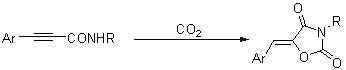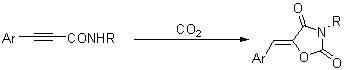Method for synthesizing 1,3-oxazole-2,4-diketone compounds
A technology of diketones and compounds, which is applied in the field of synthesizing 1,3-oxazole-2,4-diketones, can solve the problems of low yield, high toxicity, low atom economy, etc. Effects of regioselectivity, low toxicity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Potassium carbonate (54.5 mg, 0.39 mmol) was added to the reaction tube, pumped and roasted three times, carbon dioxide gas was introduced, cooled to room temperature, and then added N -Benzyl-3-phenylpropynamide (46.2 mg, 0.20 mmol), and 2 mL dimethyl sulfoxide, stirred, carbon dioxide was introduced into the solution of dimethyl sulfoxide through the catheter, and then the reaction tube was placed at 30 o After stirring in an oil bath of C for 11 hours, take out the carbon dioxide catheter, add 10 mL of water to quench the reaction, extract with ether (15 mL × 3), combine the organic phases, wash with saline once, dry over anhydrous sodium sulfate, and concentrate the solution. Flash column chromatography (petroleum ether / ethyl acetate=20 / 1), obtains solid product ( Z )- N -Benzyl-5-benzoylidene-1,3-oxazole-2,4-dione 34.0 mg, yield 62%, melting point: 158.2-159.2 o C (n-hexane / dichloromethane).
[0023] 1 H NMR (300 MHz, CDCl 3 ) d 7.79-7.70 (m, 2H), 7.50-7.29 (...
Embodiment 2
[0026] By the method described in Example 1, the difference is that the substrate and reagent used are: N -Butyl-3-phenylpropynamide (40.1 mg, 0.20 mmol), potassium carbonate (55.8 mg, 0.40 mmol), the product ( Z )- N -Butyl-5-benzoylidene-1,3-oxazole-2,4-dione 39.0 mg, yield 80%. The product is a solid, melting point: 82.7-83.4 o C (n-hexane / dichloromethane).
[0027] 1 H NMR (300 MHz, CDCl 3 ) d 7.80-7.71 (m, 2H), 7.50-7.36 (m, 3H), 6.76 (s, 1H), 3.65 (t, J = 7.4 Hz, 2H), 1.78-1.62 (m, 2H), 1.46-1.30 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H); 13 C NMR (75 MHz, CDCl 3 ): d 162.4, 152.3, 137.5, 131.0, 130.7, 130.4, 129.0, 113.3, 40.0, 29.6, 19.8, 13.5; MS (m / z): 246 (M + +1, 4.12), 245 (M + , 25.65), 118 (100); IR (KBr, cm -1 ): 1816, 1721, 1666, 1495, 1449, 1413, 1354, 1315, 1264, 1236, 1187, 1082, 1006; Anal. Calcd. for C 14 h 15 NO 3 : C 68.56, H 6.16, N 5.71; Found: C 68.88, H 6.40, N 5.72.
[0028]
Embodiment 3
[0030] By the method described in Example 1, the difference is that the substrate and reagent used are: N -Ethyl-3-phenylpropynamide (34.2 mg, 0.20 mmol), potassium carbonate (55.4 mg, 0.40 mmol), the product ( Z )- N -Ethyl-5-benzoylidene-1,3-oxazole-2,4-dione 28.4 mg, yield 66%. The product is a solid, melting point: 123.6-124.3 o C (n-hexane / dichloromethane).
[0031] 1 H NMR (300 MHz, CDCl 3 ) d 7.85-7.68 (m, 2H), 7.52-7.35 (m, 3H), 6.75 (s, 1H), 3.71 (q, J = 7.2 Hz, 2H), 1.32 (t, J = 7.2 Hz, 3H); 13 C NMR (75 MHz, CDCl 3 ): d 162.1, 152.0, 137.5, 131.0, 130.7, 130.4, 129.0, 113.2, 35.3, 13.0; MS (m / z): 218 (M + +1, 5.78), 217 (M +, 43.70), 118 (100); IR (KBr, cm -1 ): 1813, 1735, 1682, 1496, 1452, 1441, 1415, 1372, 1347, 1320, 1246, 1211, 1169, 1115, 1080, 1045; Anal. Calcd. for C 12 h 11 NO 3 : C 66.35, H 5.10, N 6.45; Found: C 66.31, H 5.33, N 6.46.
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com