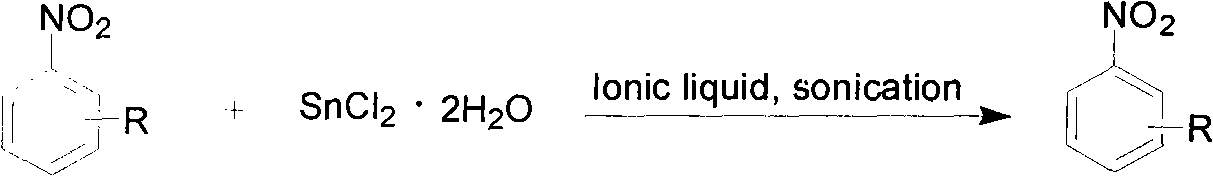Method for synthesizing aromatic amine
A technology of aromatic amine and aromatic trifluoromethanesulfonate, which is applied in the field of synthesizing aromatic amine, can solve the problems of poor stability, cumbersome operation, and long reaction time, and achieve mild reaction conditions, wide application range, and short reaction time. Effect
Inactive Publication Date: 2011-06-15
BEIJING INSTITUTE OF TECHNOLOGYGY
View PDF1 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In summary, in the known synthetic methods of aromatic amines, either the temperature is higher, or the reaction time is longer, or the required reagents are expensive, difficult to preserve, poor in stability, and cumbersome to use and operate.
These deficiencies have brought a lot of inconvenience to the synthesis of this type of compound, especially industrialized production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a method for synthesizing aromatic amine. A reaction formula is shown in the specifications, wherein when X is nitrogen, a substituent group R can be 3- / 5-nitro, 3- / 5-cyan, 3- / 5-trifluoromethyl or 3- / 5-halo (fluorine, chlorine, bromine or iodine); when X is carbon, R can be 2- / 4-nitro, 2- / 4-cyan, 2- / 4-trifluoromethyl or 2-nitro-4-chlorine; R1 and R2 refer to hydrogen, alkyl, alkyl alcohol, benzyl or-(CH2)n-(n=2-6) naphthenic base respectively; reaction alkali is caesium carbonate, potassium phosphate, pyridine, triethylamine, sodium bicarbonate, potassium carbonate, sodium / potassium hydroxide or sodium / potassium alkoxide; a reaction solvent is dioxane, toluol, dimethyl sulfoxide, dimethyl formamide, acetonitrile, tetrahydrofuran or acetone; and the reaction is implemented in the conventional heating state. In the method, raw materials are readily available, the process is simple, the reaction condition is mild, the application range is wide, and a plurality of aromatic amine compounds can be synthesized by different substrates.
Description
A kind of method of synthesizing aromatic amine (1) Technical field The invention relates to a synthesis method for preparing aromatic amine (arylamine in English) through the reaction of aromatic trifluoromethanesulfonate and zinc chloride complex of secondary amine. (2) Background technology Aromatic amine compounds are extremely important organic raw materials, widely used in the production of dyes, medicines, agricultural chemicals, additives, surfactants, textile auxiliaries, chelating agents, polymers, flame retardants, etc. (Catal.Commun., 2007, 8, 629). For example, p-nitrodimethylaniline (p-NDMA) can be used for the determination of sliding arc discharge degradation methyl violet (Journal of Zhejiang University <Engineering Science Edition>, 2009, 43, 931); RT base (N-phenyl-1 , 4-phenylenediamine) is an important intermediate for the production of various rubber antioxidants with excellent properties (Journal of Nanjing Institute of Chemical Technology, 19...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07B43/04C07D295/073C07D213/74C07D213/73C07C209/18C07C211/52C07C215/16C07C213/02
Inventor 李加荣徐娟史大昕张奇
Owner BEIJING INSTITUTE OF TECHNOLOGYGY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com