Preparation method of 2-methyl-8-aminoquinoline
An aminoquinoline and methyl technology, which is applied in the field of preparation of 2-methyl-8-aminoquinoline, can solve the problems of difficulty in preparing 2-methyl-8-aminoquinoline, difficulty in carrying out amino substitution reaction, and the like, To achieve the effect of novel preparation process, increased activity, and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
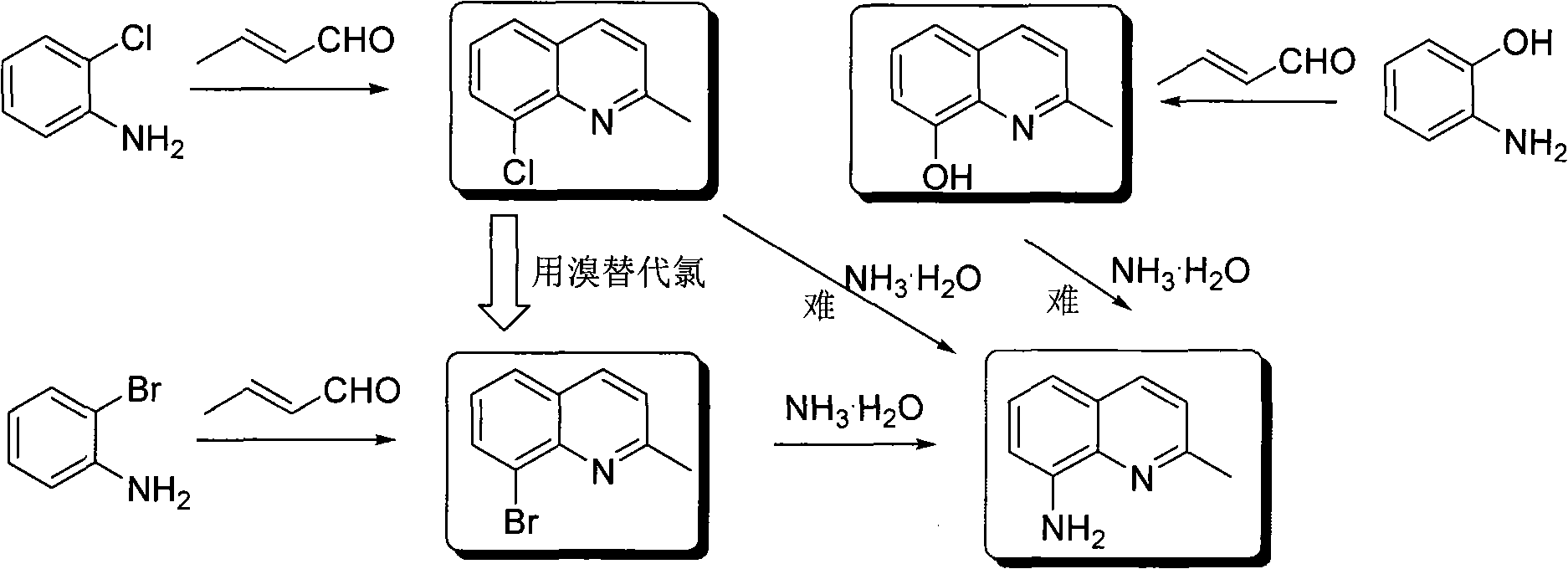
[0020] The preparation of embodiment 1 2-methyl-8-aminoquinoline
[0021] (1) Preparation of 2-methyl-8-bromoquinoline
[0022]
[0023] 8.60g (0.05mol) o-bromoaniline, heat up to about 40°C, add 50mL 18% HCl solution and 3.10g (0.05mol) boric acid, stir and heat up to about 100°C, add dropwise 2.02g (0.01mol) 2-nitro The mixture of bromobenzene and 4.20g (0.06mol) crotonaldehyde reacted for about 3.5h. The reaction solution was post-treated to obtain 2-methyl-8-bromoquinoline with a yield of 52.0% and a melting point of 69-71°C. 1 H NMR (CDCl 3 , 400MHz) δ: 2.82 (s, 3H, CH 3 ), 7.33 (m, 2H, quinoline ring), 7.73 (dd, J=8.0, J=1.2Hz, 1H, quinoline ring), 8.02 (m, 2H, quinoline ring).
[0024] (2) Preparation of 2-methyl-8-aminoquinoline
[0025]
[0026] 4.44g (20mmol) 2-methyl-8-bromoquinoline, 0.53g (2mmol) copper acetylacetonate, Cs 2 CO 3 Add 13.04g (40mmol), 0.80g (8mmol) acetylacetone and 80mL N-methylpyrrolidone into the reaction tank, quickly add 12mL 25%...
Embodiment 2
[0027] The preparation of embodiment 2 2-methyl-8-aminoquinoline
[0028] (1) Preparation of 2-methyl-8-bromoquinoline
[0029]
[0030] 8.60g (0.05mol) o-bromoaniline, heat up to about 40°C, add 50mL 18% HCl solution and 0.05mol glacial acetic acid, stir and heat up to about 100°C, add dropwise 0.015mol 2-nitrobromobenzene and 4.20g (0.06 mol) a mixture of crotonaldehyde, the reaction is about 4.0h. The reaction solution was post-treated to obtain 2-methyl-8-bromoquinoline with a yield of 62.1% and a melting point of 69-71°C.
[0031] (2) Preparation of 2-methyl-8-aminoquinoline
[0032]
[0033] 4.44g (20mmol) 2-methyl-8-bromoquinoline, 0.53g (2mmol) copper acetylacetonate, 40mmol cesium hydroxide, 0.80g (8mmol) acetylacetone and 80mL N, N-dimethylformamide were added to the reaction In the tank, add 12mL of 25% concentrated ammonia water, stir, slowly raise the temperature to 85°C, and keep it warm for 36h. The reaction solution was post-treated to obtain 2-methyl...
Embodiment 3
[0034] The preparation of embodiment 3 2-methyl-8-aminoquinoline
[0035] (1) Preparation of 2-methyl-8-bromoquinoline
[0036]
[0037]8.60g (0.05mol) o-bromoaniline, heat up to about 40°C, add 50mL 18% HCl solution, 5mL sulfuric acid and 0.05mol ferric sulfite, stir and heat up to about 100°C, add 0.012mol cerium ammonium nitrate and dropwise add 4.20g (0.06mol) a mixture of crotonaldehyde, the reaction is about 3.0h. The reaction solution was post-treated to obtain 2-methyl-8-bromoquinoline with a yield of 61.0% and a melting point of 69-71°C.
[0038] (2) Preparation of 2-methyl-8-aminoquinoline
[0039]
[0040] Add 4.44g (20mmol) 2-methyl-8-bromoquinoline, 2mmol iron acetylacetonate, 40mmol potassium carbonate, 0.80g (8mmol) acetylacetone and 80mL dimethyl sulfoxide into the reaction tank, add 12mL 25% concentrated ammonia water , stirred, and slowly heated to 120°C. Insulation reaction 48h. The reaction solution was post-treated to obtain 2-methyl-8-aminoquin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com