonate, lead halide, oleic acid, oleylamine and octadecene into a container to form a precursor solution,allowing a mole ratio of cesium carbonate to lead halide to be 1:(2-4), allowing a volume ratio of oleic acid, oleylamine and octadecene to be 1:1:(18-22), when adding 1mmol cesium carbonate, adding0.5ml oleic acid correspondingly, performing ultrasound treatment on the precursor solution, and centrifuging the precursor solution to form supernate, namely dispersing liquid of the inorganic lead halide perovskite quantum dot. The method is simple in preparation process, low in energy consumption, high in yield and facilitates massive production.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
Dinaphthalene azobenzene ring-type photosensitive chiral molecule and preparation method and application thereof
ActiveCN109810129ARealize reversible regulationGood compatibilityMaterial analysis by optical meansGroup 3/13 element organic compoundsN dimethylformamideBoronic acid
The invention discloses a dinaphthalene azobenzene ring-type photosensitive chiral molecule and a preparation method and application thereof. The preparation method comprises the steps that 6,6'dibromo-1,1'-bi-2-naphthol, potassium carbonate, potassium iodide and 2-(2-chloroethoxy)ethanol are dissolved in N,N-dimethylformamide, thus a midbody 1 is obtained, the midbody 1 and [4'-(pentyloxy)[1,1'-biphenyl]-4-yl]boronic acid are dissolved in 1,4-dioxane to react with a potassium carbonate aqueous solution and tetrakispalladium to obtain a midbody 2, the midbody 2 is dissolved through dichloromethane, and then triethylamine, 4-dimethylaminopyridine and paratoluensulfonyl chloride are added and react to obtain a midbody 3; and cesium carbonate is added into the midbody 3, 2,2'-dihydroxyazobenzene and dibenzo-18-crown ether-6 under the nitrogen atmosphere for reacting, and thus a target product T is obtained. The dinaphthalene azobenzene ring-type photosensitive chiral molecule is easy to synthesize and good in stability, the screw pitch of a cholesteric liquid crystal can be adjusted and controlled through photoinduced cis-trans isomerization, and good compatibility between the dinaphthalene azobenzene ring-type photosensitive chiral molecule and liquid crystal molecules is achieved.
Owner:HEFEI UNIV OF TECH
Method for producing halogenated caesium lead material for perovskite solar cell
InactiveCN106449990ASimple processHigh purity productSolid-state devicesSemiconductor/solid-state device manufacturingChemical reactionHole transport layer
The invention relates to a method for producing a halogenated caesium lead material that is obtained based on solid-liquid two-phase chemical reaction of a halogenated ammonium lead material and cesium carbonate and has advantages of low production cost, high stability and easy dispersion. A halogenated ammonium lead, cesium carbonate, and C1-C4 fatty alcohol are mixed uniformly according to a molar ratio 1 to 0.5-0.55 to 10-20; while heating is carried out slowly at a temperature of 70 to 110 DEG C, reaction is carried out for 2 to 6 hours, so that the cesium carbonate reacts with the halogenated ammonium lead material to obtain a halogenated caesium lead material and ammonium carbonate as a by product decomposes and volatilizes; and a reaction product is cooled to be reach a temperature of 50 to 60 DEG C, a polar solvent as a cosolvent of a halogenated caesium lead crystal and silicone oil as a surface treating agent are added, a supernatant solution is stirred continuous for 6 to 12 hours, thereby forming a halogenated caesium lead crystal particle with characteristics of uniform particle diameter and surface hydrophobicity. The method provided by the invention has characteristics of simple process and secure environment protection and is easy to expanded produce and industrial produce. The product obtained by using the method can be used as a material for a light absorption layer of a perovskite solar cell and a material of a hole transport layer.
Owner:TIANJIN VOCATIONAL INST
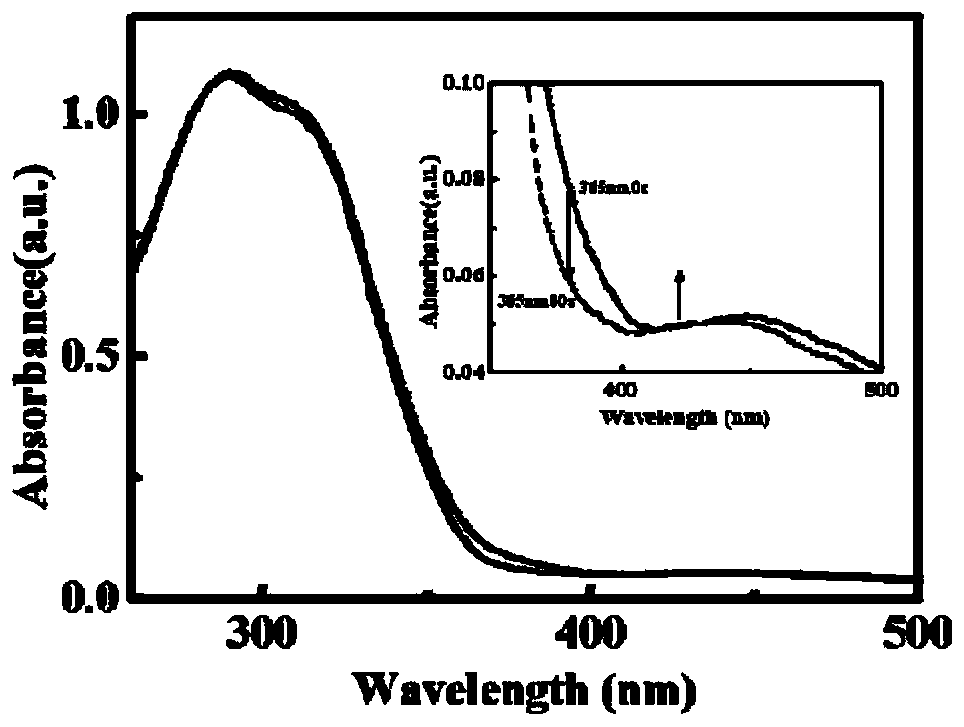
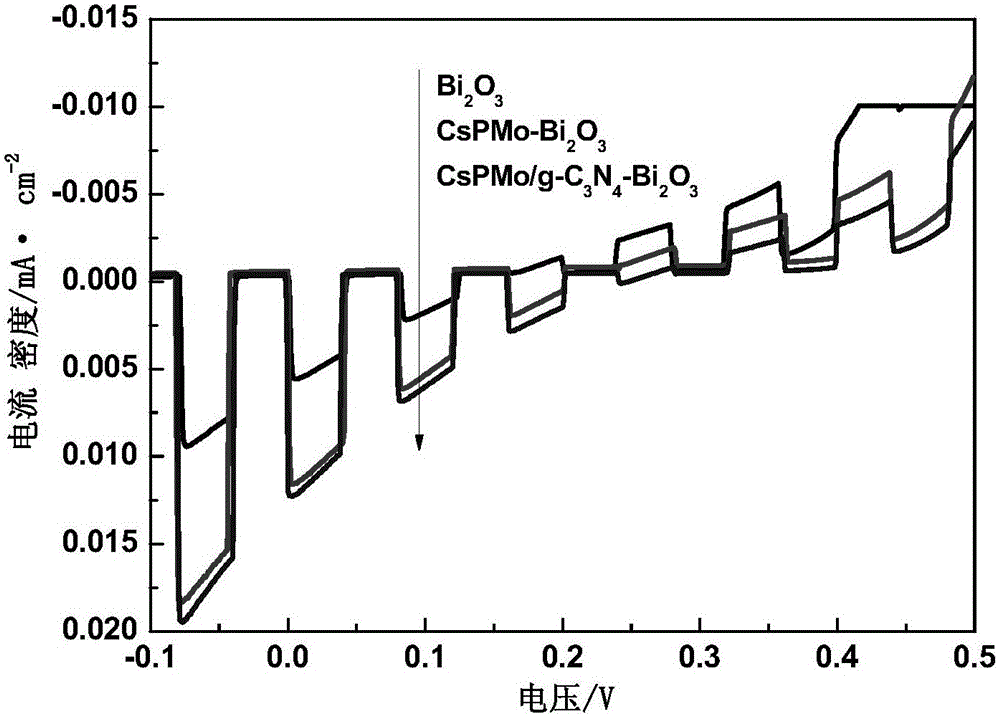
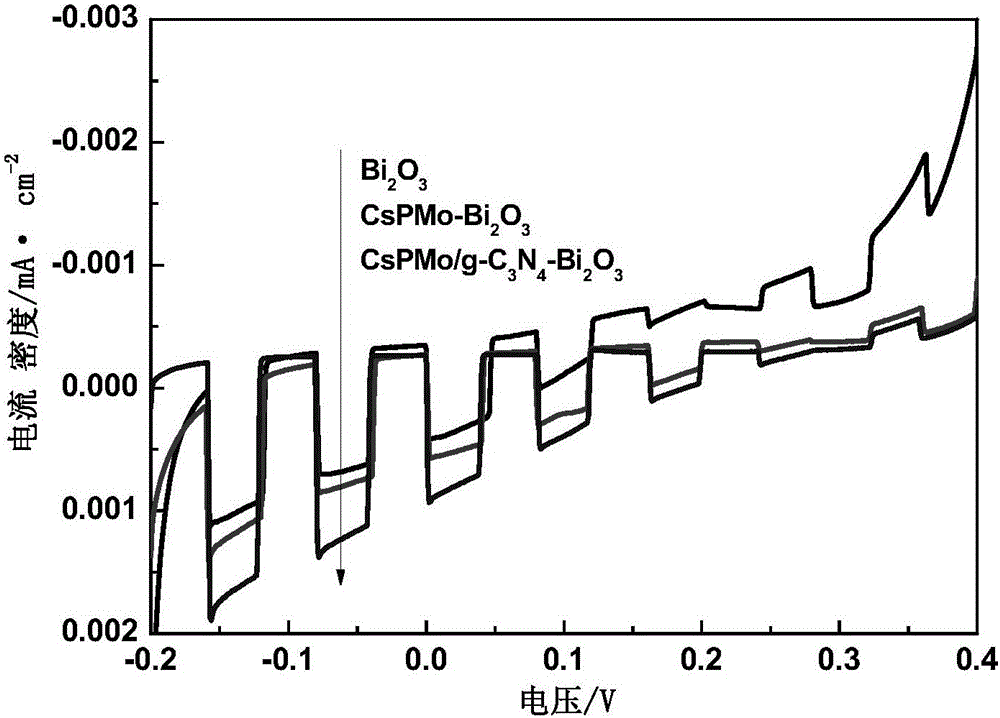
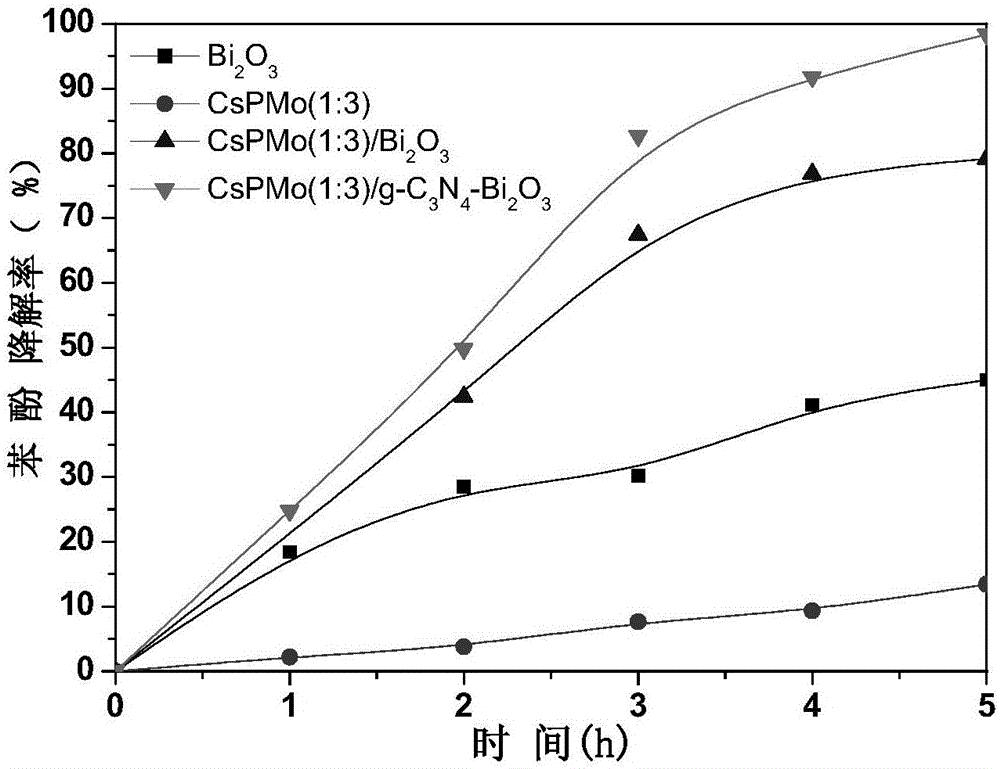
CsPMo/g-C3N4-Bi2O3 photocatalyst and preparation method therefor and application thereof in phenolic wastewater treatment
InactiveCN105032463AImproved visible light responseEasy to handlePhysical/chemical process catalystsWater/sewage treatment by irradiationPhosphomolybdic acidPhoto irradiation
The invention discloses a CsPMo / g-C3N4-Bi2O3 photocatalyst and a preparation method therefor and application thereof in phenolic wastewater treatment. The preparation method for the CsPMo / g-C3N4-Bi2O3 photocatalyst comprises the steps: (1) thoroughly dispersing g-C3N4 into water, then, sequentially adding Bi2O3, phosphomolybdic acid and cesium carbonate into the dispersion, carrying out uniformly mixing, and carrying out reaction to produce yellow precipitates; and (2) drying and grinding the obtained yellow precipitates, thereby obtaining the CsPMo / g-C3N4-Bi2O3 photocatalyst. The photocatalyst disclosed by the invention is applied to the photocatalytic treatment of phenolic wastewater and is added into the phenolic wastewater, stirring is carried out at a dark place until adsorption equilibrium is achieved, and reaction is carried out under visible light irradiation. The preparation method for the photocatalyst, disclosed by the invention, is simple, the degree of visible light response is increased, the photoproduction electron and hole separating effect is good, the effect of treating the phenolic wastewater is good, and no secondary pollution is caused.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Process for making 5-lipoxygenase inhibitors having varied heterocyclic ring systems
InactiveUS6344563B1Organic active ingredientsAntipyreticPhosphonium saltTetra-n-butylammonium chloride
process is described for preparing a compound of Formula (1.3.0):comprising: establishing a reaction mixture consisting ofin an aprotic solvent; in the presence of a strong base in solid form selected from the group consisting of sodium hydroxide, NaOH; and potassium hydroxide, KOH;-and optionally-in the presence of a catalytic amount of cesium carbonate, Cs2CO3, or of a phase transfer catalyst, especially a quaternary ammonium salt or a phosphonium salt-followed by-heating said reaction mixture under a nitrogen atmosphere; whereby there is produced a compound of Formula (1.3.0); and in a preferred embodiment the aprotic solvent is DMSO, the strong base in solid form is NaOH in powder or pellet form, and the phase transfer catalyst is tetra-n-butylammonium chloride (TBAC), which is used to prepare a preferred compound, useful as a 5-lipoxygenase inhibitor, of the formula:especially the substantially pure methanesulfonate salt thereof.
Owner:NORRIS TIMOTHY +2
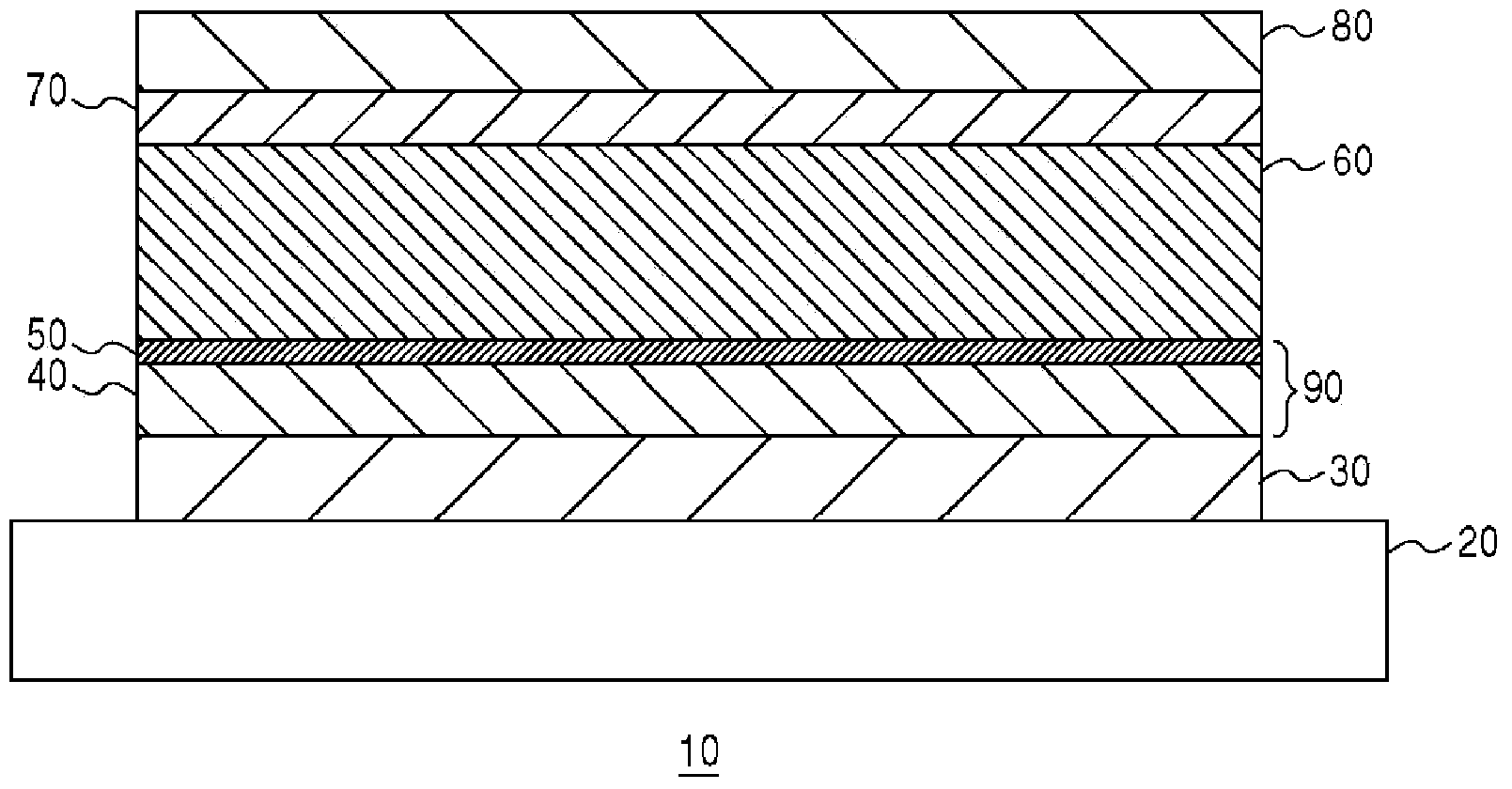
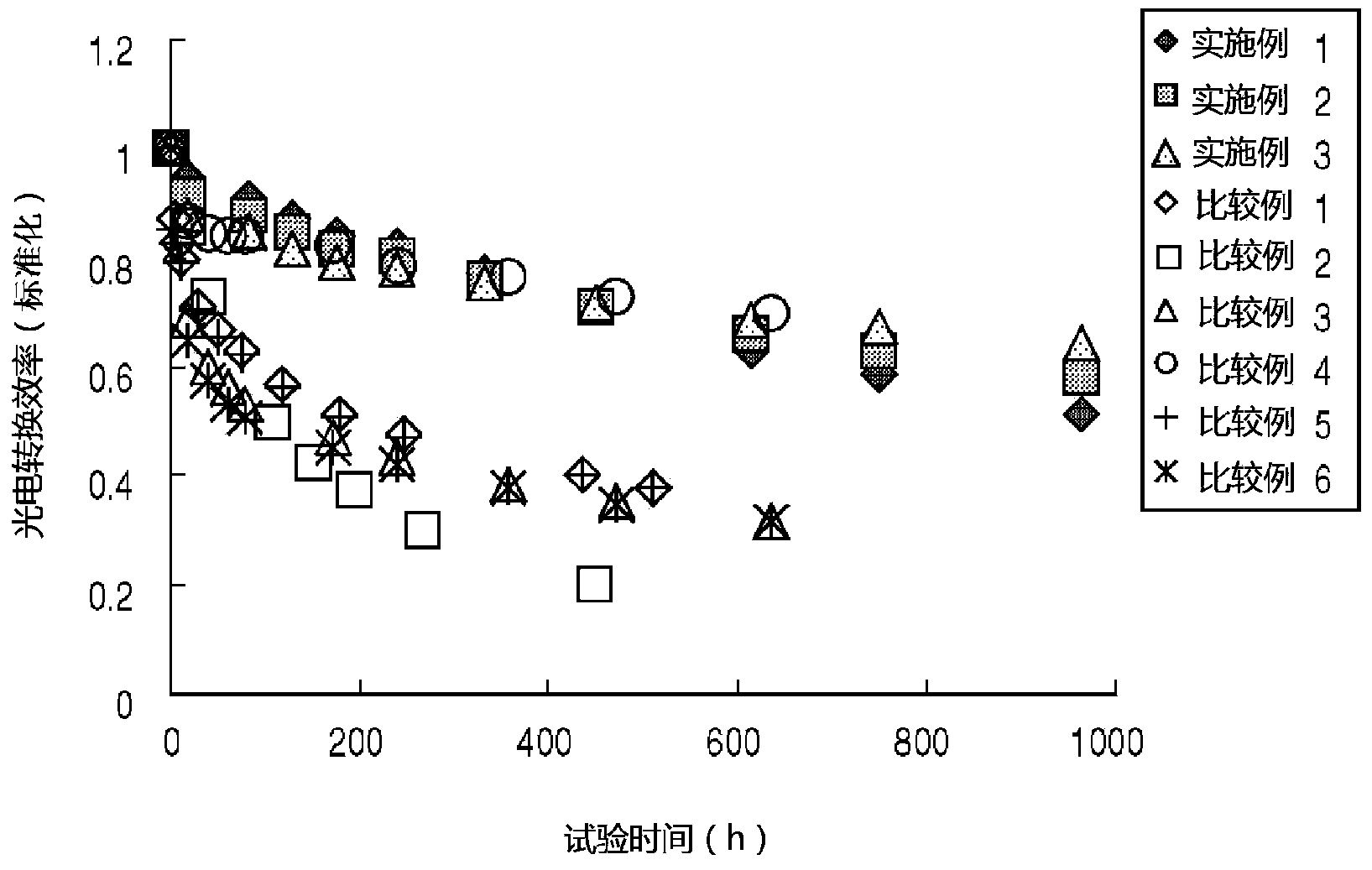
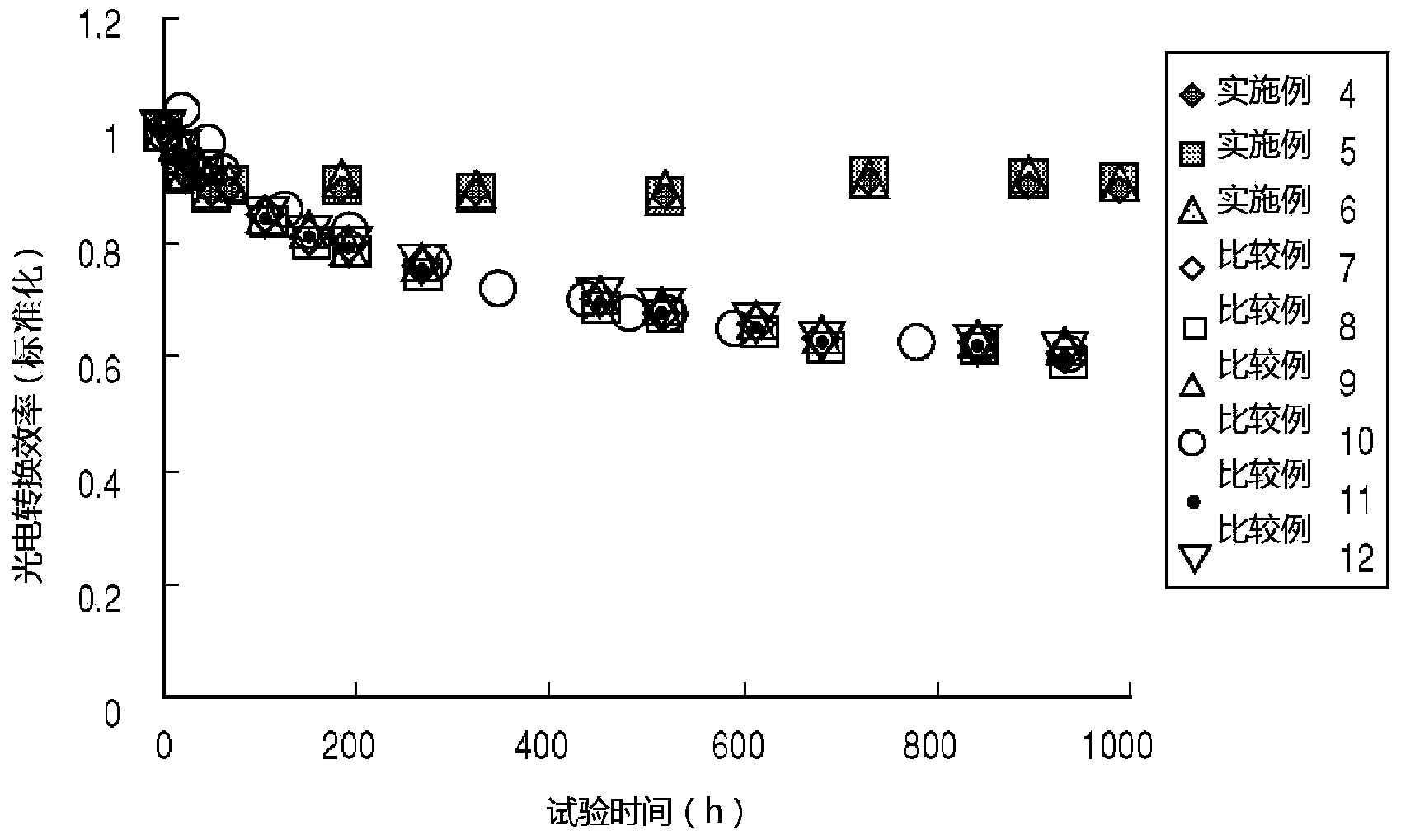
Photovoltaic conversion element and method of manufacture thereof
InactiveCN103931008AImprove photoelectric conversion efficiencyOrganic chemistryFinal product manufactureHole transport layerZinc Acetate Dihydrate
A photovoltaic conversion element (10) has a construction wherein an electron transport layer (40), a conduction zone bottom end energy adjustment layer (50), a photovoltaic conversion layer (60) and a positive hole transport layer (70) are sandwiched in this order between a first electrode (30) and a second electrode (80). The conduction zone bottom end energy adjustment layer (50) is formed by for example caesium carbonate. Also, the electron transport layer (40) is formed of zinc acetate.
Owner:JX NIPPON OIL & ENERGY CORP
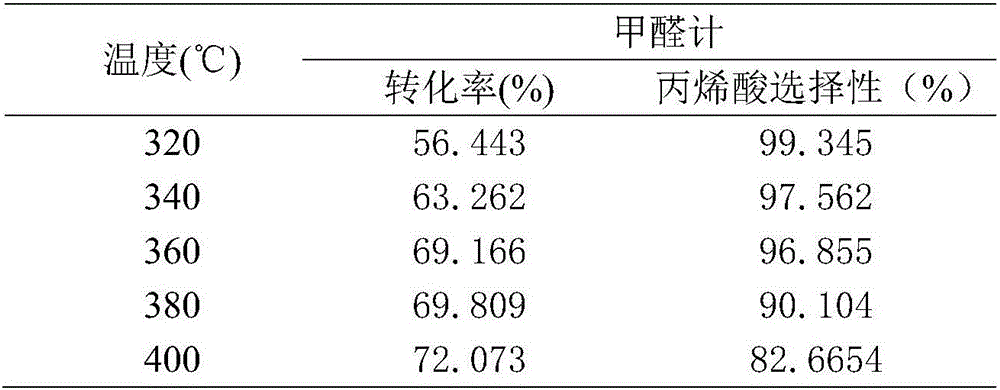
Cs-VPO/SiO2 catalyst, preparation method therefor and use of Cs-VPO/SiO2 catalyst in preparation of acrylic acid through catalyzing condensation of acetic acid and trioxymethylene
InactiveCN106582749AHigh catalytic activityImprove catalytic stabilityPhysical/chemical process catalystsOrganic compound preparationIsobutanolFiltration
The invention provides a Cs-VPO / SiO2 catalyst, a preparation method therefor and use of the Cs-VPO / SiO2 catalyst in preparation of acrylic acid through catalyzing condensation of acetic acid and trioxymethylene. The preparation method comprises the steps: (1) firstly, weighing vanadium pentoxide, adding the weighed vanadium pentoxide into a three-mouthed flask, then, adding isobutanol and benzyl alcohol into the three-mouthed flask, and carrying out constant-temperature stirring refluxing until the vanadium pentoxide is completely dissolved; and weighing phosphoric acid, slowly adding the weighed phosphoric acid into the flask, and then, continuing to carry out a constant-temperature refluxing reaction until the solution is dark blue, so as to obtain a vanadium-phosphorus oxide, i.e., VPO; and (2) adding SiO2 into the three-mouthed flask in the step (1), then, adding an aqueous solution of cesium carbonate into the three-mouthed flask, and carrying out a stirring reaction; and taking out a sample after the reaction is completed, carrying out suction filtration, carrying out washing with isobutanol and acetone, then, carrying out drying, grinding and roasting, tabletting the sample, and carrying out screening, thereby preparing the Cs-VPO / SiO2 catalyst. The Cs-VPO / SiO2 catalyst provided by the invention has the remarkable characteristic of having good catalytic activity and stability in a reaction process.
Owner:JIANGSU UNIV
Method for synthesizing 1,3-oxazole-2,4-diketone compounds
The invention discloses a method for synthesizing 1,3-oxazole-2,4-diketone compounds. 3-aryl-2-alkyneamide reacts with carbon dioxide under the action of potassium carbonate or cesium carbonate so as to generate a 1,3-oxazole-2,4-diketone compound. The method is easy to operate, raw materials and reagents are readily available and the reaction has high regioselectivity; the product is easy to separate and purify and has low toxicity, high yield and readily available raw materials; and the method is suitable for synthesizing various substituted 1,3-oxazole-2,4-diketone compounds.
Owner:ZHEJIANG UNIV
Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate
ActiveCN111620869AReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionPalladium on carbonNonane
The invention relates to a synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following seven steps: 1, reacting a compound 1 with ethyl malonate added into a solvent ethanol to obtain a compound 2; 2, reacting the compound 2 with lithium borohydridein tetrahydrofuran to obtain a compound 3; 3, reacting the compound 3 with p-toluenesulfonyl chloride in dichloromethane to obtain a compound 4; 4, adding cesium carbonate into the compound 4 in acetonitrile serving as a solvent for cyclization to obtain a compound 5; 5, adding magnesium chips into the compound 5 in methanol serving as a solvent for reduction to obtain a compound 6, 6, reacting the compound 6 with Boc anhydride in dichloromethane to obtain a compound 7, and 7, reacting the compound 7 with palladium on carbon in methanol to obtain a final compound 8.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
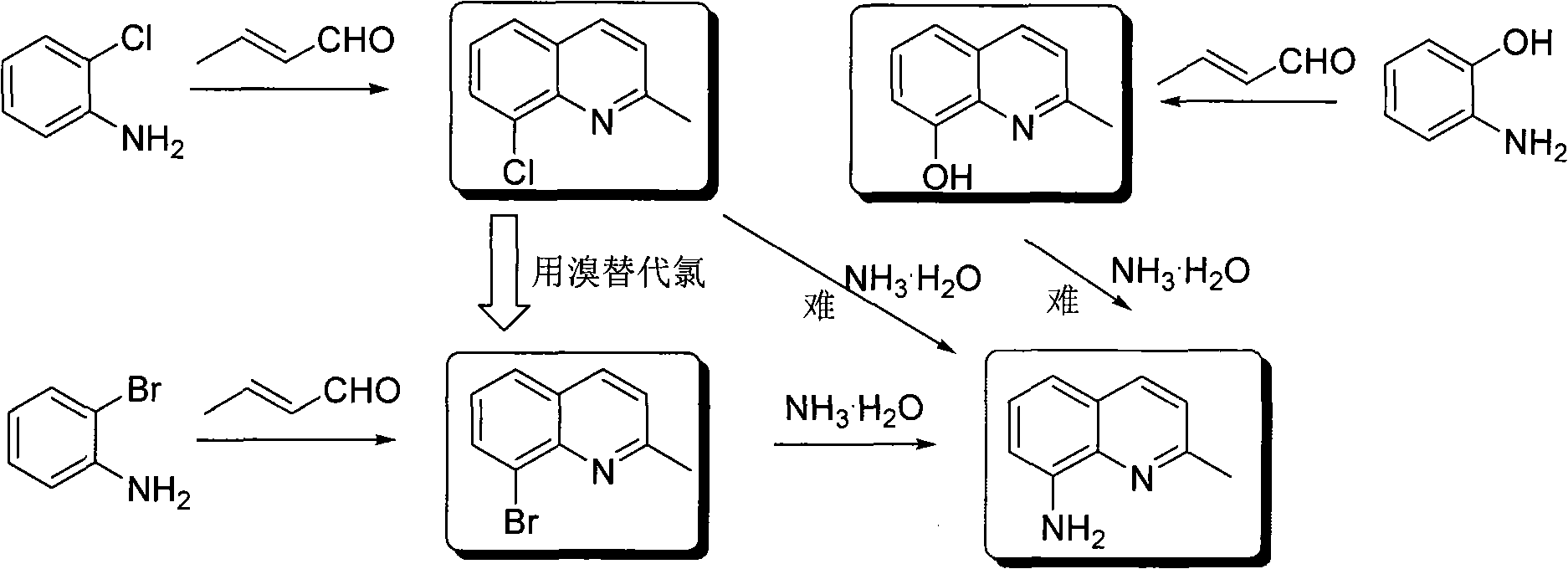
Preparation method of 2-methyl-8-aminoquinoline
InactiveCN101602723AIncrease the activity of the substitution reactionImprove responseOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChlorobenzeneCrotonaldehyde
The invention discloses a preparation method of 2-methyl-8-aminoquinoline. The preparation method of 2-methyl-8-aminoquinoline comprises the step of ammoniating 2-methyl-8-bromoquinoline in a solvent at 60-120 DEG C under the action of catalyst and strong alkali to obtain the 2-methyl-8-aminoquinoline, wherein the catalyst is one or more selected out of copper acetylacetonate, iron acetylacetonate, cobalt acetylacetonate and zinc acetylacetonate, the solvent used is one or more selected out of dimethylsulfoxide, N, N-dimethylformamide, acetylacetone, tetrahydrofuran and N-methylpyrrolidone, and the strong alkali is one or more selected out of caesium carbonate, caesium hydroxide, potassium carbonate and potassium hydroxide. The preparation method of the intermediate 2-methyl-8-bromoquinoline comprises the step of performing a cyclization reaction on o-bromoaniline and crotonic aldehyde in a solvent in the presence of oxidant and mollient, so as to obtain the 2-methyl-8-bromoquinoline, wherein the oxidant is selected out of one or more of nitrobenzene, 2-nitrobromobenzene, ammonium ceric nitrate, vanadic acid and iron oxide, the mollient is selected out of one or more of glacial acetic acid, hydrochloric acid, ferrous sulfate and boric acid, and the solvent is selected out of one or more of hydrochloric acid, sulfuric acid and chlorobenzene.
Owner:HUNAN UNIV
Method for preparing salts of polyene macrolide esters
InactiveUS6613889B2Promote conversionReducing methylationSugar derivativesGlycosidesCarbonateTetrahydrofuran
Owner:BIOSOURCE PHARM INC
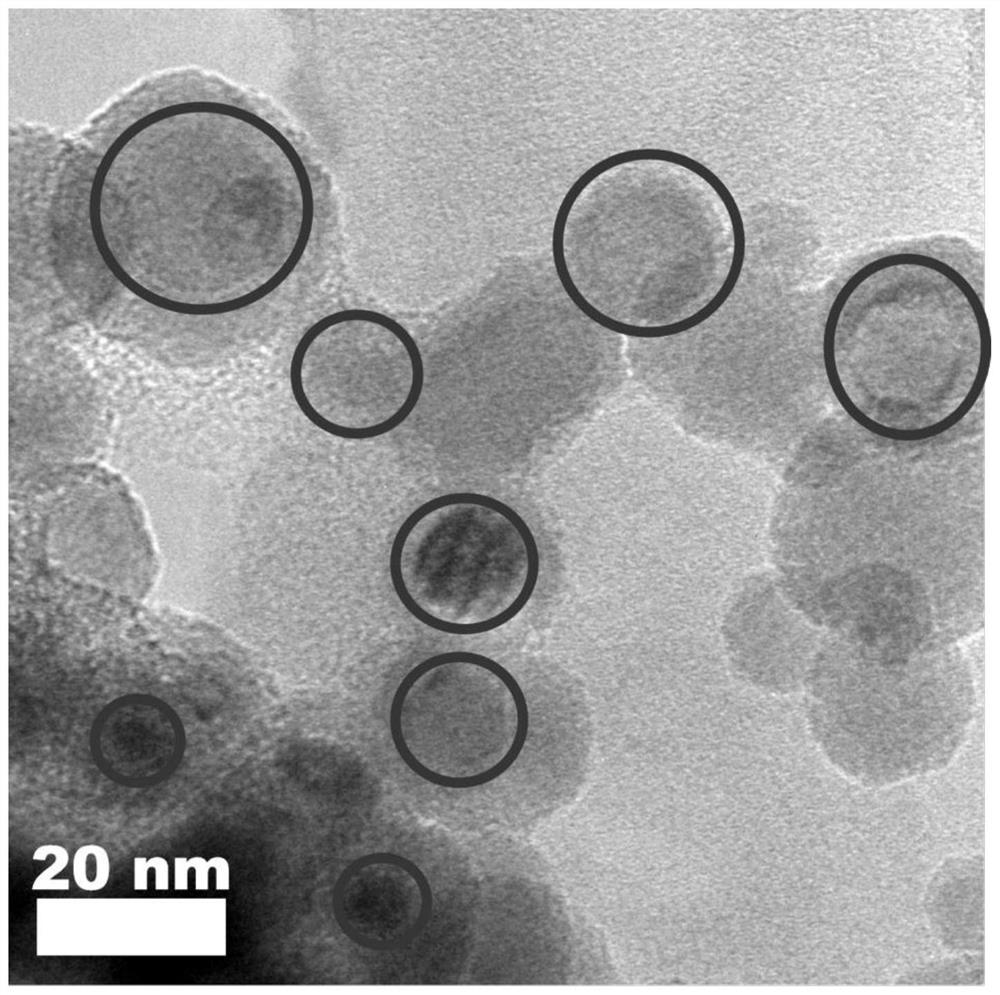
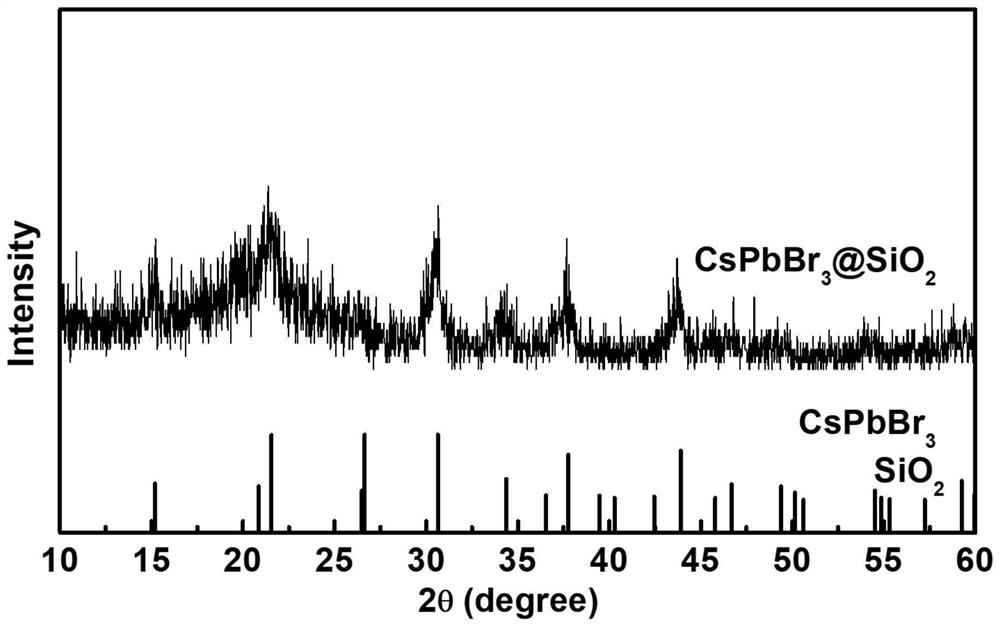
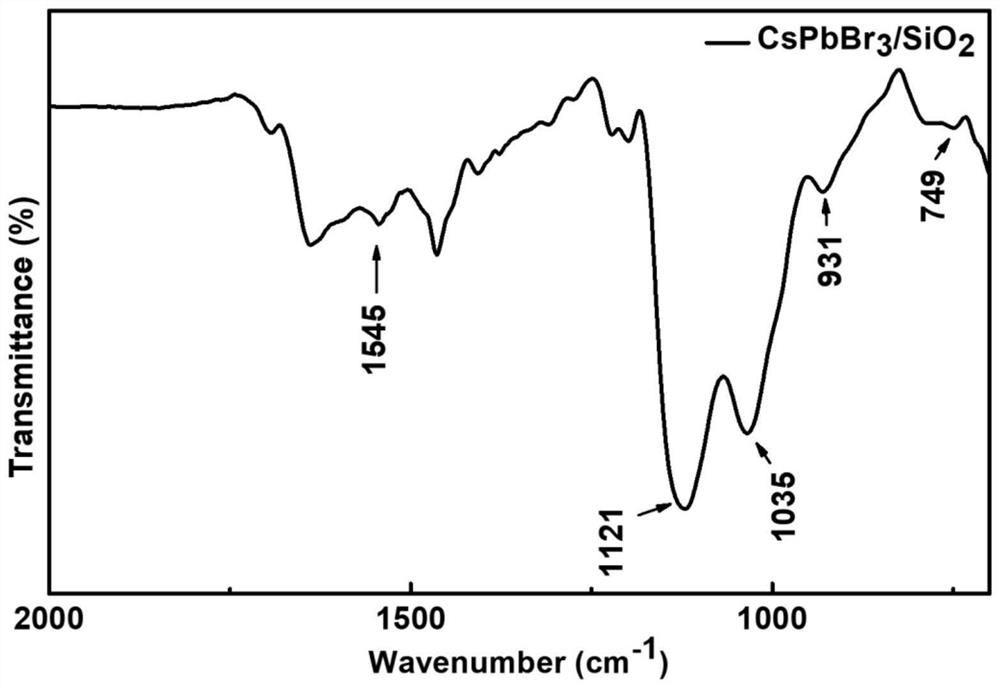
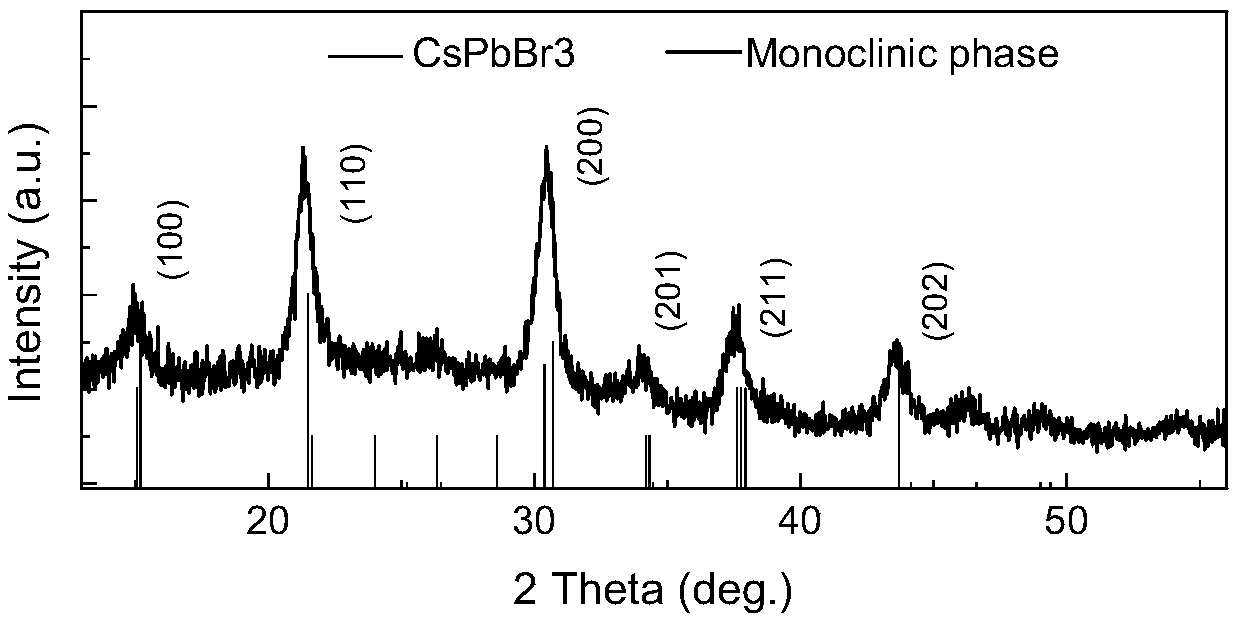
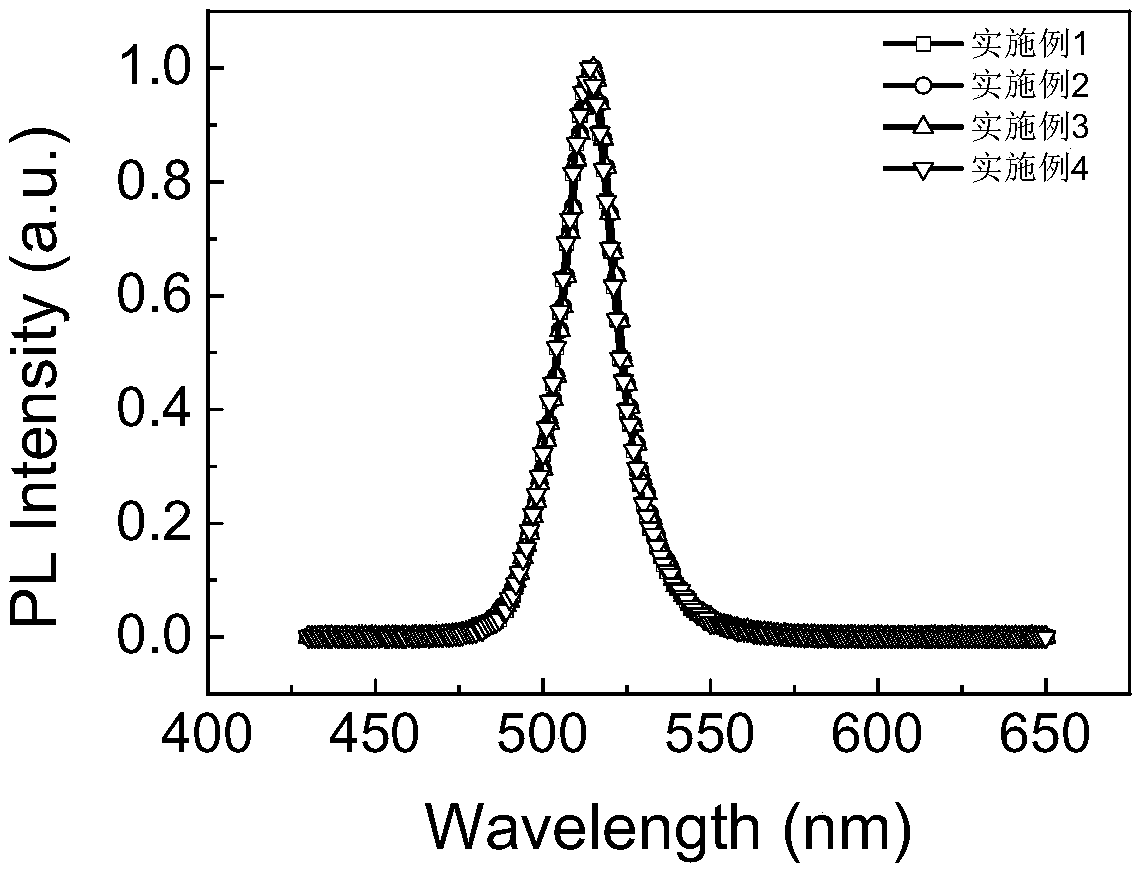
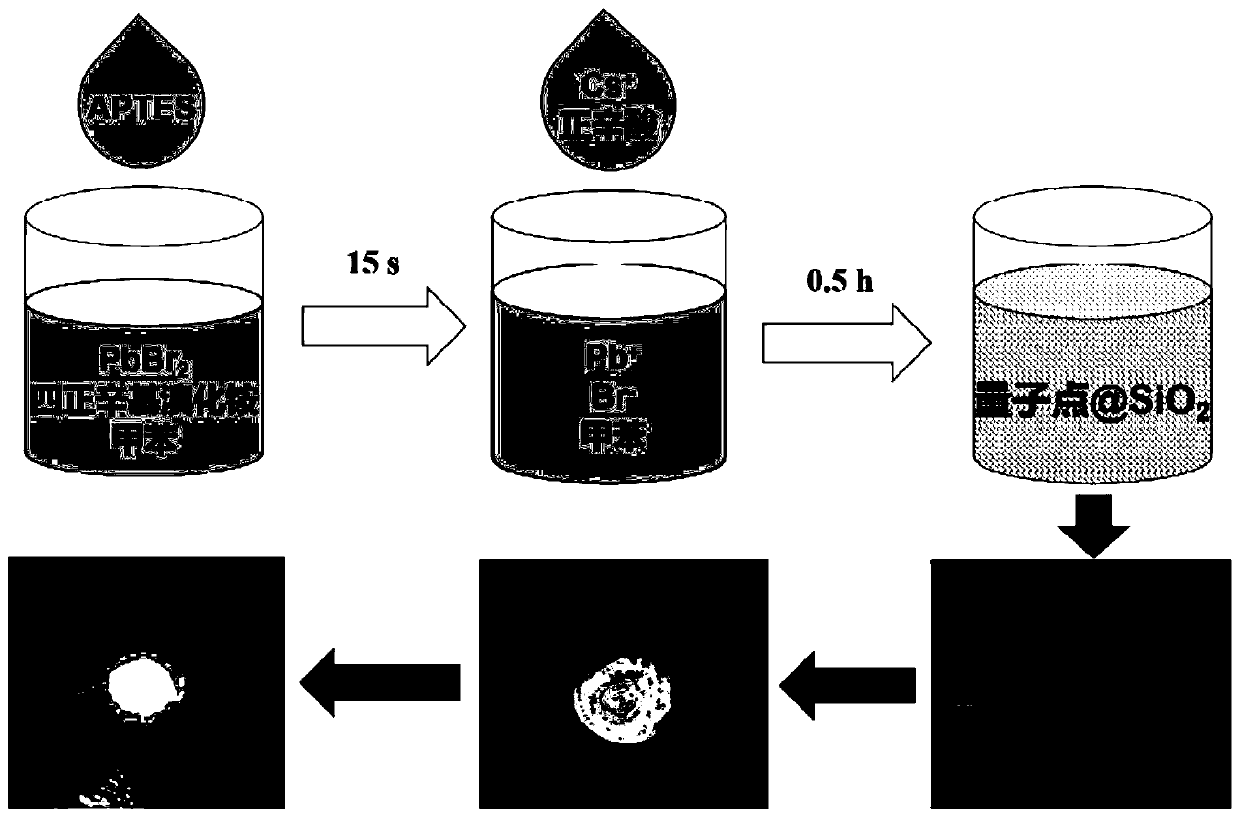
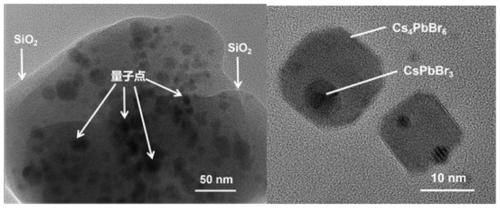
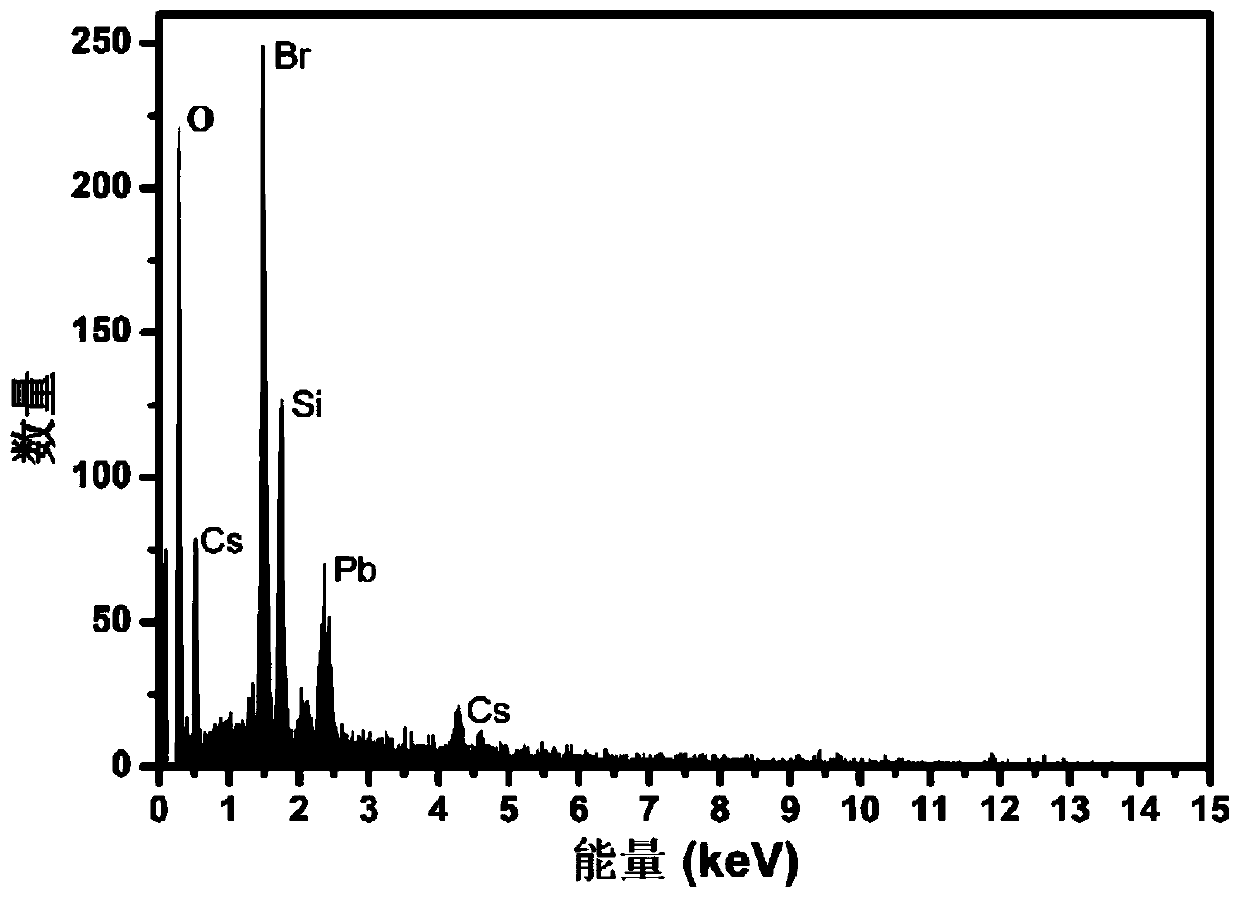
Preparation method for CsPbBr3@SiO2 nanoparticles with ultrahigh water stability
InactiveCN111978950AImprove performanceImprove water stabilityMaterial nanotechnologyNanoopticsSio2 nanoparticlePhysical chemistry
The invention relates to a preparation method for CsPbBr3@SiO2 nanoparticles with ultrahigh water stability. The preparation method comprises the following steps: adding 0.1 g of cesium carbonate and0.5-1.5 mL of oleic acid into a reaction flask, adding 1-octadecene into the reaction flask in a nitrogen environment, and carrying out heating to 110-130 DEG C until the cesium carbonate is completely dissolved into a transparent solution so as to obtain cesium oleate; adding PbBr2 into the reaction flask, adding 1-octadecene into the reaction flask in a nitrogen environment, carrying out heatingto 110 -130 DEG C, then adding oleic acid, oleylamine and APTES, and continuing heating until the PbBr2 is completely dissolved; and carrying out heating to 160-180 DEG C, weighing a small amount ofcesium oleate, and quickly adding the cesium oleate into a PbBr2 precursor solution. When a reaction is carried out for 5-15 s, the flask is rapidly subjected to an ice-water bath; when a liquid is gradually changed from yellow to yellow-green, the CsPbBr3 nanoparticles are successfully prepared; and the structural stability and the highly-efficient fluorescence performance are maintained for at least 48 h under the water environment condition.
Owner:NANJING UNIV +2
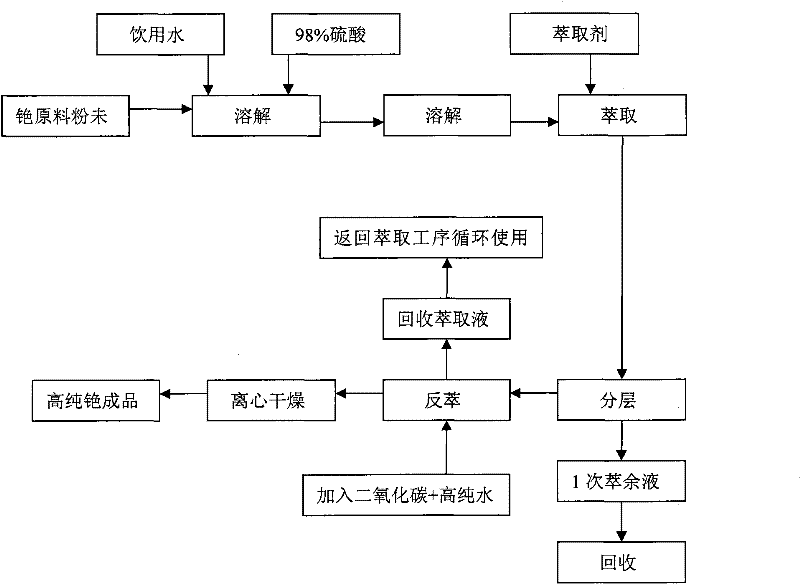
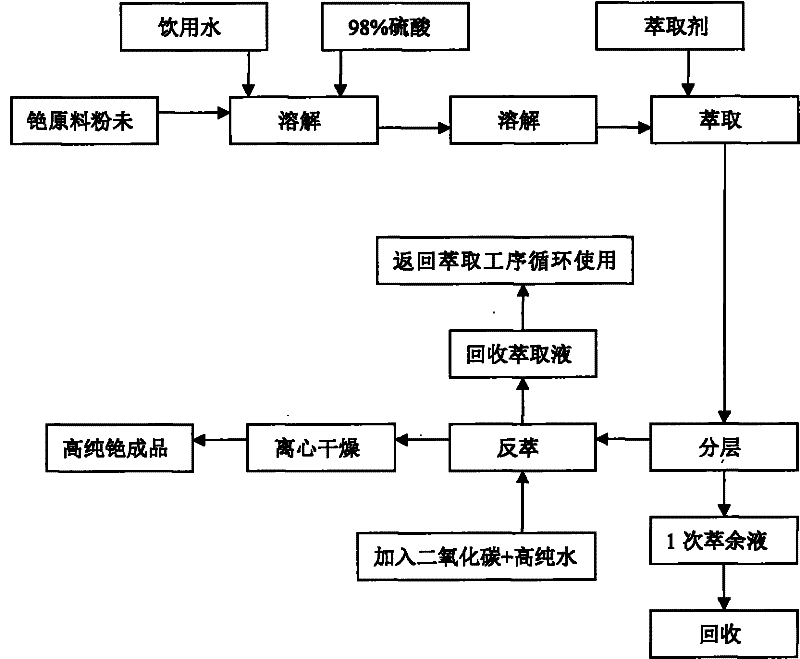
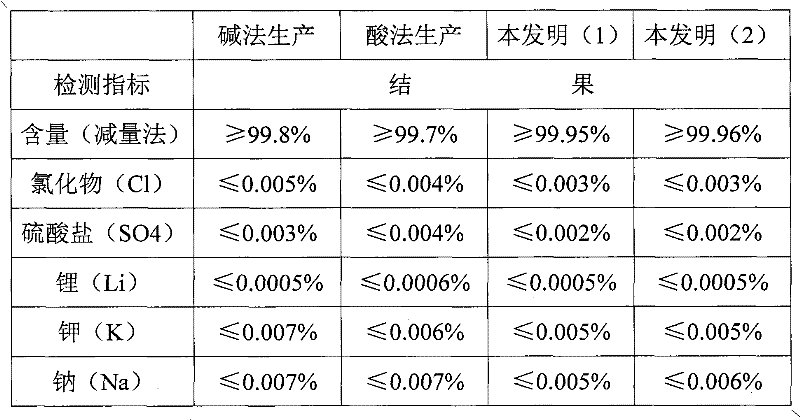
Preparation method of cesium carbonate
ActiveCN102241409AReduce manufacturing costReduce high energy consumptionRubidium/caesium/francium compoundsHigh energyToxic material
The invention relates to a preparation method of cesium carbonate. The cesium carbonate is prepared from raw materials of cesium powder, 98% of sulfuric acid and water, wherein a weight ratio of cesium powder to 98% of sulfuric acid to water is 1:0.7 to 0.8:1.8 to 2.2. The preparation method comprises the steps that: cesium powder is pretreated, such that a leached stock solution is obtained; the pH value of the stock solution is regulated; the stock solution is extracted, layered, back-extracted, dried by centrifugation, dried by baking, and packaged. After the processes, a finished product is obtained. The cesium carbonate preparation method provided by the invention is advantaged in that: (1) a high energy consumption of traditional alkali production methods is reduced, a requirement to equipment is reduced, and cesium carbonate production cost is greatly reduced; (2) generation of toxic substances during a production process with an acid method is avoided, production period is shortened, and productivity is improved; (3) a cesium ion leaching rate of the product provided by the present invention in a metal ion solution reaches 98.5%, while a cesium ion leaching rate of products provided with traditional technologies in a metal ion solution is 80%; (4) product quality is improved, and application scope of the product is enlarged.
Owner:上海实验试剂有限公司 +1
Method for synthesizing aromatic amine
InactiveCN102093150ARaw materials are easy to getSimple processOrganic compound preparationAmino group formation/introductionSodium bicarbonatePotassium carbonate
The invention provides a method for synthesizing aromatic amine. A reaction formula is shown in the specifications, wherein when X is nitrogen, a substituent group R can be 3- / 5-nitro, 3- / 5-cyan, 3- / 5-trifluoromethyl or 3- / 5-halo (fluorine, chlorine, bromine or iodine); when X is carbon, R can be 2- / 4-nitro, 2- / 4-cyan, 2- / 4-trifluoromethyl or 2-nitro-4-chlorine; R1 and R2 refer to hydrogen, alkyl, alkyl alcohol, benzyl or-(CH2)n-(n=2-6) naphthenic base respectively; reaction alkali is caesium carbonate, potassium phosphate, pyridine, triethylamine, sodium bicarbonate, potassium carbonate, sodium / potassium hydroxide or sodium / potassium alkoxide; a reaction solvent is dioxane, toluol, dimethyl sulfoxide, dimethyl formamide, acetonitrile, tetrahydrofuran or acetone; and the reaction is implemented in the conventional heating state. In the method, raw materials are readily available, the process is simple, the reaction condition is mild, the application range is wide, and a plurality of aromatic amine compounds can be synthesized by different substrates.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
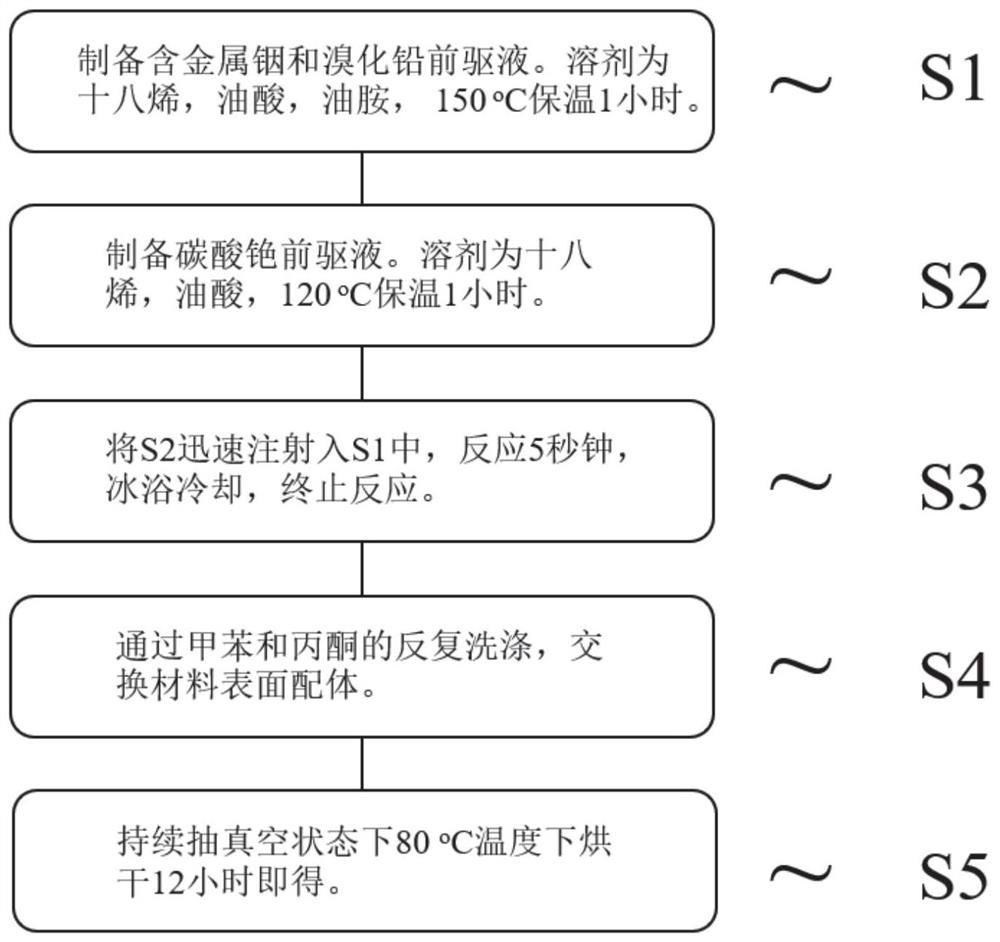
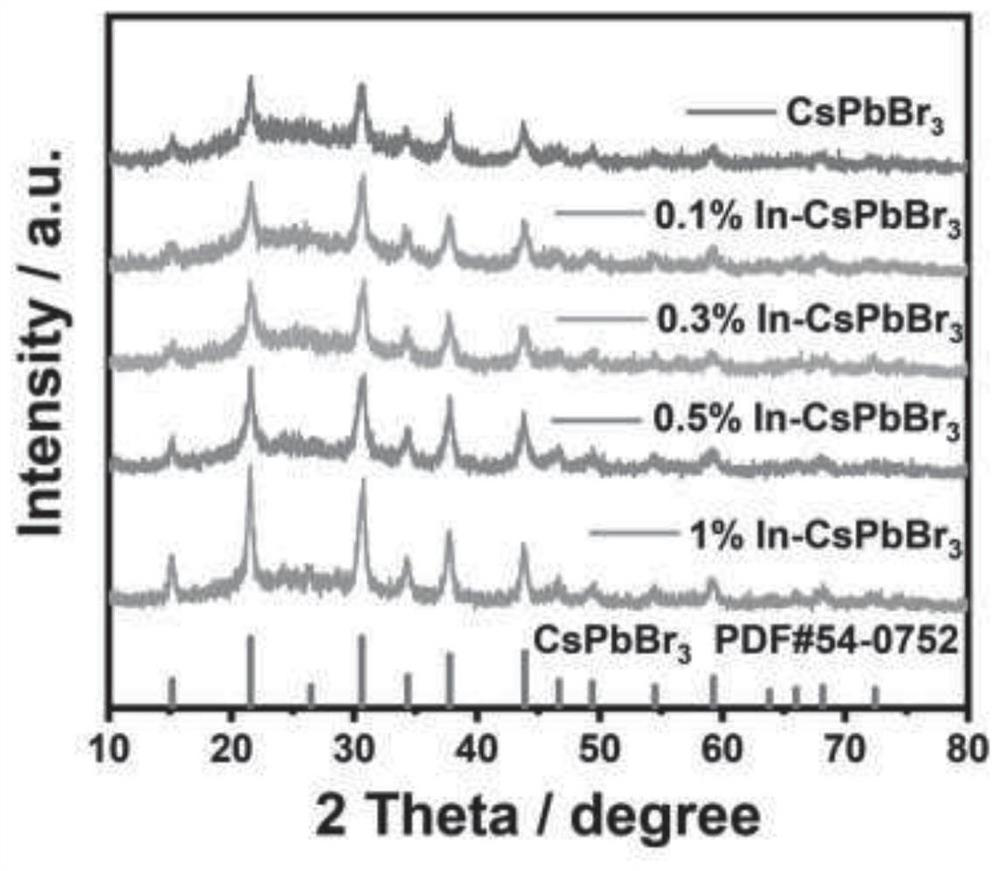
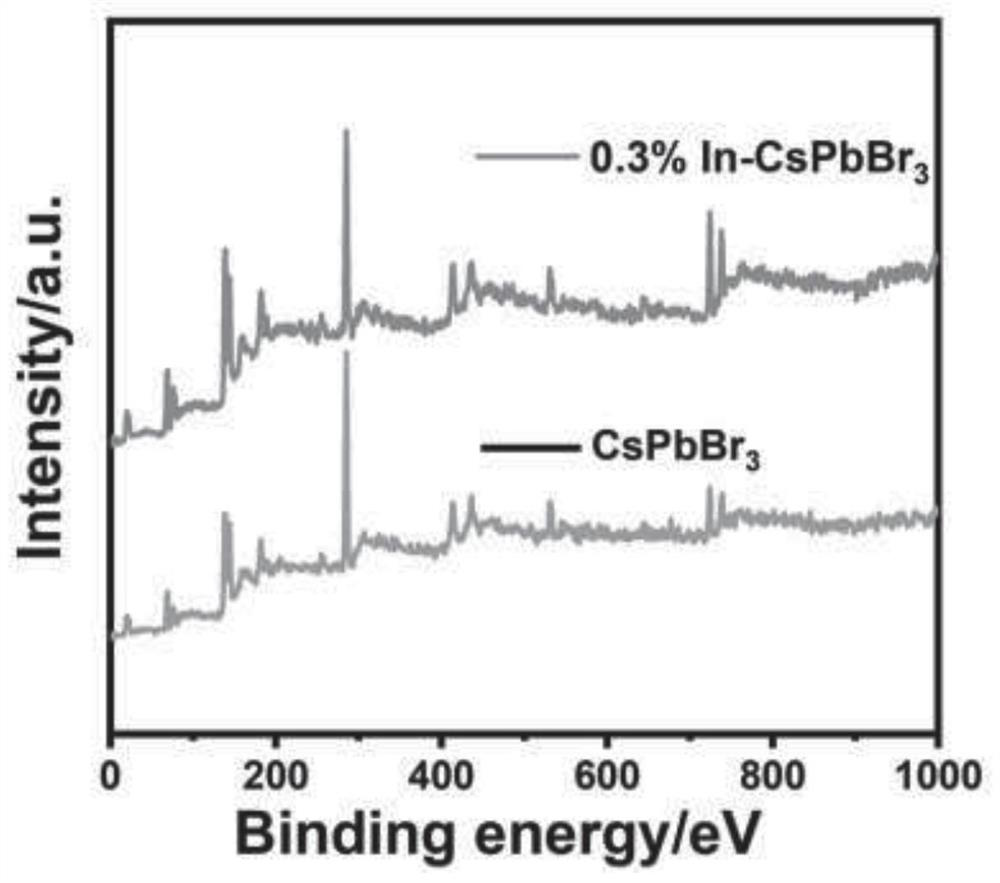
Metal indium-doped cesium lead bromide perovskite quantum dot photocatalyst and preparation method thereof, and application of metal indium-doped cesium lead bromide perovskite quantum dot photocatalyst in reduction of carbon dioxide
ActiveCN113198496AImprove stabilityInhibition of adsorptionMaterial nanotechnologyPhysical/chemical process catalystsPtru catalystHalogen
The invention discloses a metal indium-doped CsPbBr3 perovskite quantum dot photocatalyst. The photocatalyst is characterized in that halogen perovskite quantum dots are used as a carrier, and metal indium is doped in the halogen perovskite quantum dots to serve as an active center. A preparation method of the photocatalyst comprises the following steps: S1, preparing a precursor solution containing metal indium and lead bromide; S2, preparing a cesium carbonate precursor solution; S3, rapidly injecting the cesium carbonate precursor solution into the precursor solution containing the metal indium and the lead bromide for a reaction, conducting cooling in an ice bath, and then terminating the reaction; S4, exchanging a ligand on the surface of a catalyst through repeated washing of methylbenzene and acetone; and S5, conducting drying in a continuous vacuumizing state to obtain the photocatalyst. The photocatalytic carbon dioxide reduction performance of the metal indium-doped CsPbBr3 perovskite quantum dot photocatalyst disclosed by the invention is 1.48 to 3.25 times the photocatalytic carbon dioxide reduction performance of a pure-phase CsPbBr3 perovskite quantum dot photocatalyst, and the stability of the CsPbBr3 perovskite quantum dot photocatalyst is greatly improved.
Owner:YANGTZE DELTA REGION INST OF UNIV OF ELECTRONICS SCI & TECH OF CHINE HUZHOU
(S)-/(R)-difurodinaphthalene as well as derivatives thereof and preparation method
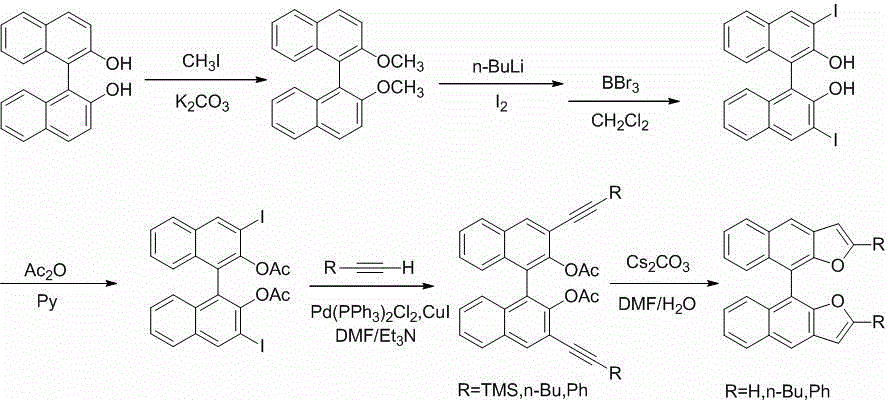
The invention discloses (S)- / (R)-difurodinaphthalene as well as derivatives thereof and a preparation method. The compounds have two spatial configurations which are (S)- / (R)-, and in the structure, the site 2 of furan has substituent groups which are hydrogen, and linear or branched alkyl groups or phenyl groups of C4 or below. The preparation method comprises the following steps of: reacting (S)- / (R)-binaphthol as a raw material and potassium carbonate as alkali with iodomethane in acetone so as to obtain (S)- / (R)-2, 2'-dimethoxy-1, 1'-dinaphthalene; then carrying out diiodo-reaction of a product obtained in the previous step, removing protecting groups of phenolic hydroxyl groups, and esterifying with acetic anhydride, thus obtaining (S)- / (R)-3, 3'-diiodo-2, 2'-diacetoxy-1, 1'-dinaphthalene; carrying out Sonogashira coupled reaction of (S)- / (R)-3, 3'-diiodo-2, 2'-diacetoxy-1, 1'-dinaphthalene with alkyne under the catalysis action of palladium to obtain a series of (S)- / (R)-3, 3'-di(trimethylsilylacetylene, hexynyl or phenyl ethynyl)-2, 2'-diacetoxy-1, 1'- dinaphthalene; and finally under the action of cesium carbonate, carrying out ring closing reaction to obtain the (S)- / (R)-double furodinaphthalene and derivatives thereof. A series of compounds provided by the invention broaden the range of organic electroluminescence materials.
Owner:EAST CHINA NORMAL UNIV
Methods To Recover Cesium Formate From A Mixed Alkali Metal Formate Blend
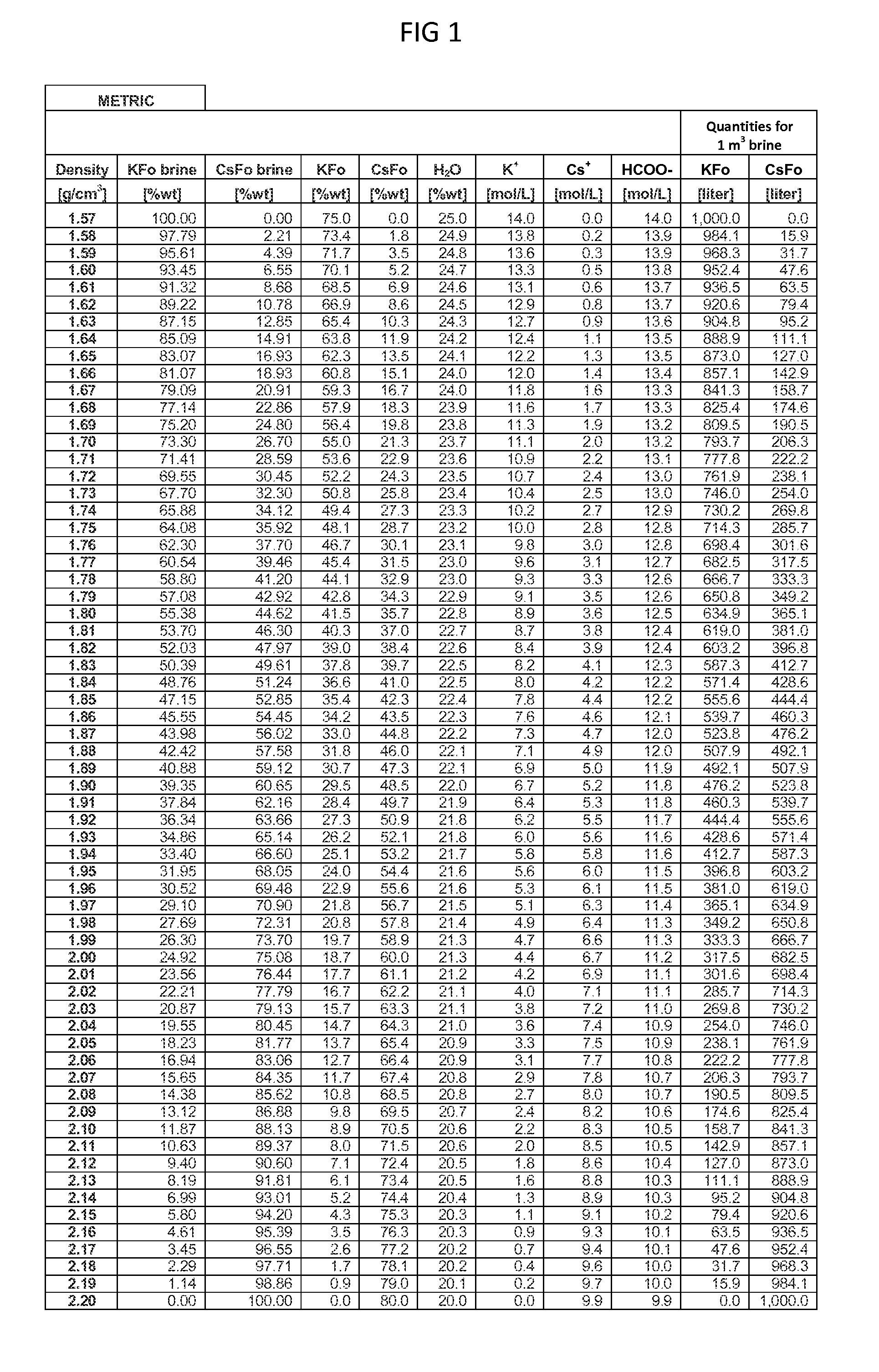
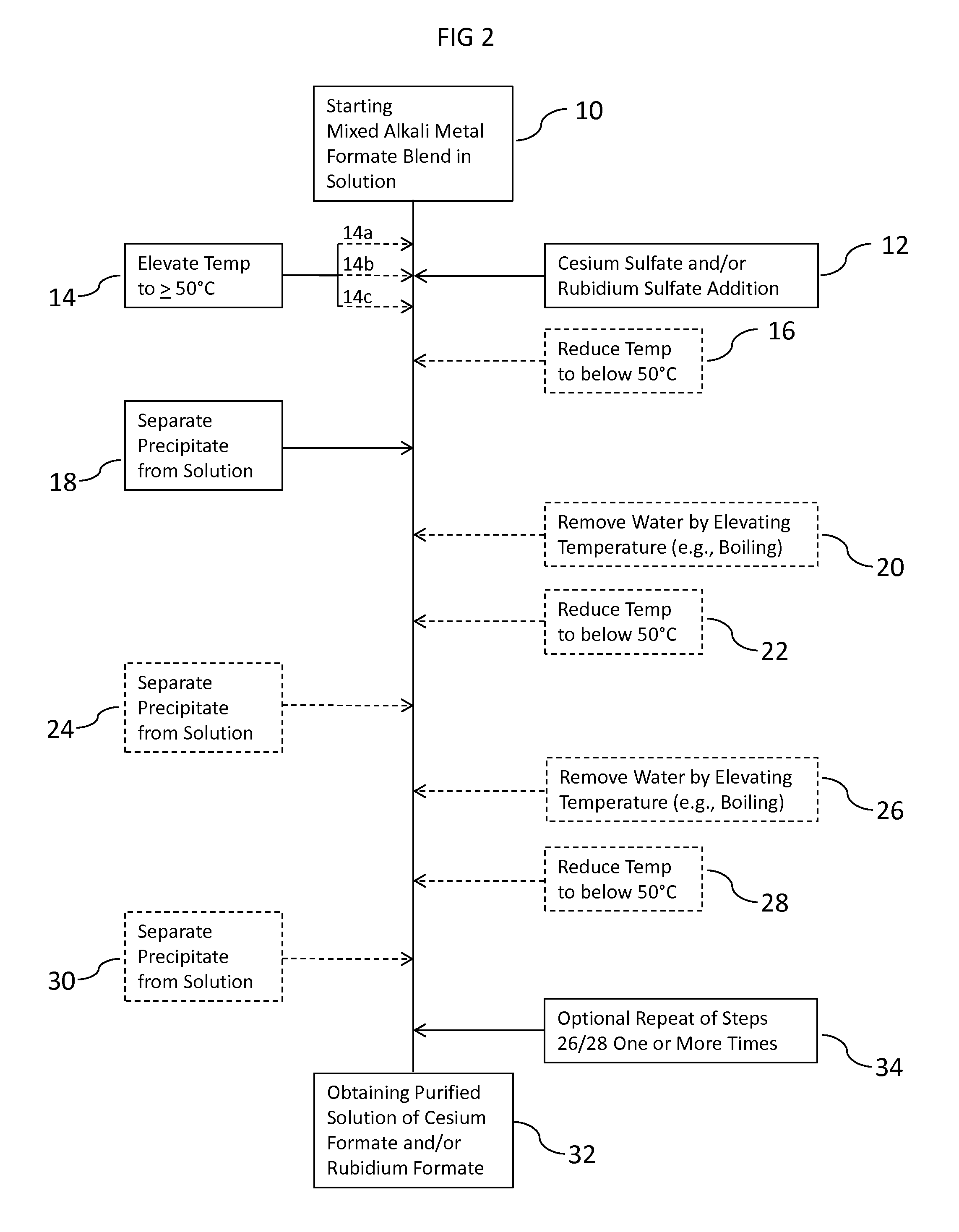
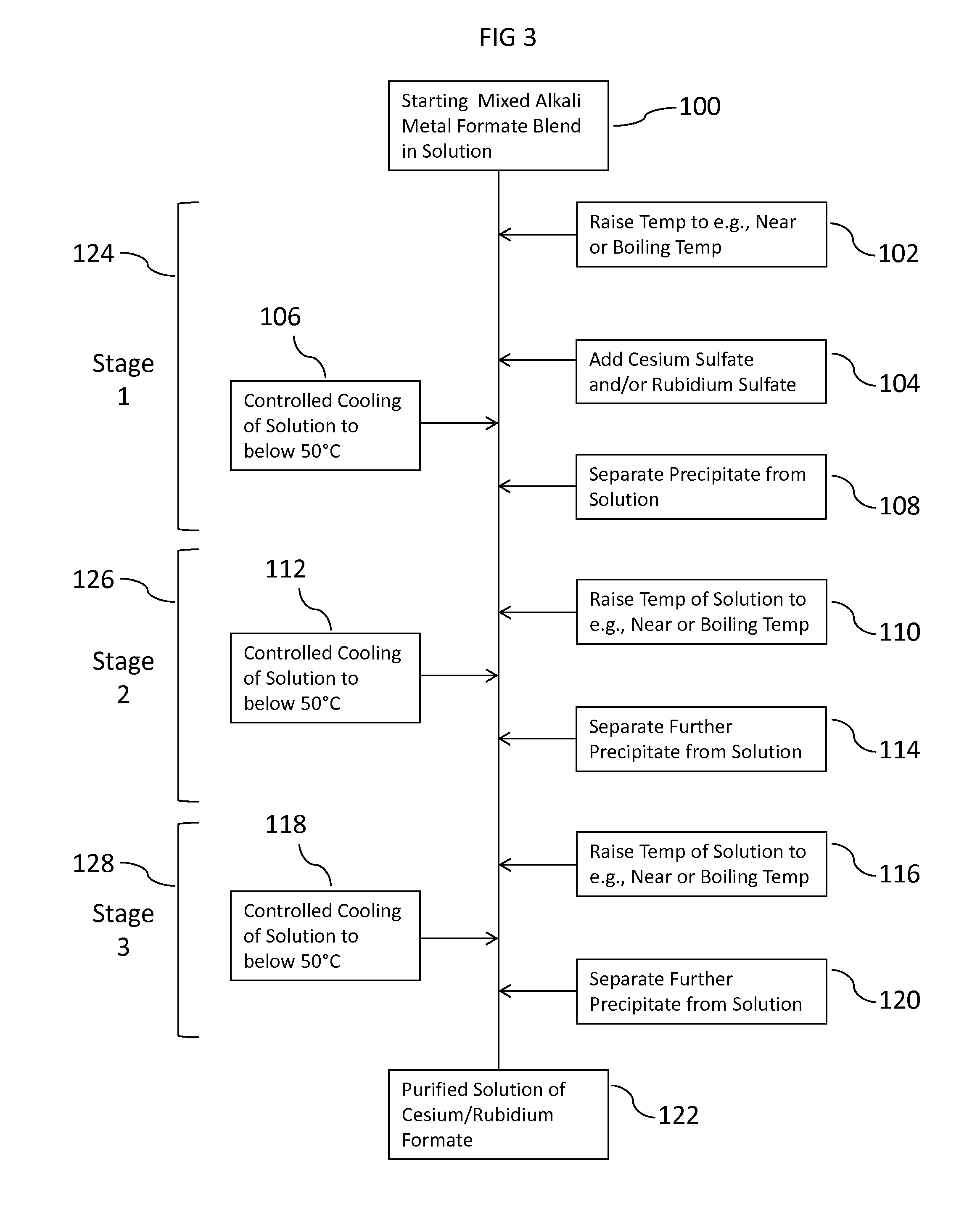
ActiveUS20150152033A1Efficient and inexpensivePreparation from carboxylic acid saltsOrganic compound preparationSodium bicarbonateFormate
Methods to recover or separate cesium formate or rubidium formate or both from a mixed alkali metal formate blend are described. One method involves adding cesium sulfate or rubidium sulfate to the mixed alkali metal formate blend in order to preferentially precipitate potassium sulfate from the mixed alkali metal formate blend. Another method involves adding cesium carbonate or cesium bicarbonate or both to preferentially precipitate potassium carbonate / bicarbonate and / or other non-cesium or non-rubidium metals from the mixed alkali metal blend. Further optional steps are also described. Still one other method involves converting cesium sulfate to cesium hydroxide.
Owner:CABOT SPECIALTY FLUIDS
Method for preparing salts of polyene macrolide esters
InactiveUS20030040610A1Promote conversionReducing methylationSugar derivativesGlycosidesCarbonateTetrahydrofuran
This method is for preparing salts of amphotericin B methyl ester and other polyene macrolide esters. The steps of the process involve methylation by use of cesium carbonate for converting to methyl ester and significantly reducing side products, which are over methylation products. The free base-Schiff base mixture is converted to amphotericin B methyl ester hydrochloride or some other salt form, by using tetrahydrofuran-water to dissolve the mixture for acid treatment to obtain the salt. The aldehyde liberated during salt formation is removed by centrifuging, as to any precipitated aldehyde, and the aldehyde remaining in solution is removed by eluting the solution through a reverse phase adsorbent to obtain amphotericin B methyl ester hydrochloride as a yellow powder.
Owner:BIOSOURCE PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


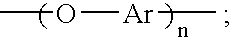
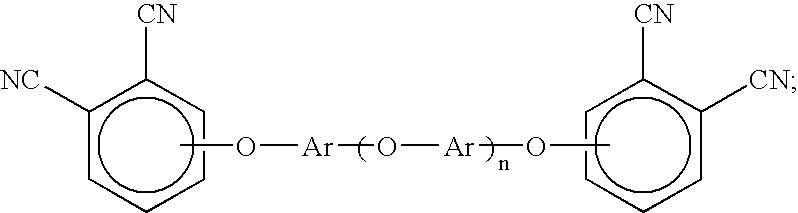
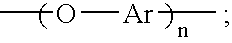








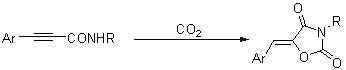
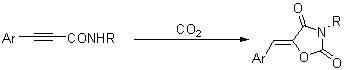
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/204216DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/349392DEST_PATH_IMAGE002.png)