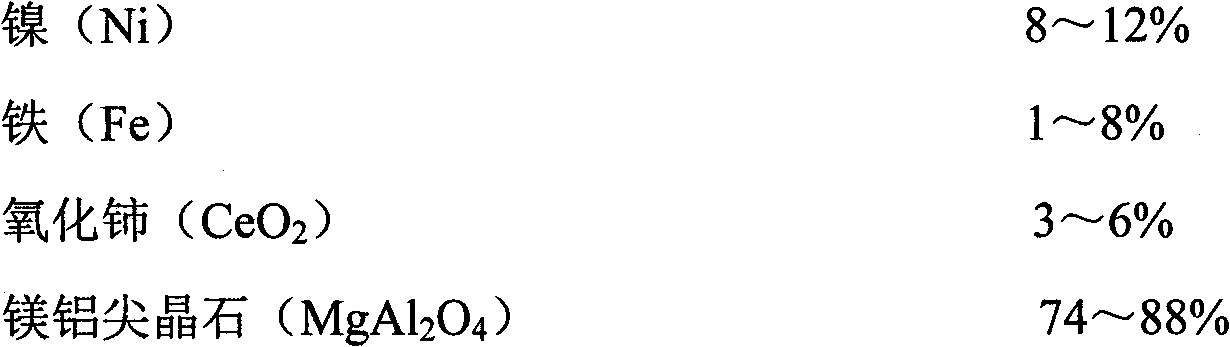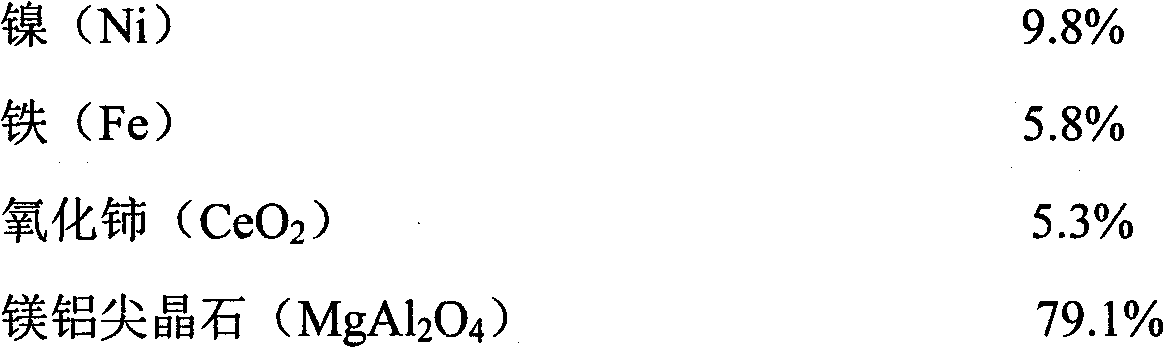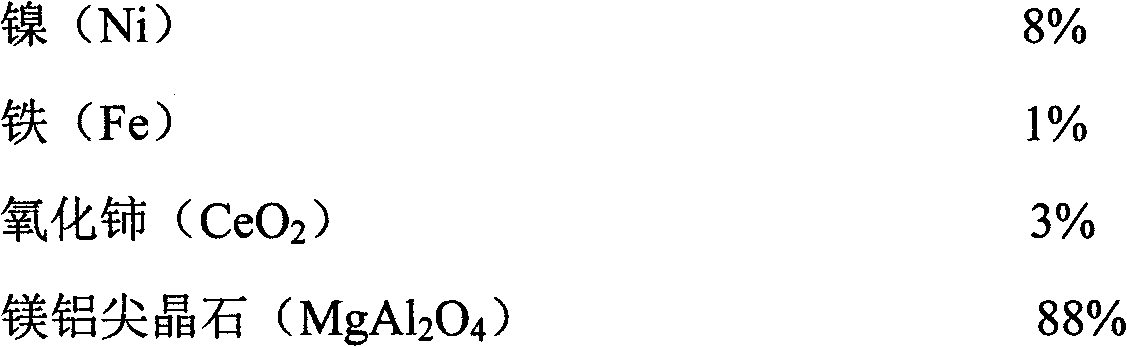Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
384 results about "Non precious metal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Base metals-Non-precious metals such as copper, zinc, tin, nickel, lead, and iron. Bead setting-Setting style with gems held in place by rounded beads, usually pushed up from the surrounding metal. Benefit-The value a feature holds for a customer. [] Base Metal: Non-precious metals such as steel, zinc, lead, tin and copper.
Catalytic process for converting renewable resources into paraffins for use as diesel blending stocks
A process for converting renewable resources such as vegetable oil and animal fat into paraffins in a single step which comprises contacting a feed which is a renewable resources with hydrogen and a catalyst which comprises a non-precious metal and an oxide to produce a hydrocarbon product having a ratio of odd-numbered hydrocarbons to even-numbered hydrocarbons of at least 2:1.
Owner:REFINING TECH SOLUTIONS LLC
Catalytic process for converting renewable resources into paraffins for use as diesel blending stocks
InactiveUS20080312480A1Hydrocarbon from carbon oxidesFatty acid chemical modificationAlkaneVegetable oil
A process for converting renewable resources such as vegetable oil and animal fat into paraffins in a single step which comprises contacting a feed which is a renewable resources with hydrogen and a catalyst which comprises molybdenum, a non-precious metal and an oxide to produce a hydrocarbon product having a ratio of even-numbered hydrocarbons to odd-numbered hydrocarbons of at least 2:1.
Owner:EI DU PONT DE NEMOURS & CO
Catalytic process for converting renewable resources into paraffins for use as diesel blending stocks
ActiveUS20080308458A1Fatty acid chemical modificationMolecular sieve catalystsParaffin waxVegetable oil
A process for converting renewable resources such as vegetable oil and animal fat into paraffins in a single step which comprises contacting a feed which is a renewable resources with hydrogen and a catalyst which comprises a non-precious metal a first oxide and optionally a second oxide wherein at least one of the first oxide or second oxide comprises a zeolite, through hydrodeoxygenation and one or both of hydroisomerization and hydrocracking.
Owner:REFINING TECH SOLUTIONS LLC
Non-mercuric catalyst used in hydrochlorination of acetylene and method for preparing vinyl chloride by using catalyst
InactiveCN102029189AGood stability in continuous useSolution to short lifePreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsPhosphatePotassium
The invention relates to a non-mercuric catalyst used in hydrochlorination of acetylene and a method for preparing vinyl chloride by using the catalyst. The non-mercuric catalyst used in hydrochlorination of acetylene for preparing vinyl chloride comprises main active ingredients-gold salts, auxiliary active ingredients-non precious metal salts and a carrier, wherein the main active ingredients are gold salts and can be halides, complexes and the like of gold; gold in the gold salts accounts for 0.1-10% by weight of the catalyst; the auxiliary active ingredients are non precious metal salts and can be halides, acetates, phosphates, complexes and the like of potassium, barium, lanthanum and copper; the non precious metal salts account for 0.1-10% by weight of the catalyst; and the carrier is activated carbon, comprising coconut shell carbon, coal carbon, nutshell carbon or silica gel. The non-mercuric catalyst can be prepared by the conventional impregnation method, has simple preparation method, is environment-friendly and has the advantages of less by-products, good stability and long service life. The conversion rate of acetylene can be 90-99% and the selectivity of vinyl chloride is not lower than 99%.
Owner:EAST CHINA UNIV OF SCI & TECH
Method of hydrogenating liquefied petroleum gas to prepare ethylene cracking feed
ActiveCN102311787AEffective temperature controlTemperature controlLiquid hydrocarbon mixtures productionTreatment with hydrotreatment processesHydrogenAlkene
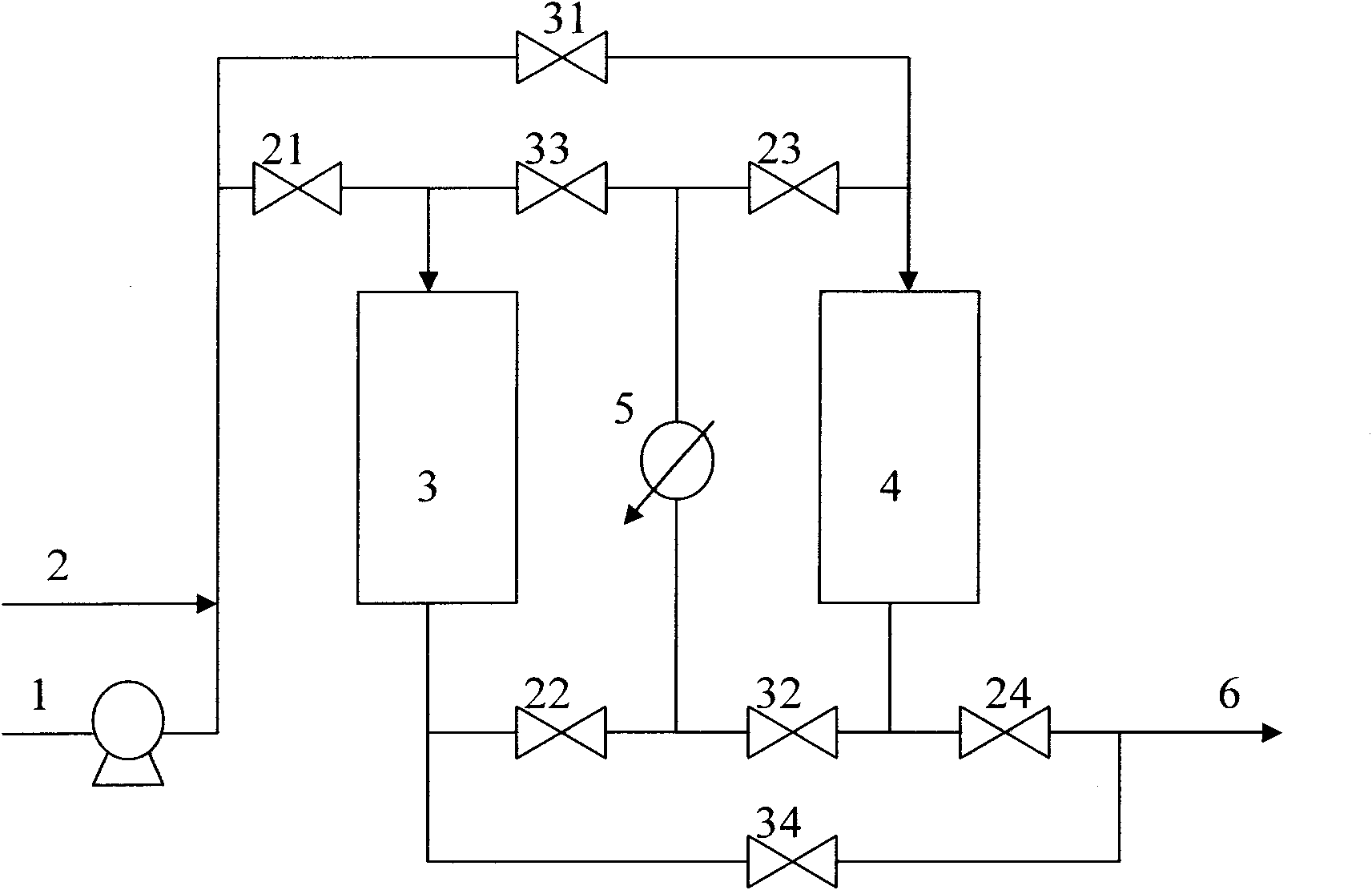
The invention discloses a method of hydrogenating liquefied petroleum gas to prepare ethylene cracking feed, which is provided with two reaction sections. Reaction material cooling operation is carried out between the two reaction sections, the mixture of C4 fraction raw material and hydrogen is cooled after passing through the first reaction section, then enters the second reaction section, non-precious metal hydrofining catalysts are arranged in the two reaction sections, and a reaction material flow switching device is arranged between an inlet and an outlet of the two reaction sections to change the sequence of the reaction material flow entering the two reaction sections. The method disclosed by the invention solves the problem that the olefin content does not reach the standard due to thermodynamic control factors, simultaneously the catalysts are entirely fully utilized, and the operating cycle can be prolonged.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nitrogen and phosphorus co-doped porous carbon catalyst and preparation method thereof
InactiveCN105457666AAperture controllableImprove adsorption capacityPhysical/chemical process catalystsCell electrodesPorous carbonAniline
The invention provides a preparation method and an application of a nitrogen and phosphorus co-doped porous carbon catalyst and belongs to the field of oxygen reduction catalysts for a fuel cell cathode. Nitrogen and phosphorus are introduced with an in-situ doping method, and the nitrogen and phosphorus doping amount is changed by adjusting the content of a nitrogen and phosphorus precursor. Besides, nitrogen and phosphorus co-doped porous carbon is prepared with a hard template method, and controllability of pore diameters of the porous carbon is realized by adjusting a hard template. The method comprises steps as follows: an earlier polymer of aniline monomers, a phosphorus precursor, a silica-based hard template and non-precious metal salt is prepared; the earlier polymer is calcined, and solids are obtained; the solids are etched, cleaned and dried, and the carbon material is obtained. More importantly, the prepared nitrogen and phosphorus co-doped porous carbon material has an excellent oxygen reduction electro-catalytic property under the acid condition and has huge application potential.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Nitrogen-doped graphene catalyst and preparation method and application thereof
InactiveCN103611555AReduce manufacturing costEasy to makePhysical/chemical process catalystsCell electrodesMicrobial fuel cellFuel cells
The invention provides a nitrogen-doped graphene catalyst and a preparation method and application thereof. The nitrogen-doped graphene catalyst is characterized by being prepared by roasting a nitrogen-doped graphene precursor; the nitrogen-doped graphene precursor is prepared from the raw materials including at least one of non-precious metal salt and hydrate thereof, graphite oxide and nitrogen-containing small organic molecules; in the raw materials, the mass percent of the graphite oxide is 10-89wt%, the mass percent of the nitrogen-containing small organic molecules is 10-89wt%, and the mass percent of at least one of non-precious metal salt and hydrate thereof is 1-10wt%. The preparation process of the nitrogen-doped graphene provided by the invention is simple, the process is easy to operate, the activity is high, the cost is low, and industrial production is easy to implement; the nitrogen-doped graphene can be applied to the fields of fuel cells, metal-air cells, microbial fuel cells and the like.
Owner:DONGHUA UNIV
Non-precious Metal-based Hyrdosilylation Catalysts Exhibiting Improved Selectivity
ActiveUS20130158281A1Silicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsManganeseHydrosilylation
Disclosed herein is the use of manganese, iron, cobalt, or nickel complexes containing tridentate pyridine di-imine ligands as hydrosilylation catalysts. These complexes are effective for efficiently catalyzing hydrosilylation reactions, as well as offering improved selectivity and yield over existing catalyst systems.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC +1
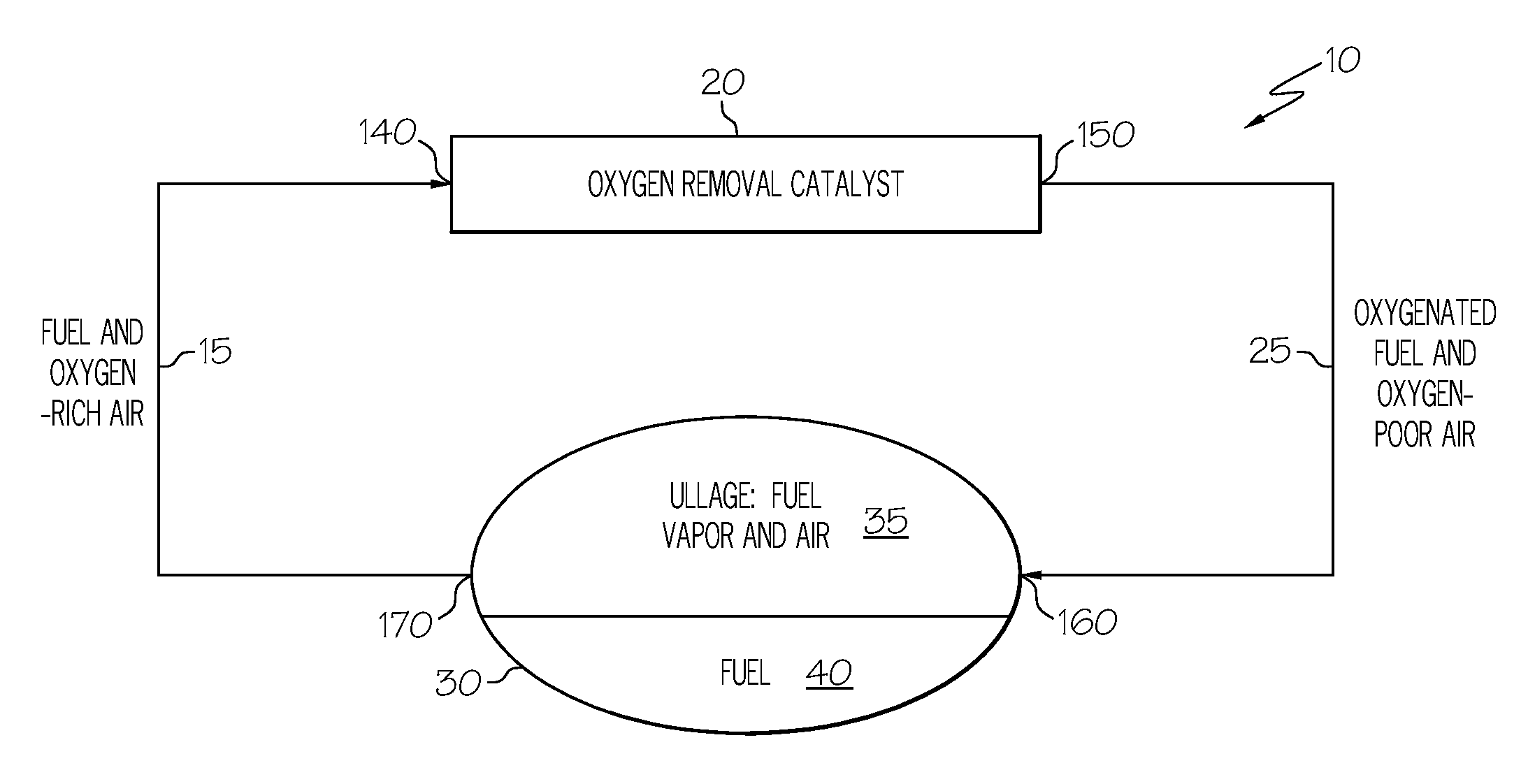
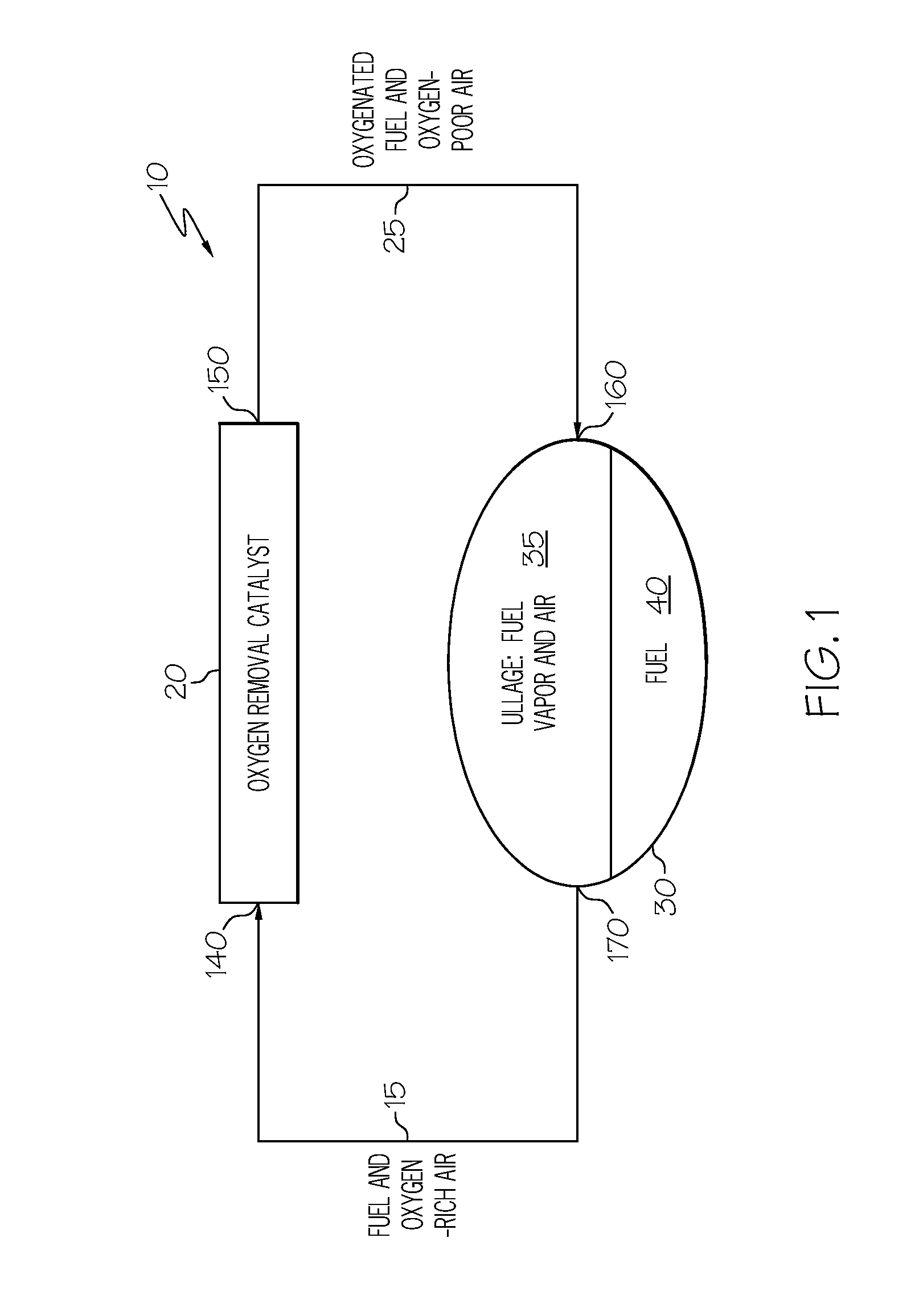
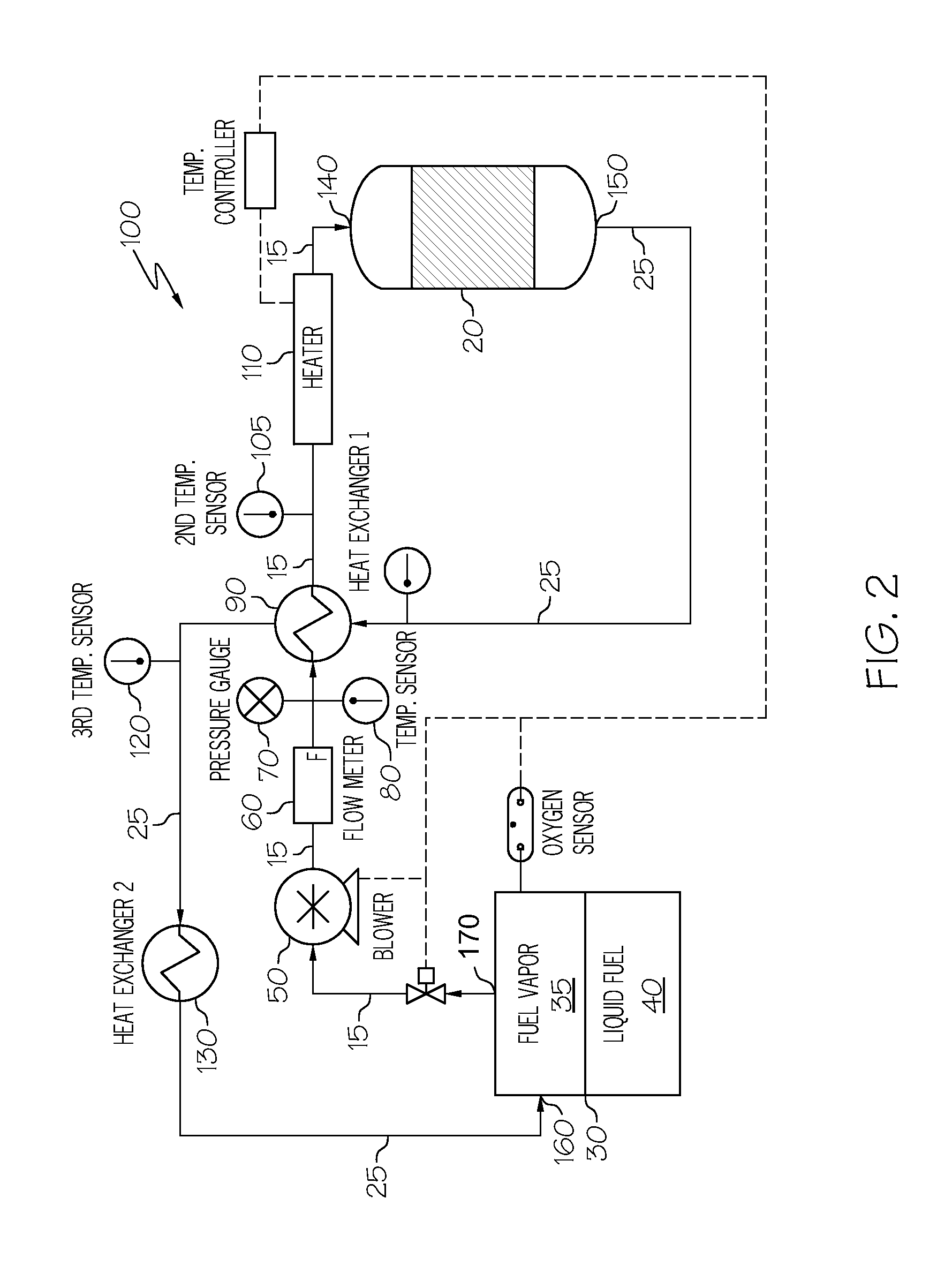
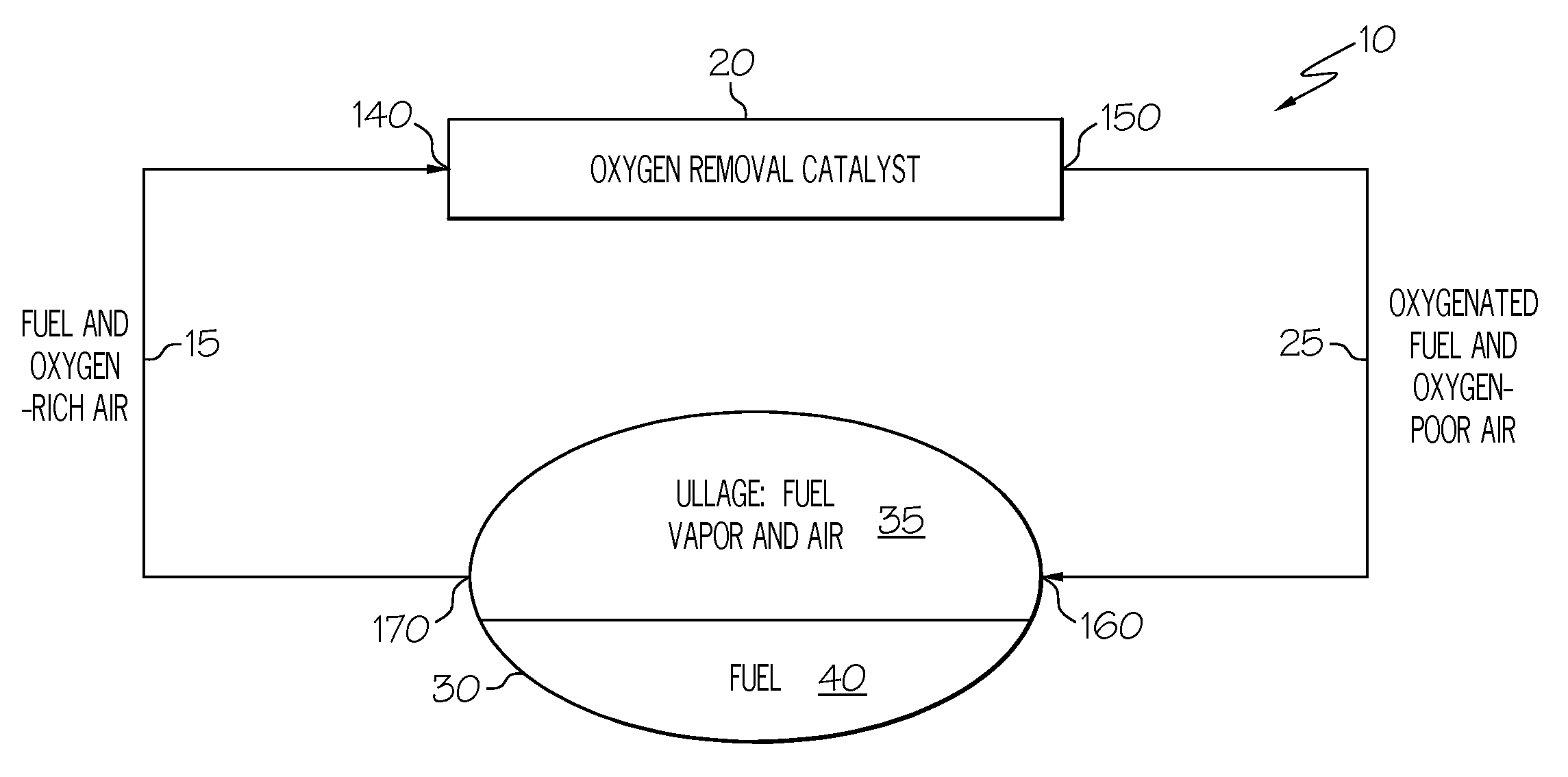
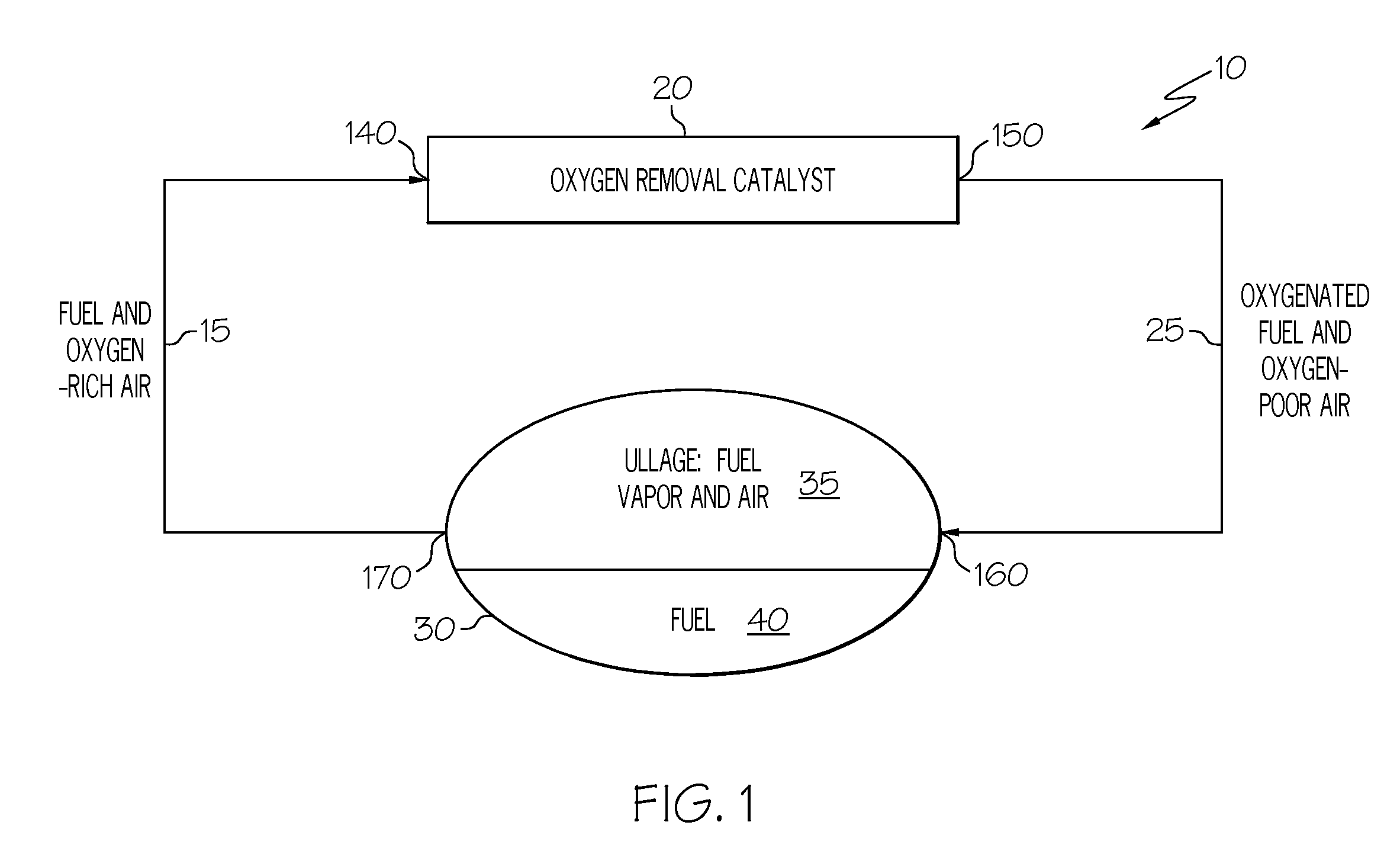
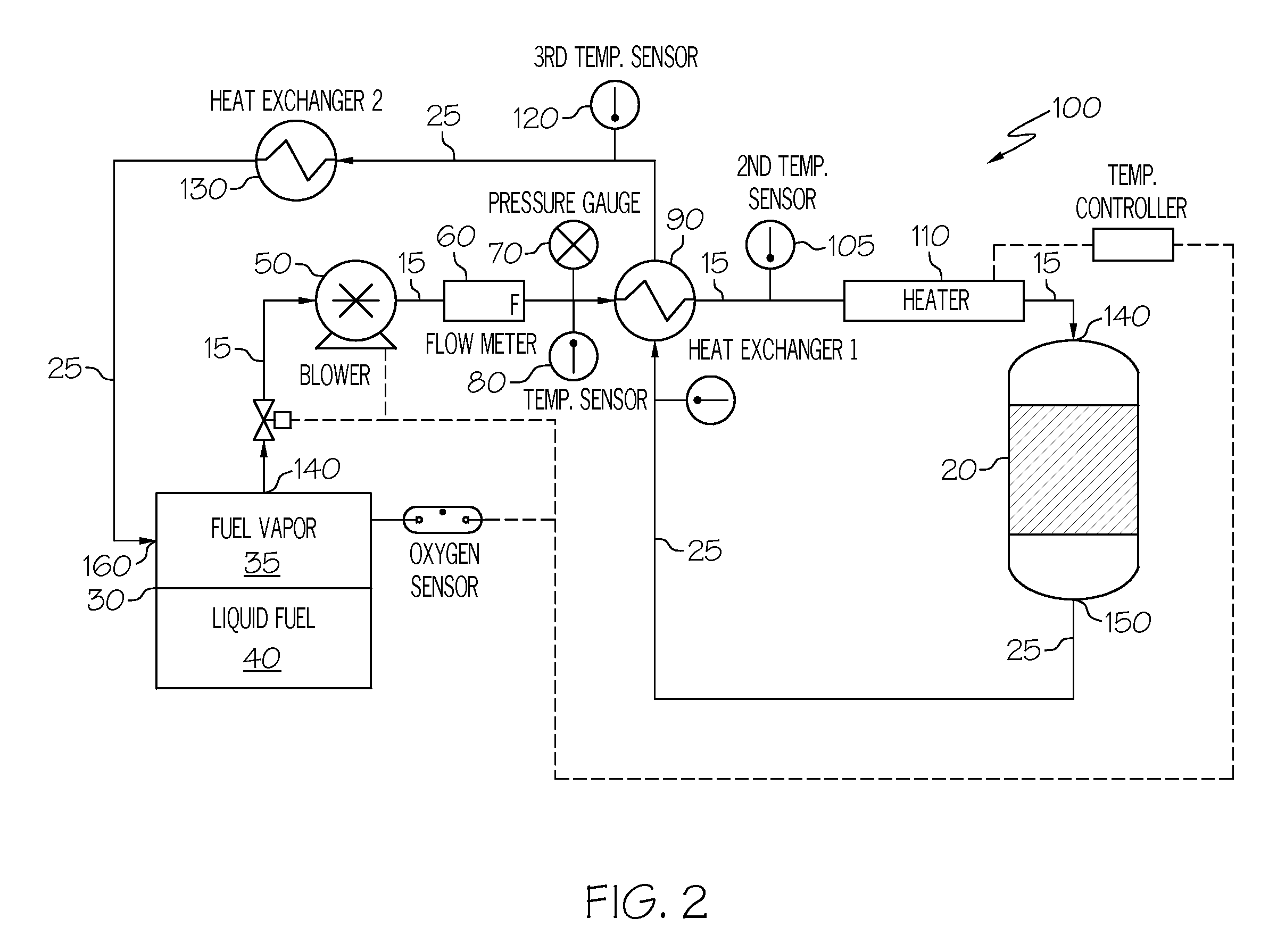
Oxygen removal system
A system and process to deplete or remove oxygen in the ullage of a fuel tank to reduce the oxygen / fuel vapor ratio below a lower explosion limit by exposing the ullage compounds to an oxygen removal catalyst active at a relatively low temperature. The oxygen removal catalyst may be a non-precious metal catalyst. A selective reaction of the oxygen by the catalyst forms primarily alcohols, aldehydes, and / or ketones and produces less than about 5% water by volume. The selective reaction, occurring at the relatively low temperature, reduces the risk of flammability of the fuel.
Owner:HONEYWELL INT INC
Process and apparatus for production of hydrogen using the water gas shift reaction
InactiveUS20080128655A1Lower carbon dioxide levelsCatalytic gas-gas reactionHydrogenForming gasWater-gas shift reaction
A process and a reactor vessel for production of hydrogen via the water gas shift reaction at CO / CO2 ratios above 1.9, and steam to gas rations below 0.5, are disclosed. The process includes first reacting a feed gas mixture of carbon monoxide and steam in the presence of a precious metal catalyst on a structural support, yielding a resultant gas, and then reacting the resultant gas in the presence of a non-precious metal catalyst on a support medium. The reactor vessel includes a chamber having an inlet duct and an outlet. A structural support having the precious metal catalyst is positioned upstream of the non-precious metal catalyst positioned within the chamber. The structural support may be positioned within the inlet duct or within the chamber. The support medium may be a granular medium or a structural support.
Owner:AIR PROD & CHEM INC
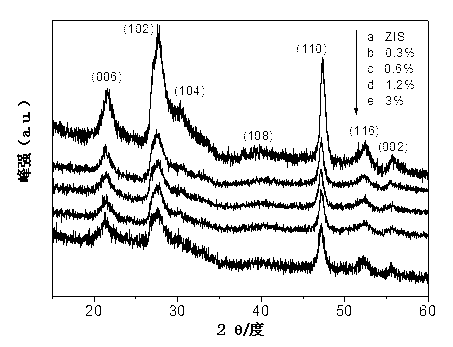
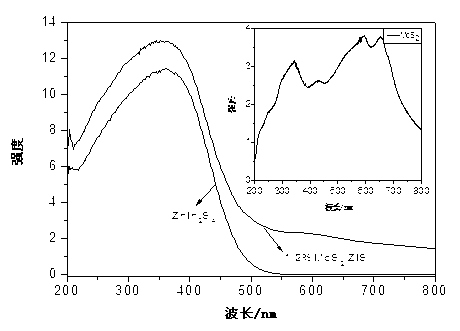
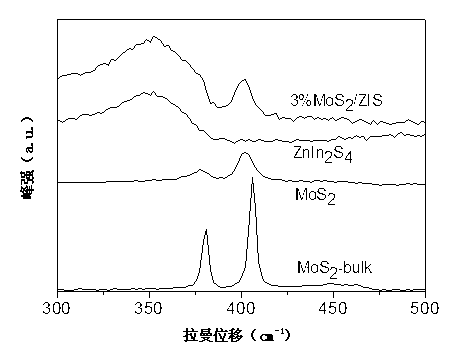
Hydrogen-production photocatalyst MoS2/ZnIn2S4 and preparation method thereof
InactiveCN103071513ALow costImprove photocatalytic hydrogen production performancePhysical/chemical process catalystsHydrogen productionInorganic ChemicalNon precious metal
The invention discloses a hydrogen-production photocatalyst MoS2 / ZnIn2S4 and a preparation method and application thereof, and belongs to the technical fields of inorganic chemistry and photocatalysis. The photocatalyst is a multiplex photocatalyst of non TiO2 by that a non-precious metal MoS2 serving as a cocatalyst is loaded on ZnIn2S4; and the chemistry formula is MoS2 / ZnIn2S4. The hydrogen-production photocatalyst MoS2 / ZnIn2S4 and the preparation method thereof solve the problem that photocatalystic performance is improved by taking rare and expensive precious metal Pt as the cocatalyst in the prior art; the cheap non-precious metal MoS2 serving as the cocatalyst is loaded on ZnIn2S4 for the first time; the obtained multiplex photocatalyst has a high-efficiency photocatalysis hydrogen production effect; the preparation method is simple, convenient and easy and is beneficial to industrial promotion on a large scale.
Owner:FUZHOU UNIV
Catalyst for preparing synthesis gases through dry methane reforming (DMR) and preparation method thereof
ActiveCN102416328AImprove conversion rateImprove anti-coking performanceHydrogenMetal/metal-oxides/metal-hydroxide catalystsSpinelNon precious metal
The invention provides a catalyst for preparing synthesis gases through dry methane reforming (DMR) and a preparation method thereof, belonging to the technical field of catalysts for preparing synthesis gases through DMR. The catalyst is formed by nickel, iron, cerium oxide and magnesia-alumina spinel. The method comprises the following steps of: firstly preparing a mesoporous MgAl2O4 spinel carrier, then preparing a nickel-based catalyst suspension and finally preparing the finished product through such simple processes as filtering, washing, drying and roasting. The catalyst and the preparation method have the following beneficial effects that: as such non-precious metal raw materials as nickel and iron are adopted and the raw material sources are wide, the cost is low; the process is simple, the equipment is simple and the production cost is low; the prepared product has high catalytic activity; the conversion rates of methane and carbon dioxide are high; and the carbon depositionresistance and stability are good. The catalyst can be widely used for preparing synthesis gases through DMR.
Owner:XUZHOU FENGTONG INFORMATION TECH CO LTD
Compound type fuel cell cathode catalyst NGPC/NCNTs and preparation method thereof
ActiveCN106159287AThe preparation method is simple and controllableLower synthesis costCell electrodesMetal-organic frameworkCarbon nanotube
The invention discloses a compound type fuel cell cathode catalyst NGPC / NCNTs and a preparation method thereof, belongs to the technical field of catalyst preparation and aims to develop a cheap and efficient non-precious metal catalyst to solve the problem of high cost of a fuel cell cathode catalyst made of a Pt-based material. An MOF (metal-organic framework) material as a C source of the NGPC / NCNTs is pre-carbonized at a low temperature firstly, a graphitization catalyst and a N source are introduced step by step, two times of corresponding high-temperature heat treatment are performed, and the NGPC / NCNTs are prepared. The active component of the NGPC / NCNTs is N-doped graphitized porous C or CNTs, a pore structure is in a micropore-mesopore compound type, and a continuous conduction framework in three-dimensional space is formed through structural connection of dispersed porous C particles by the CNTs, so that the NGPC / NCNTs have good conductivity and higher mass transfer rate and can show efficient catalytic activity, methyl alcohol tolerance and cycle stability, which are better than those of a commercial Pt / C electrocatalyst, in an alkaline medium.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Oxygen removal system
ActiveUS20080099618A1Reduce riskReduce oxygen concentrationLiquid degasificationPower plant fuel tanksAlcoholKetone
A system and process is provided to deplete or remove oxygen in the ullage of a fuel tank to reduce the oxygen / fuel vapor ratio below a lower explosion limit by exposing the ullage compounds to an oxygen removal catalyst active at a relatively low temperature. The oxygen removal catalyst may be a non-precious metal catalyst. A selective reaction of the oxygen by the catalyst forms primarily alcohols, aldehydes, and / or ketones and produces less than about 5% water by volume. The selective reaction, occurring at the relatively low temperature, reduces the risk of flammability of the fuel.
Owner:HONEYWELL INT INC
Preparation method of nitrogen-doped graphene aerogel supported non-precious metal oxygen reduction catalyst
InactiveCN104174424AAvoid reunionHigh catalytic activity for oxygen reductionPhysical/chemical process catalystsSupercritical dryingNitrogen
The invention provides a preparation method of a nitrogen-doped graphene aerogel supported non-precious metal oxygen reduction catalyst. The method comprises the following steps: stirring a mixed solution of organic amine and aldehyde for 0.5-2 hours at the temperature of 50-90 DEG C, adding graphene oxide and a non-precious salt solution, stirring for 1-24 hours at the temperature of 60-99 DEG C to obtain organogel, performing freezing or supercritical drying, heating to 500-1000 DEG C under protection of inert gas, preserving heat for 1-6 hours, and naturally cooling to room temperature, thereby obtaining the nitrogen-doped graphene aerogel supported non-precious metal oxygen reduction catalyst. The preparation method of the nitrogen-doped graphene aerogel supported non-precious metal oxygen reduction catalyst is simple, the production can be easily expanded, the cost is low, the oxygen reduction catalysis activity is high, and the commercial popularization is facilitated.
Owner:CENT SOUTH UNIV
Multilevel hole material load cobalt catalyst, preparation method and application thereof
ActiveCN101269336AChange structureRich microporous structureHydrocarbon from carbon oxidesLiquid hydrocarbon mixture productionCarbon numberThermal stability
Owner:中科潞安能源技术有限公司
Commercial power production by catalytic fusion of deuterium gas
InactiveUS20010040935A1Avoid pressure dropFree spaceNuclear energy generationNuclear explosivesCost effectivenessProduct gas
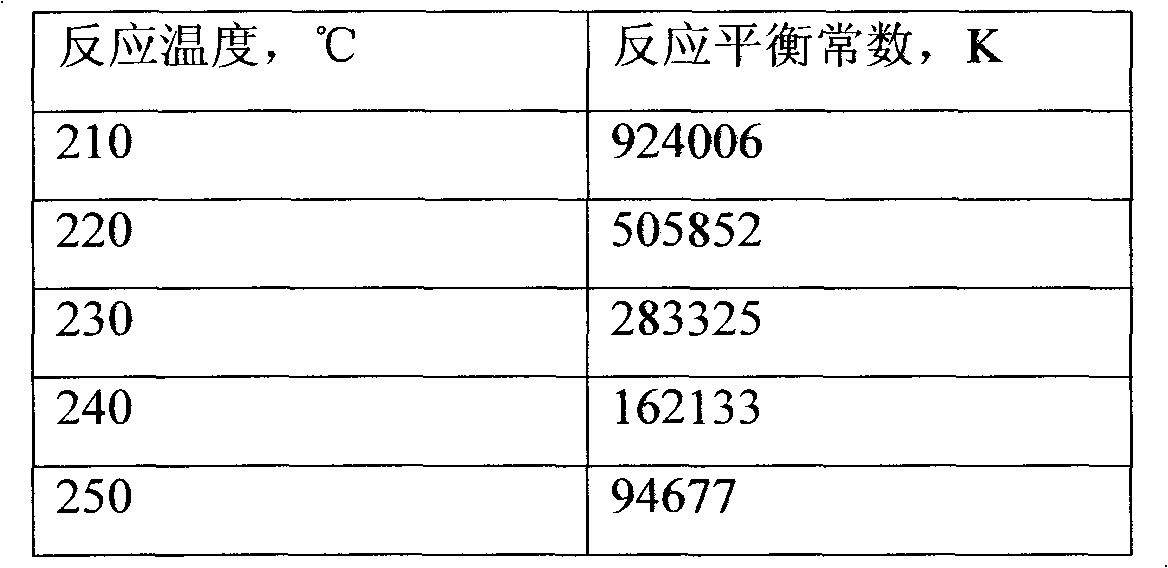
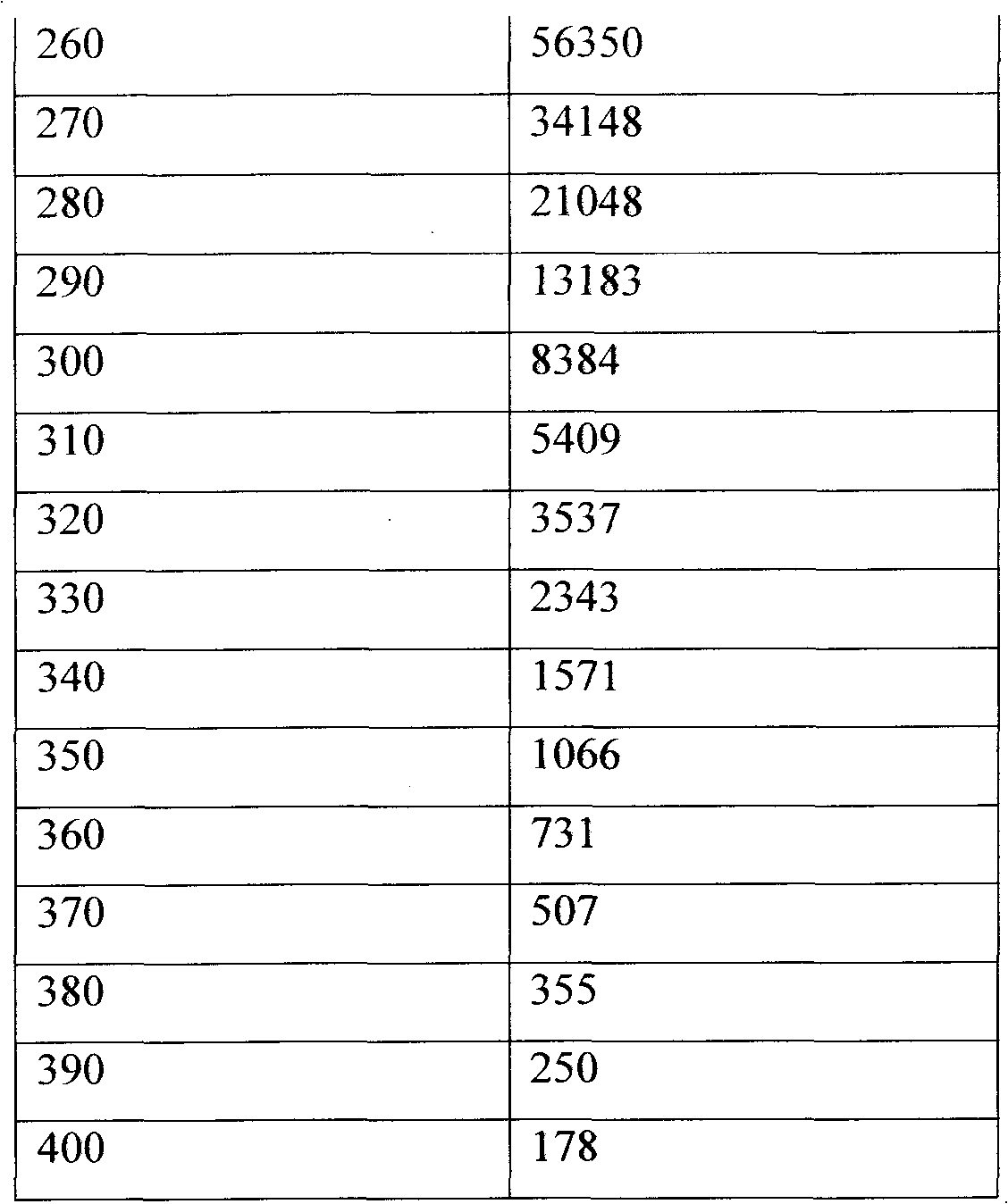
After much experimentation, I have developed, a new, cost-effective, process for commercial-scale production of power by catalytic fusion of D2 gas, under moderate conditions of temperature and pressure. This process can be scaled up to any desired size, and can employ a variety of "hydrogenation" catalysts, both precious metal, and non-precious metal. Briefly, the process comprises absorbing D2 gas in or on the selected catalyst, then bringing the temperature into the range of very roughly 150° to 250° C., and then degassing the catalyst bed under reduced pressure. The process is necessarily run on a cyclic basis, with a multiplicity of catalyst bed entities, with one or more being in the D2-absorption mode, concurrently with one or more being in the heat-generation node.
Owner:CASE LESLIE CATRON
Low temperature CO oxidation non-noble metal catalyst
InactiveCN101143321AHave substantive characteristicsLow costCatalyst carriersDispersed particle separationNickel saltIron salts
The invention provides an oxidation catalyst to purify CO in room temperature, which is prepared in coprecipitation method or deposition-precipitation method. The catalyst consists of non precious metal active components and carrier, wherein the loading of the active components is 5 to 80 percent of the conversion value of metal element. The active components come from cobalt salt liquid, iron salt liquid, nickel salt liquid, manganese salt liquid, copper salt liquid, zinc salt liquid, tin salt liquid and cerium salt liquid. The carrier comes from alumina, silica, molecular sieve, honeycomb ceramics, wire fence, cobalt oxide, iron oxide, manganese oxide, copper oxide, zinc oxide, tin oxide and cerium oxide. The precipitant is Na2CO3, K2CO3, sodium hydroxide, urea or ammonia. The catalyst preparation solution needs to be fully stirred for 1 to 8 hours and the retrogradation duration is 1 to 16 hours. The dried solution needs to be treated in in-situ remediation in air, oxygen, and hydrogen or nitrogen atmosphere in temperature from 100 to 500 degrees centigrade. The catalyst disuses precious metal as active component, so the cost is low and the preparation process is simple.
Owner:NO 63971 TROOPS PLA
Carbon-loaded kernel-shell copper-palladium-platinum catalyst for fuel battery and preparation method thereof
InactiveCN102664275AGood effectImprove stabilityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsAdditive ingredientReaction temperature
The invention provides a carbon-loaded kernel-shell copper-palladium-platinum catalyst for a fuel battery and a preparation method thereof. Conductive carbon black is used as a carrier, an active component is a copper-palladium-platinum alloy with a nuclear-shell structure, wherein the copper palladium is a kernel, the platinum is a shell, and the catalyst comprises the following ingredients by mass percent: 70 to 91 percent of conductive carbon black, 2 to 10 percent of copper, 2 to 10 percent of palladium and 5 to 10 percent of platinum. A two-step reduction method is utilized, namely, low-activity metal is firstly reduced, then the active precious metal is reduced, the precious metal is precipitated on the surface of the non-precious metal by controlling the reaction temperature and the pH value, and a kernel-shell catalyst is formed through a dealloying step; and in addition, the carbon carrier is added in the preparation process, so that the nano metal particles can directly grow on the carrier, the combination of the catalyst particles and the carbon carrier can be enhanced, and the catalyst is more stable. Due to the adoption of the preparation method, the catalyzing efficiency of the catalyst and the utilization rate of the precious metal can be greatly improved, and the development of the fuel battery can be promoted.
Owner:BEIJING UNIV OF CHEM TECH
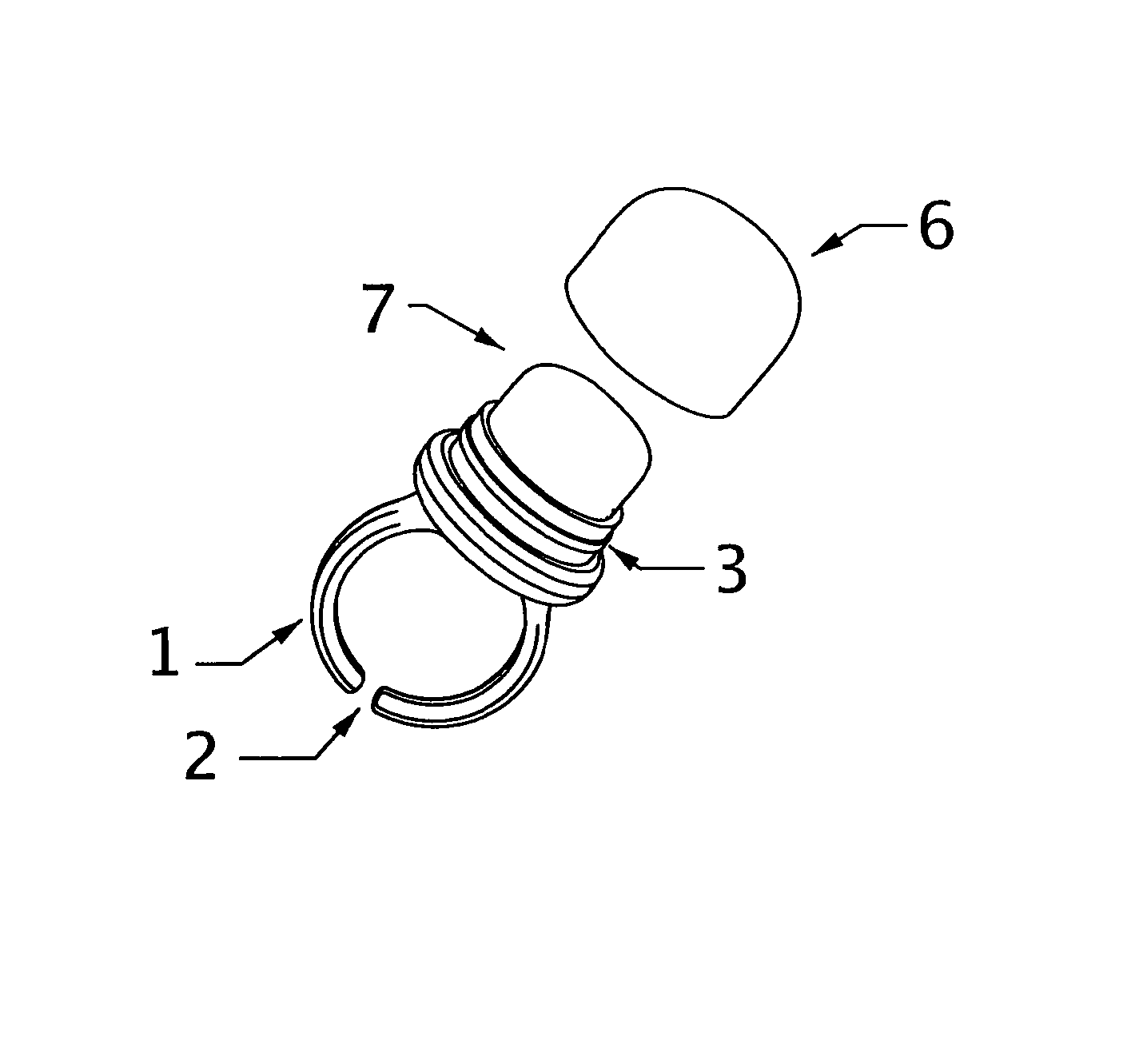
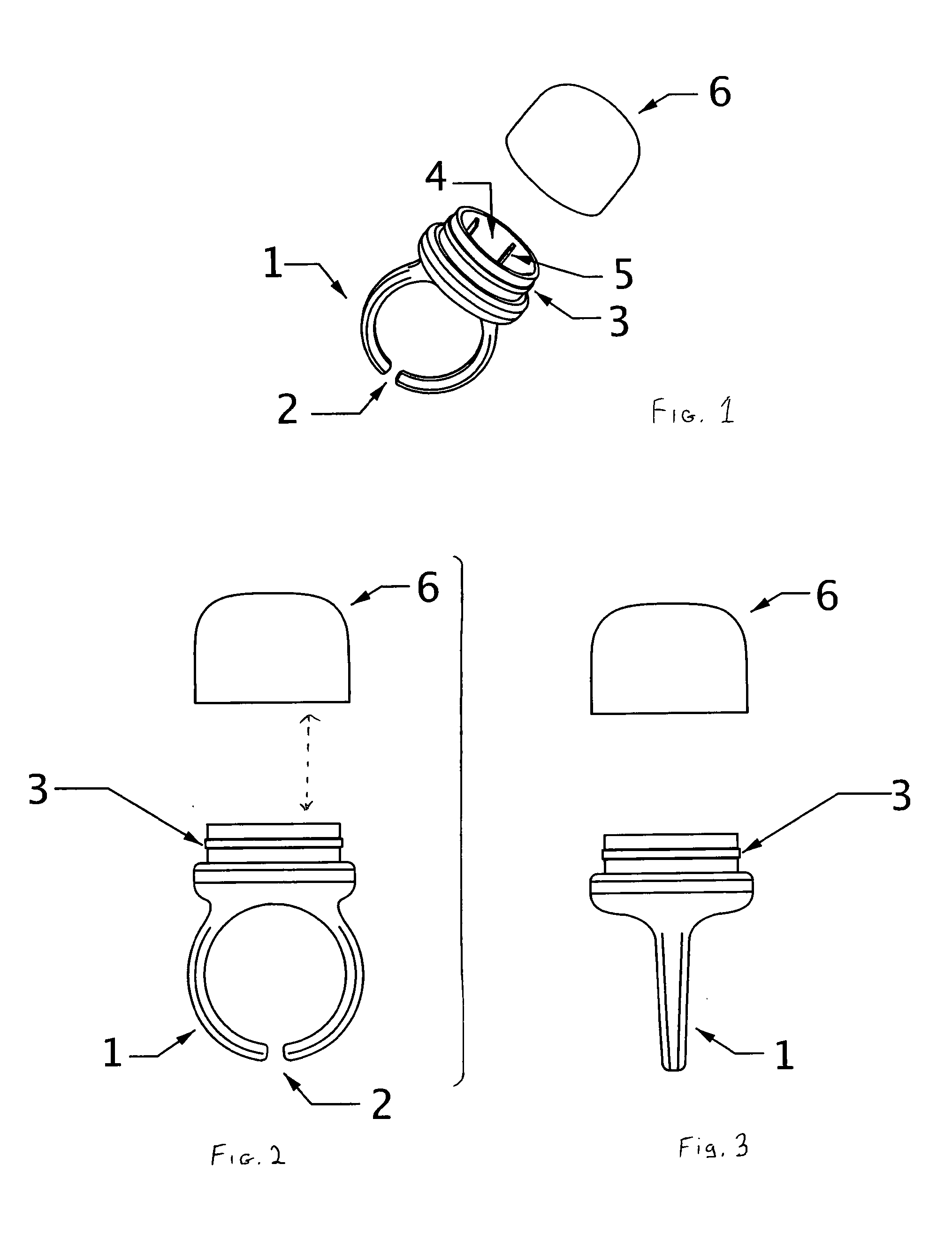
Freshening ring
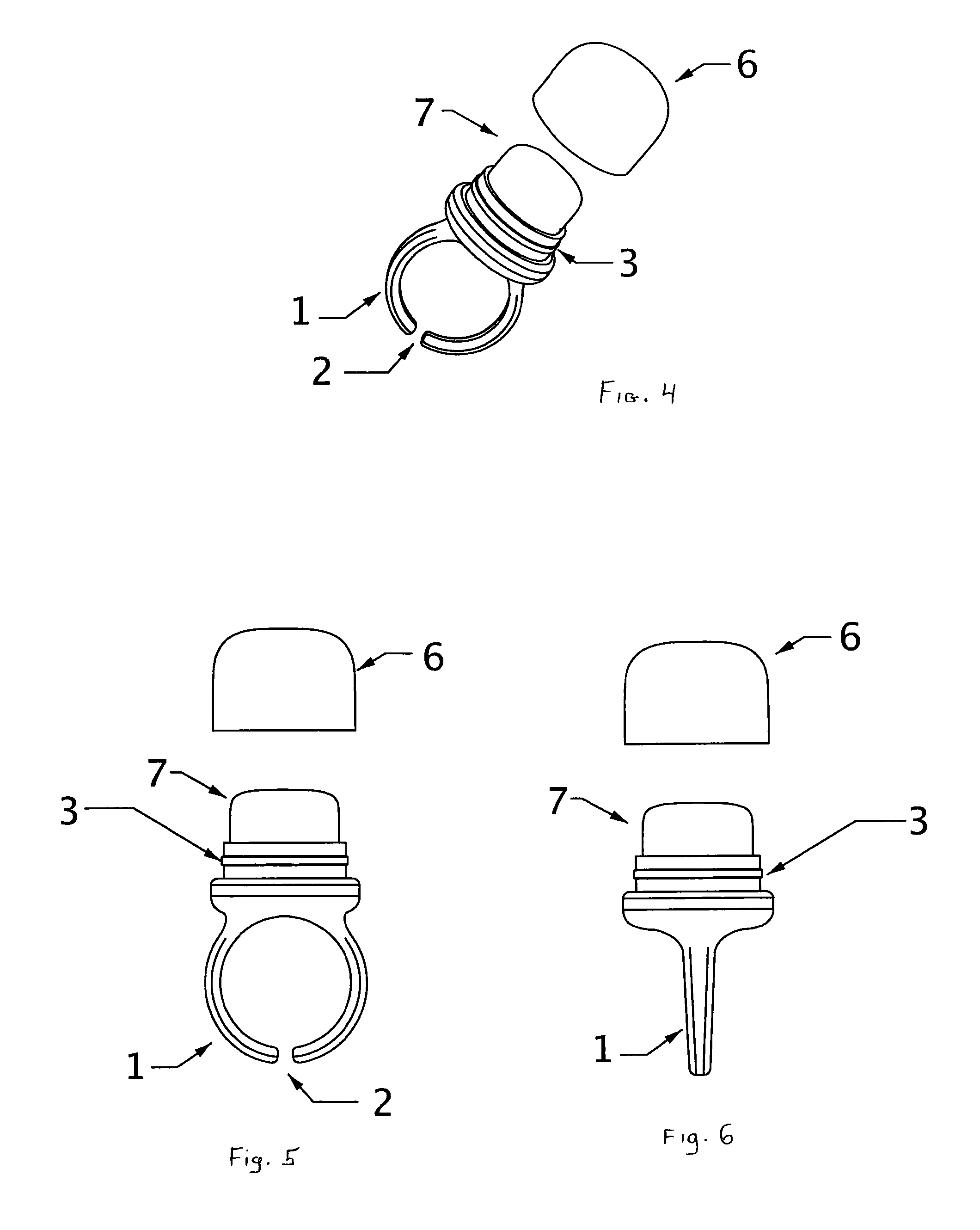
InactiveUS8747002B2Easy to useEasy accessBrush bodiesPackaging toiletriesEngineeringPersonal Care Product
The subject wearable ring assembly comprises a ring shank portion, integrally or mechanically connected to a personal care product receptacle, which is mechanically or frictionally connected to a protective cap element configured to contain and protect personal care products stored in the receptacle. The ring shank portion is sized to fit a wearer's finger or is adjustable to fit a wearer's finger. The ring is made from jewelry grade materials selected from precious metals such as gold and silver or costume jewelry grade materials selected from non-precious metals, thermo-formable plastic resins, ceramics, leather, fabrics or woven materials.
Owner:IZKOVITZ GDALYAHU
Low-bulk-density composition for reducing discharge of regenerated flue gases CO and NOx during FCC (Fluid Catalytic Cracking)
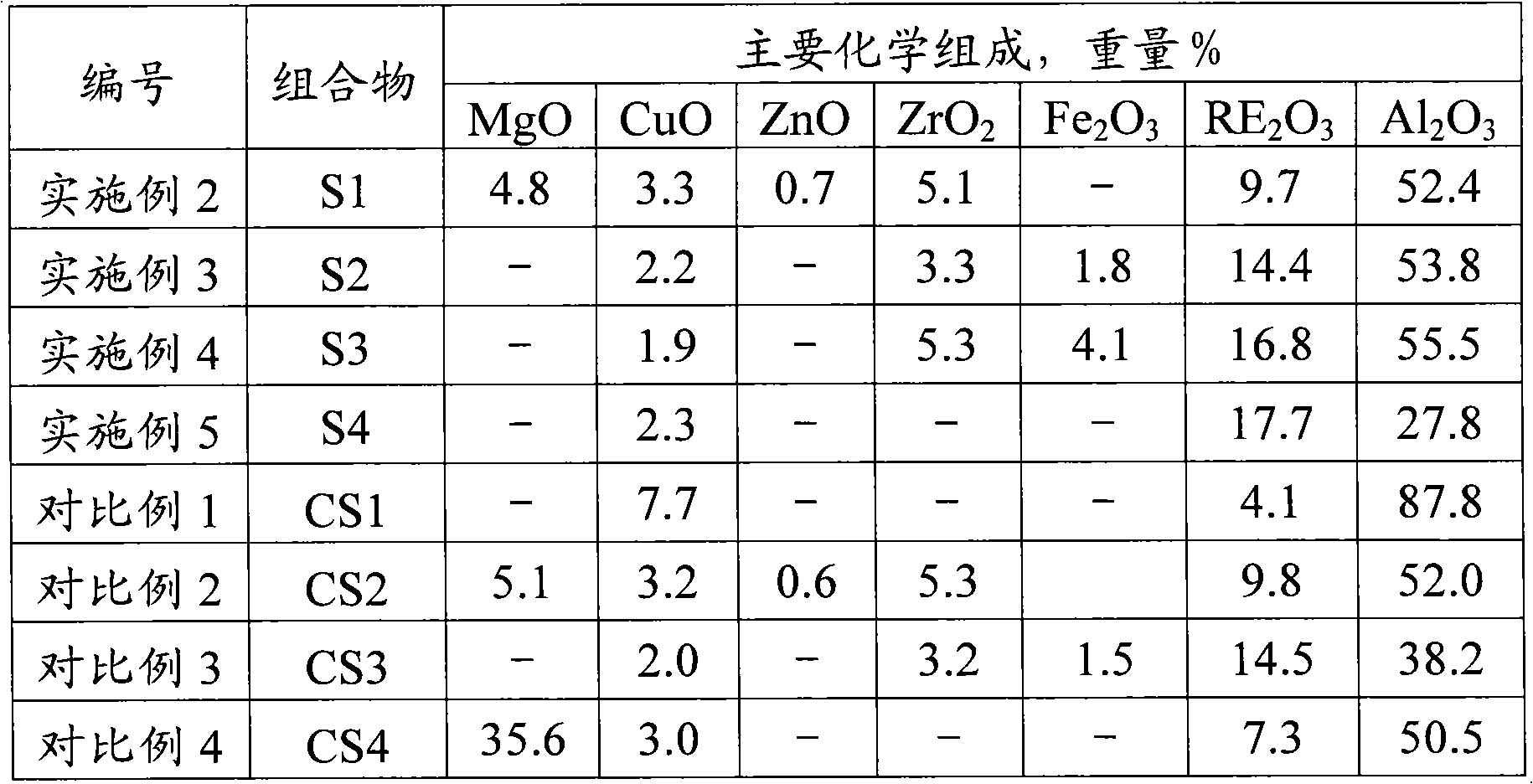
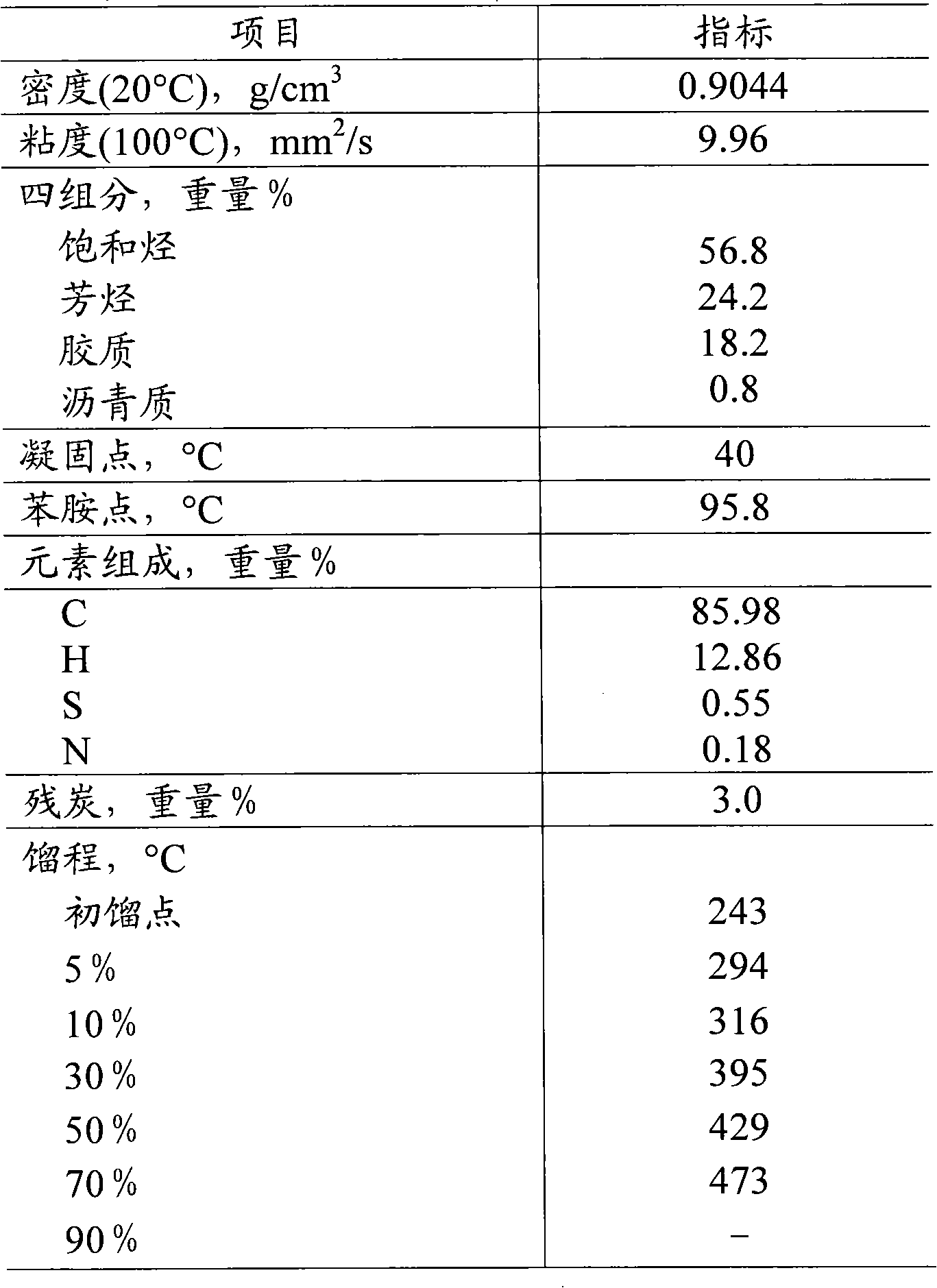
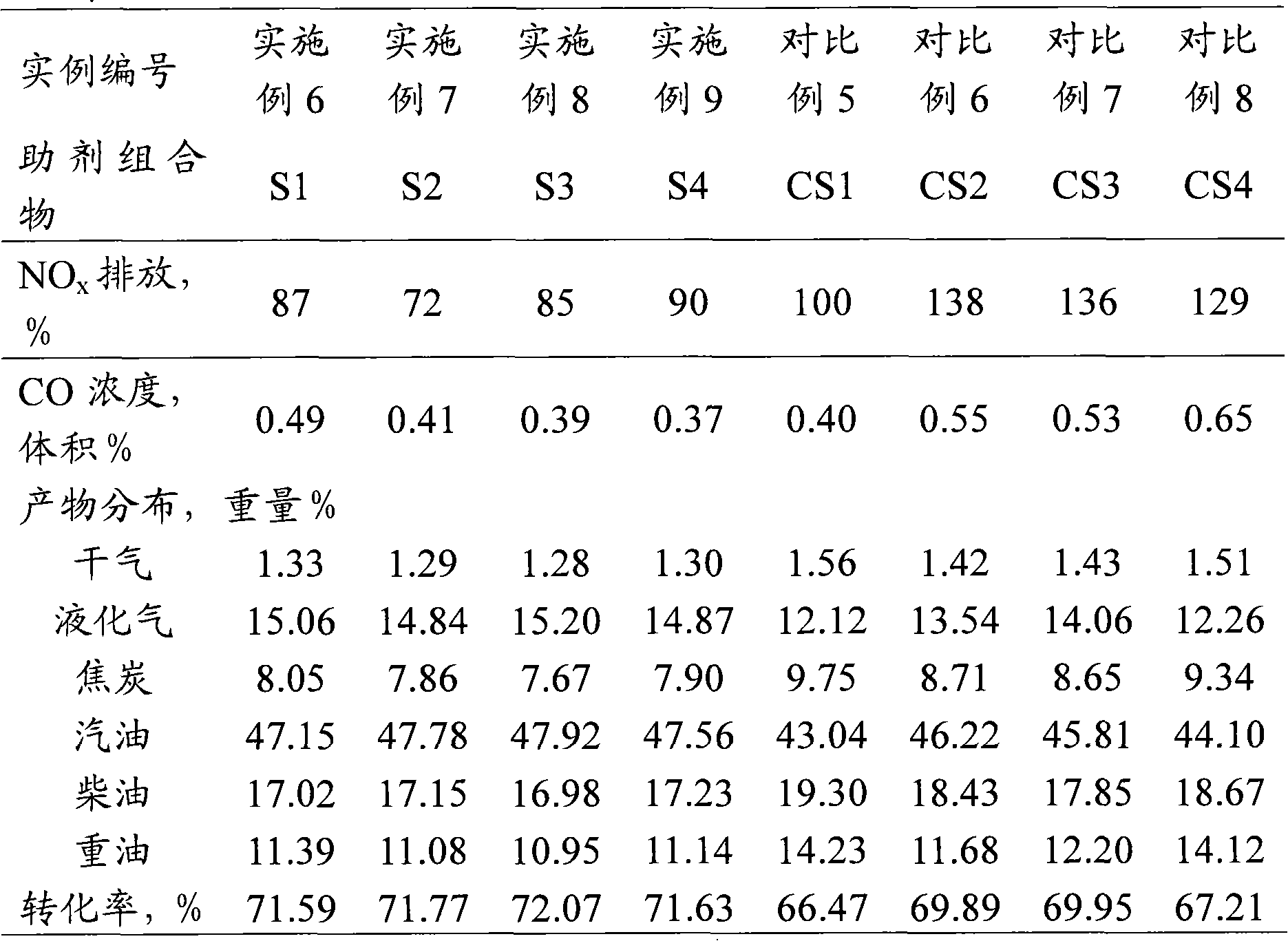
ActiveCN102371165AEfficient responseLow emission activityCatalytic crackingMolecular sieve catalystsRare-earth elementGram
The invention discloses a non-precious metal composition for reducing discharge of regenerated flue gases CO and NOx during FCC (Fluid Catalytic Cracking). The bulk density of the composition does not surpass 0.65 grams / ml. Counted by an oxide, the composition comprises the following components in percentage by weight: (1) 5-95 percent of zeolite, (2) 0.5-40 percent of one or more non-precious metal elements selected form IA, IIA, IB, IIB, IVB, VB, VIB, VIIB and VIII families, (3) 0.5-30 percent of rare-earth element and (4) 1-90 percent of inorganic oxide carrier. The low-bulk density composition is applied to catalytic cracking, can be used for remarkably reducing the discharge of regenerated flue gases CO and NOx, and has low yields of dried gas and hard coke.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalytic process for converting renewable resources into paraffins for use as diesel blending stocks
A process for converting renewable resources such as vegetable oil and animal fat into paraffins in a single step which comprises contacting a feed which is a renewable resources with hydrogen and a catalyst which comprises a non-precious metal a first oxide and optionally a second oxide wherein at least one of the first oxide or second oxide comprises a zeolite, through hydrodeoxygenation and one or both of hydroisomerization and hydrocracking.
Owner:REFINING TECH SOLUTIONS LLC
Supported catalyst and application thereof in hydrocracking reaction of xylitol
ActiveCN102019185AEfficient crackingEfficient hydrocrackingOrganic compound preparationHydroxy compound preparationGlycerolBULK ACTIVE INGREDIENT
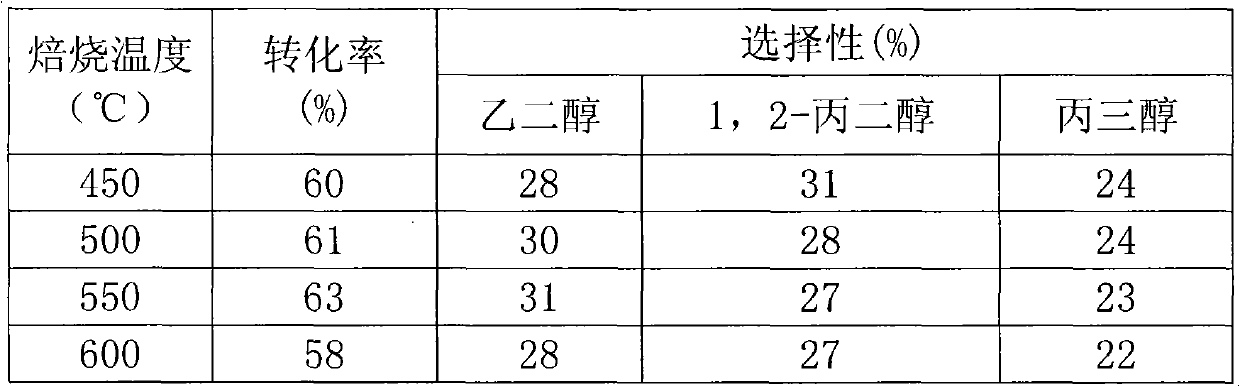
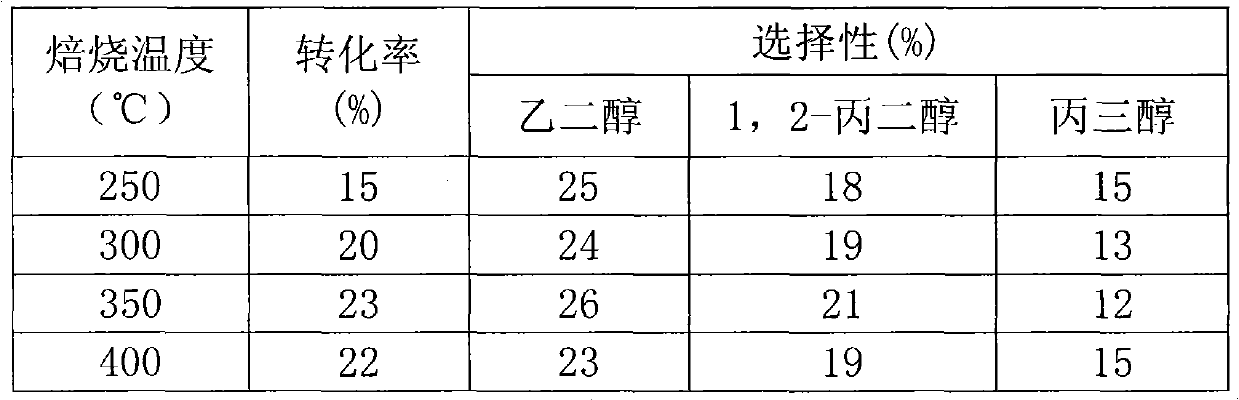
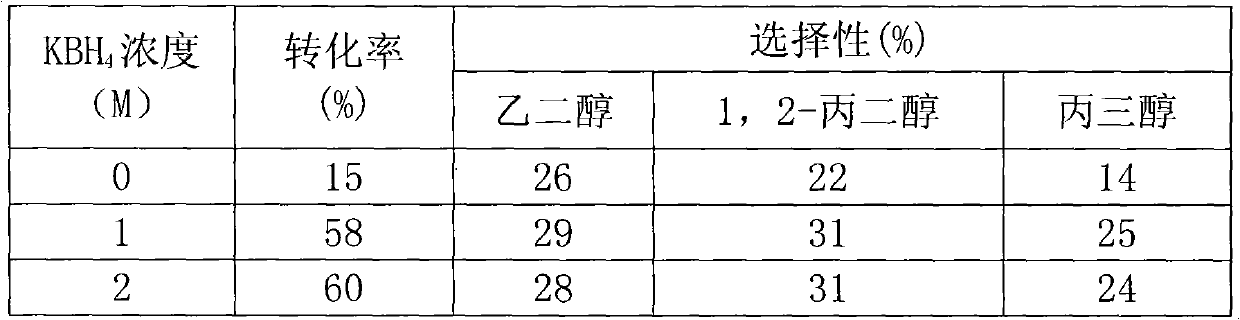
The invention relates to a catalyst for hydrocracking of xylitol, in particular to a supported catalyst and application thereof in hydrocracking reaction of xylitol. A non precious metal Ni is used as a hydrogenation active ingredient; an aid is one or more of Ru, Pd, Pt, Mo, Sn, Mg, Zn, Ce, Cu, Al and Zr; a carrier is active carbon; and a preparation method comprises the following steps of: supporting aqueous solution of soluble metal salt on the active carbon carrier; performing carbothermic reduction by roasting a metal salt precursor supported on the active carbon carrier at high temperature of between 450 and 650 DEG C to obtain a metal catalyst; and treating the metal catalyst for 1 to 4 hours by using 1-2mol / L KBH4 solution. Under the action of the catalyst, the xylitol is efficiently hydrocracked to form glycol, 1,2-propylene glycol and glycerol, the conversion rate is over 80 percent, and the total selectivity is over 90 percent.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Anti-electromagnetic interference multilayer composite material and method for producing the same
InactiveCN101175394ALow resistivityStrong electromagnetic shieldingMagnetic/electric field screeningScreening apparatusBasementElectromagnetic interference
The present invention provides an anti electromagnetic interference multilayer composite materials, which comprises a plastic basement and a metal layer loaded on the plastic basement. The metal layer comprises at least three layers which are a first metal layer, a second metal layer and a third metal layer in turn from inside to outside. The first metal layer and the third metal layer are all formed by one of stainless steel, chrome and nickel. The second metal layer is formed by one of argentums, cuprum and aluminum. The multilayer composite materials provided by the present invention has a small resistivity with a resistance less than two ohm in the largest distance in an area, strong electromagnetism shielding capacity (the electromagnetism shielding capacity reaches up to 10 to 50 Debye), strong corrosion resistance and good combining power to the plastic basement. Even under the effect of brine corrosion, the metal layer in particular to the second metal layer is still firmly attached to the plastic basement. In addition, the metals forming the metal layer of the present invention are all routine and not expensive metals, so the cost of production is relatively low.
Owner:BYD CO LTD
Nitrogen-doped graphene-iron-based nanoparticle composite catalyst and preparation method thereof
ActiveCN105170169AReduce reunionPromote generationPhysical/chemical process catalystsCell electrodesIron saltsFreeze-drying
The present invention relates to a nitrogen-doped graphene-iron-based nanoparticle composite catalyst and a preparation method thereof, wherein the catalyst is a complex of nitrogen-doped graphene and iron-based nanoparticles (including metal iron and iron nitride). The main preparation process comprises: carrying out a reaction of a graphene oxide aqueous solution and a reducing agent (hydrazine hydrate or sodium borohydride) for 1 h under an oil bath to obtain reduced graphene oxide; mixing the reduced graphene oxide aqueous solution and an iron salt, completely stirring, and carrying out freezing drying to obtain a reduced graphene oxide-iron salt aerogel precursor; and carrying out a high temperature heat treatment under a mixed atmosphere of ammonia gas and an inert gas to obtain the nitrogen-doped graphene and iron-based nanoparticle complex. Compared with the commercial platinum-carbon catalyst, the composite non-precious metal catalyst of the present invention has advantages of simple preparation process, low cost, high oxygen reduction catalysis activity, good methanol tolerance and the like, and can be used for fuel cells, lithium-air batteries and other oxygen reduction catalysis reaction systems.
Owner:TSINGHUA UNIV
Nitrogen-doped graphene/ ferrocobalt hydrotalcite-like compound difunctional oxygen-reduction catalyst and preparing method and application thereof
ActiveCN105826574AImprove conductivityImprove compound efficiencyMaterial nanotechnologyPhysical/chemical process catalystsFerrocobaltNitrogen doped graphene
The invention discloses a nitrogen-doped graphene / ferrocobalt hydrotalcite-like compound non-precious metal difunctional oxygen-reduction catalyst and a preparing method and application thereof. A bimetallic oxide is used as a precursor, the memory effect of a hydrotalcite-like compound is utilized, oxidized graphene and the ferrocobalt hydrotalcite-like compound are assembled, and then the compound is doped with carbon nitride nanosheets under the reduction condition to obtain a nitrogen-doped graphene / ferrocobalt hydrotalcite-like compound composition. The oxygen-reduction catalyst has high catalytic activity to oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) at the same time under the alkaline condition and has high stability and excellent methyl alcohol tolerance, the cost of adopted raw materials is low, the preparing method is simple, operation is easy, and large-scale production can be achieved conveniently.
Owner:JIANGSU SANJI IND
Fuel-cell system and method of generating energy from crude fuel
ActiveUS20170263945A1Improve performanceImprove adsorption capacityCell electrodesFuel cellsFuel cellsIon-exchange membranes
The present invention relates to a fuel-cell system. This system includes an anode electrode; a cathode electrode; a separator positioned between the anode electrode and the cathode electrode, wherein the separator is not an ion exchange membrane; an anode catalyst; and a cathode catalyst, wherein the cathode catalyst is a non-precious metal catalyst or metal-free catalyst. The present invention also relates to a method of generating energy from crude fuel. This method involves providing a fuel-cell system and contacting the fuel-cell system with a crude fuel under conditions effective to generate energy from the crude fuel.
Owner:IOWA STATE UNIV RES FOUND
Platinum-non-precious metal alloy nanowire, and aqueous-phase synthesis method and application thereof
ActiveCN109014237AAchieve co-reductionHigh yieldMaterial nanotechnologyTransportation and packagingPlatinum saltsNanowire
The invention discloses a platinum-non-precious metal alloy nanowire, and an aqueous-phase synthesis method and application thereof, and belongs to the field of nanometer material science. According to the aqueous-phase synthesis method, coreduction of platinum salt and non-precious metal salt in an aqueous solution is achieved through a ligand, the controllable ultrafine platinum-non-precious metal alloy nanowire is successfully synthesized, and the technical problems are solved. A preparing method is simple, the yield is high, the steps are less, controllability is good, the nanowire in a product is small in diameter and uniform in structure, the surface of the ultrafine platinum-non-precious metal alloy nanowire prepared through the preparing method is easy to clean, and the ultrafine platinum-non-precious metal alloy nanowire has superior performance in hydrogen production through electrolytic water.
Owner:XI AN JIAOTONG UNIV
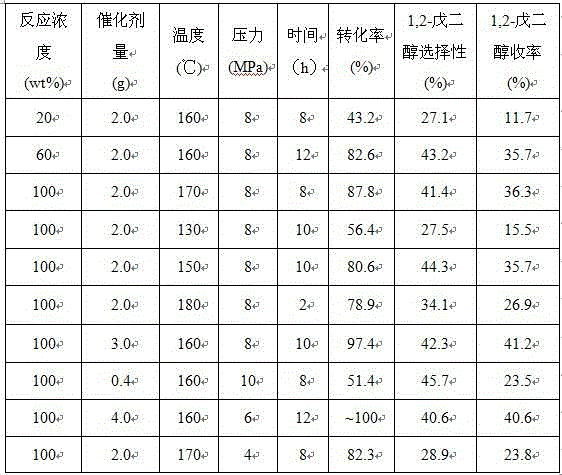
Method for preparing 1,2-pentanediol by furfuryl alcohol liquid phase selectivity and hydrogenolysis
ActiveCN104370702AHigh activityHigh selectivityMolecular sieve catalystsPreparation by oxygen reductionHigh concentrationFurfural
The invention discloses a method for preparing 1,2-pentanediol by furfuryl alcohol liquid phase selectivity and hydrogenolysis. According to the invention, a non-precious metal catalyst with environmental protection and high efficiency is selected, which concretely relates to the supported Bu-based catalyst, and then furfural is used for preparing 1,2-pentanediol with high activity and high selectivity under mild hydrogenation condition. The catalyst of the present invention uses furfuryl alcohol with high concentration even pure furfuryl alcohol as a raw material, so that energy consumption for separating a solvent can be reduced.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
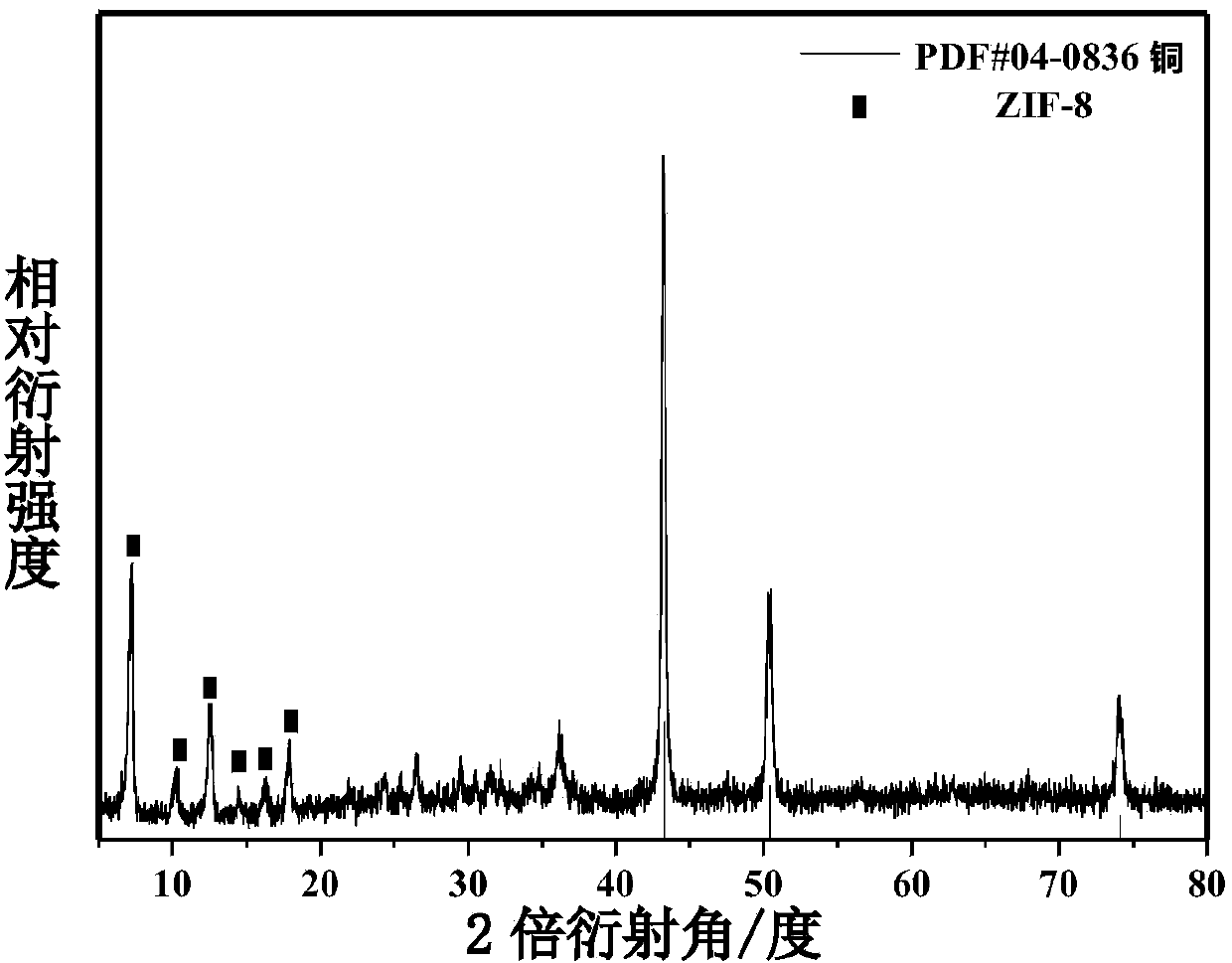
Preparation method and application of environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst
InactiveCN104001547AImprove stabilityHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionN dimethylformamideSynthesis methods
The invention discloses an environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst and a preparation method and application of the environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst. A microwave induction coring heating synthesis method is adopted, N, N-dimethylformamide is used as a solvent, polyvinylpyrrolidone is used as a trapping agent, a copper wire is used as a one-dimensional structure loading carrier, the environment-friendly core shell one-dimensional nanometer copper wire-organic metallic framework ZIF-8 composite catalyst is synthesized fast, the decomposition rate of the catalyst on ammonia borane in an ammonia borane decomposition catalytic reaction is 71 percent, and the good catalytic activity is shown. The preparation method of the catalyst is simple, and environmental pollution is small. An organic metallic framework ZIF-8 is combined with a non-precious metal copper nanometer wire with the good conductivity and certain catalytic performance, and the catalytic performance of the material of this kind is greatly improved. The material is potentially applied to energy storage, pollution gas absorption, sewage treatment, new energy development and other fields.
Owner:SHANGHAI NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com