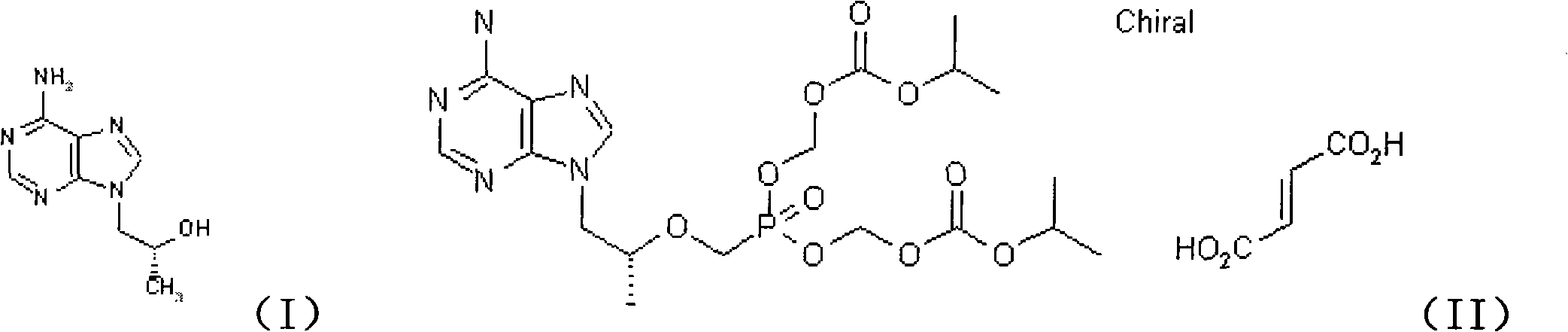
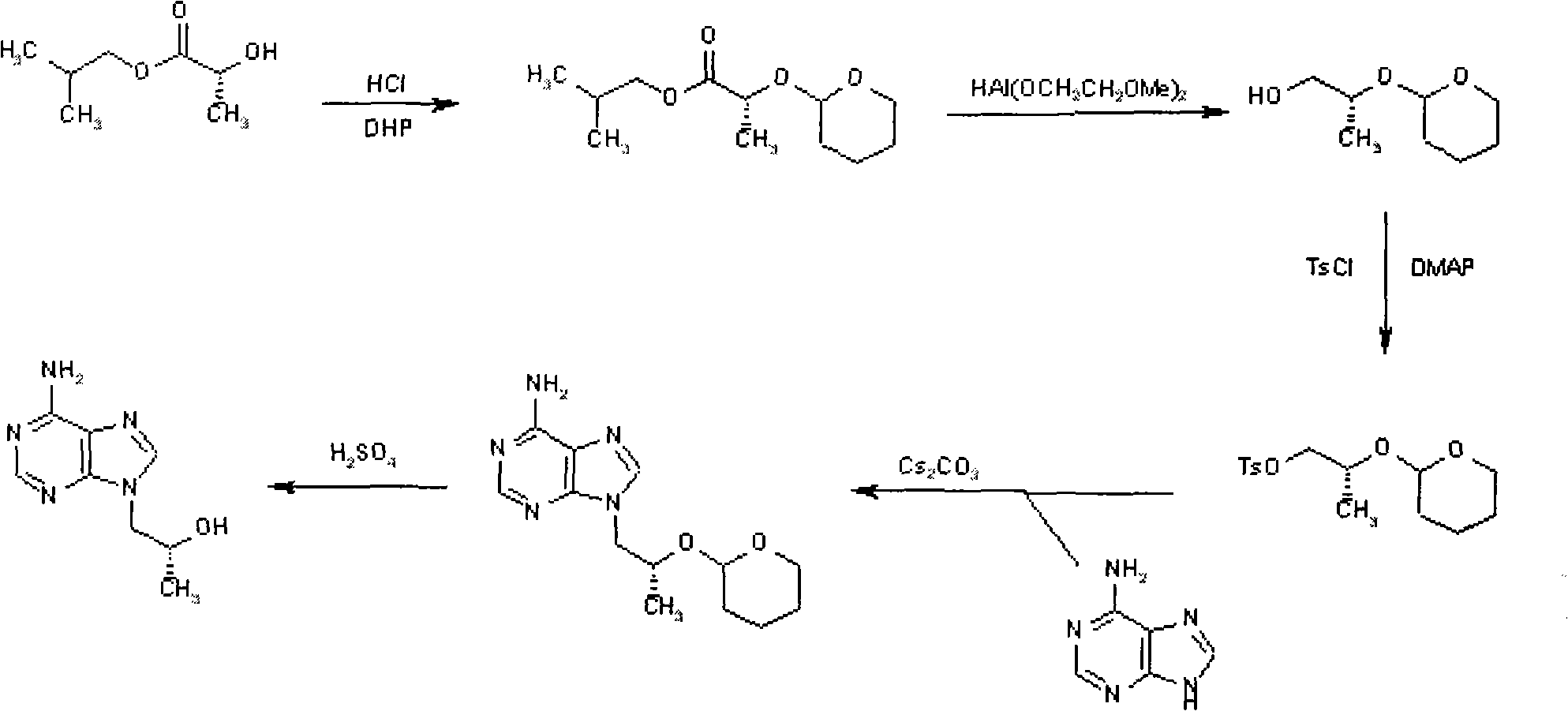
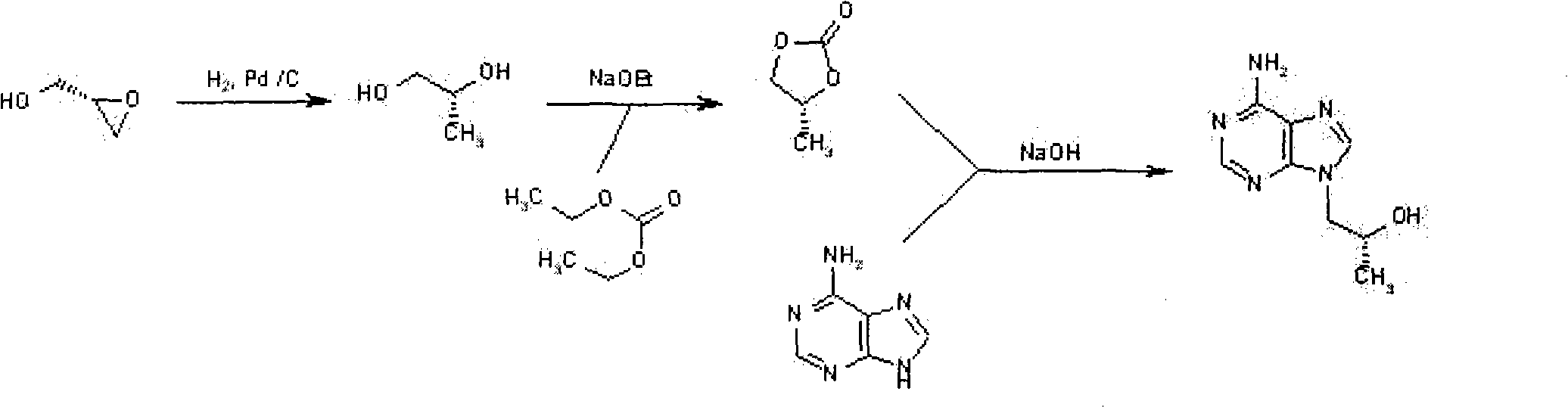
Novel method for synthesizing 9-substituted adenine compound
An adenine and compound technology, which is applied in the field of synthesis of 9-substituted adenine compounds, can solve the problems of limited product cost advantage, high price and the like, and achieves the effects of cheap raw materials, mild reaction conditions and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of (R)-2-Benzyloxypropanol
[0022] Add 1.0kg of benzyl alcohol and 50g of ZrO2 / SO4 into a three-necked flask, heat the reaction solution to 50°C, slowly add 580g of (R)-propylene oxide dropwise, keep the temperature not exceeding 50°C, and continue to keep React at high temperature for 2 hours, remove the solid by filtration, rectify the residue under reduced pressure with a glass rectification column, collect fractions from 124 to 130°C (12mmHg), and obtain 369g of a colorless oily liquid, which is (R)-2-benzyl Oxypropanol solidifies as a solid at about 20°C with a yield of 24%.
Embodiment 2
[0024] Synthesis of (R)-2-benzyloxypropanol p-toluenesulfonate
[0025] Mix 166g (R)-2-benzyloxypropanol with 1000ml DMF, add 200g triethylamine, 200g p-toluenesulfonyl and 2g 4-dimethylaminopyridine, heat to 80°C for 4 hours, cool to room temperature ,
[0026] Add 10L of water to it, extract with ethyl acetate, 1L each time, three times in total, combine the ethyl acetate layers, wash with water, dry and evaporate to dryness to obtain a light yellow solid, which is (R)-2-benzyloxypropanol p-toluene Sulfonic acid ester, weight is 278g, and yield is 87%.
Embodiment 3
[0028] Synthesis of (R)-9-(2-benzyloxypropyl)adenine
[0029] Mix 133g of adenine with 1500ml of DMSO, add 350g of (R)-2-benzyloxypropanol p-toluenesulfonate and 700g of cesium carbonate, stir and react at 100°C for 10 hours, filter after cooling, and add the filtrate to 20L of water , filtered the precipitated white solid, fully washed with water, and dried in vacuo to obtain a white solid, namely (R)-9-(2-benzyloxypropyl)adenine, with a weight of 177g and a yield of 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com