(S)-/(R)-difurodinaphthalene as well as derivatives thereof and preparation method
A derivative, difuran technology, applied in the field of new organic electroluminescent materials, to achieve the effect of broadening the range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The reagents used are all commercially available products, and the solvent is conventionally dried; Reagent description: PE-petroleum ether; DCM-dichloromethane; EA-ethyl acetate; DMF-N.N-dimethylformamide, TEA-triethylamine .
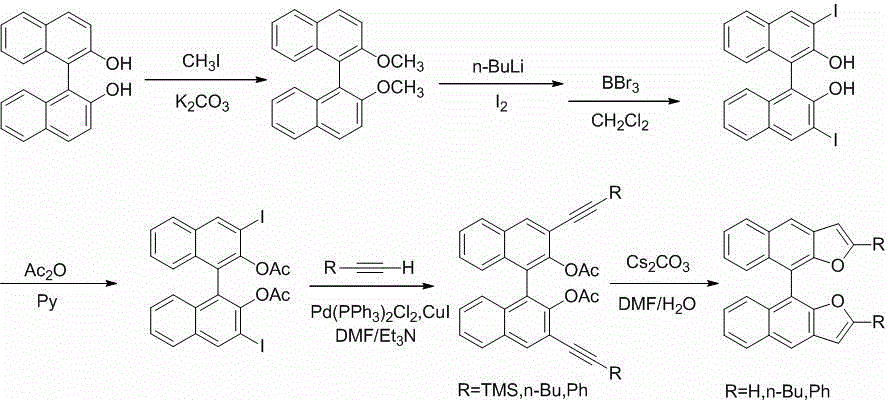
[0025] refer to figure 1 , the invention discloses a ( S) - / ( R) - bisfuranobinaphthalene and derivatives thereof, characterized in that binaphthol has ( S) - / ( R) -Two kinds of steric configurations, through a series of synthetic steps to obtain the target compound with the same steric configurations. Its preparation is carried out as follows:
[0026] (S) - / ( R) Preparation of -2,2'-dimethoxy-1,1'-binaphthyl (a)
[0027] (1)( S) --2,2'-Dimethoxy-1,1'-binaphthyl (1a)
[0028] 4.0g (14.0mmol) (S) -Binaphthol was dissolved in 60mL of acetone, 7.0g (50mmol) of potassium carbonate and 7.8g (55mmol) of methyl iodide were added to the solution, and the mixture was refluxed for 24h; then 3.5g (25mmol) of potassium carbonate and 3.5g (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com