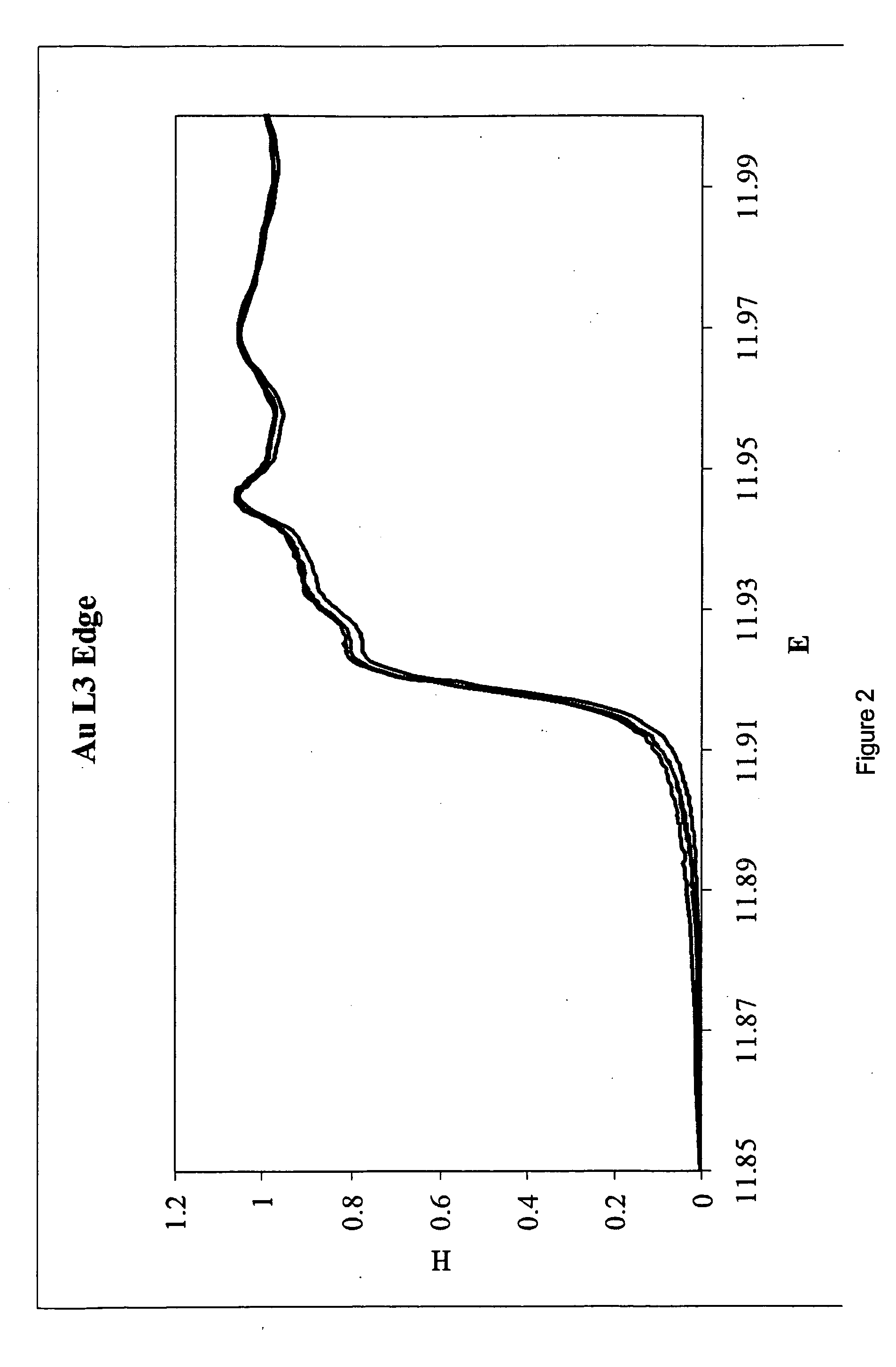
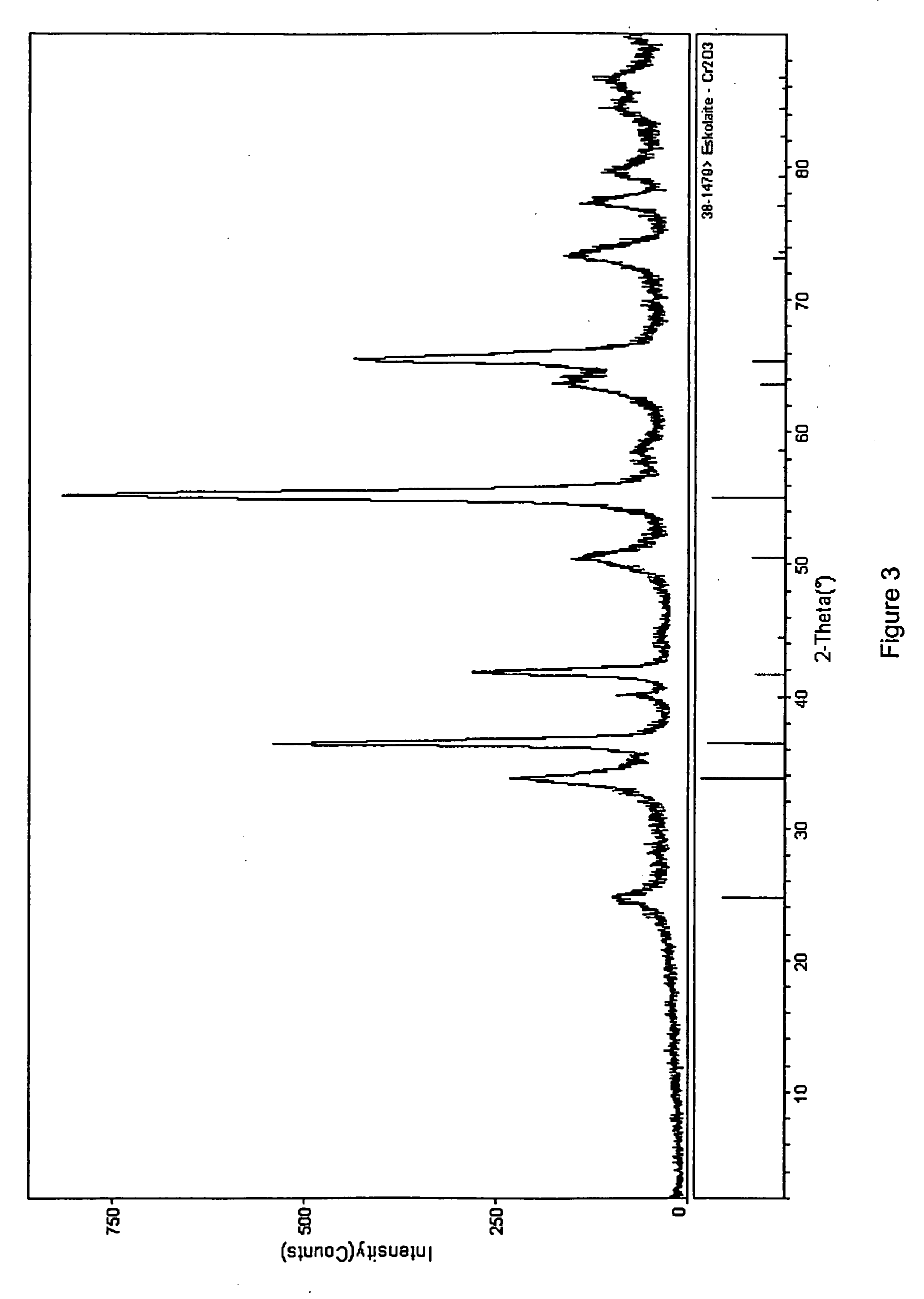
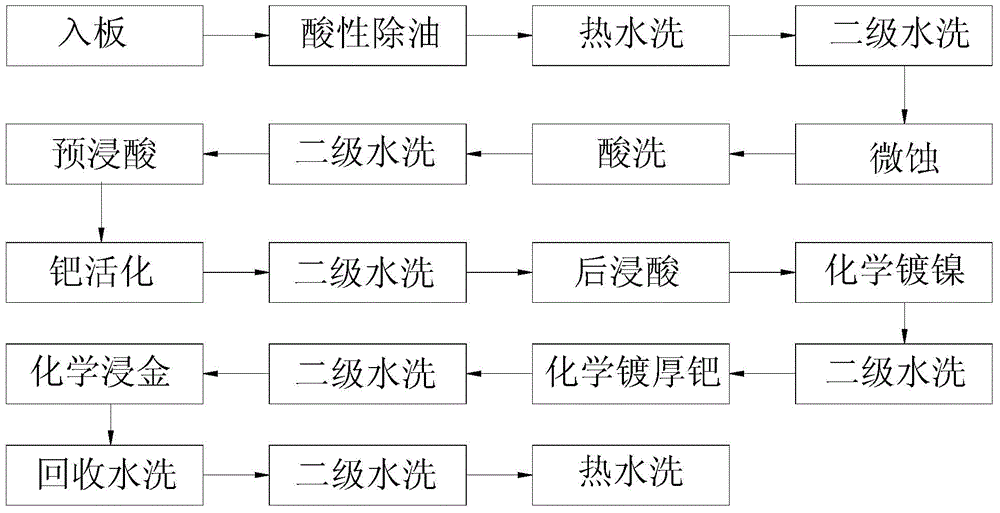
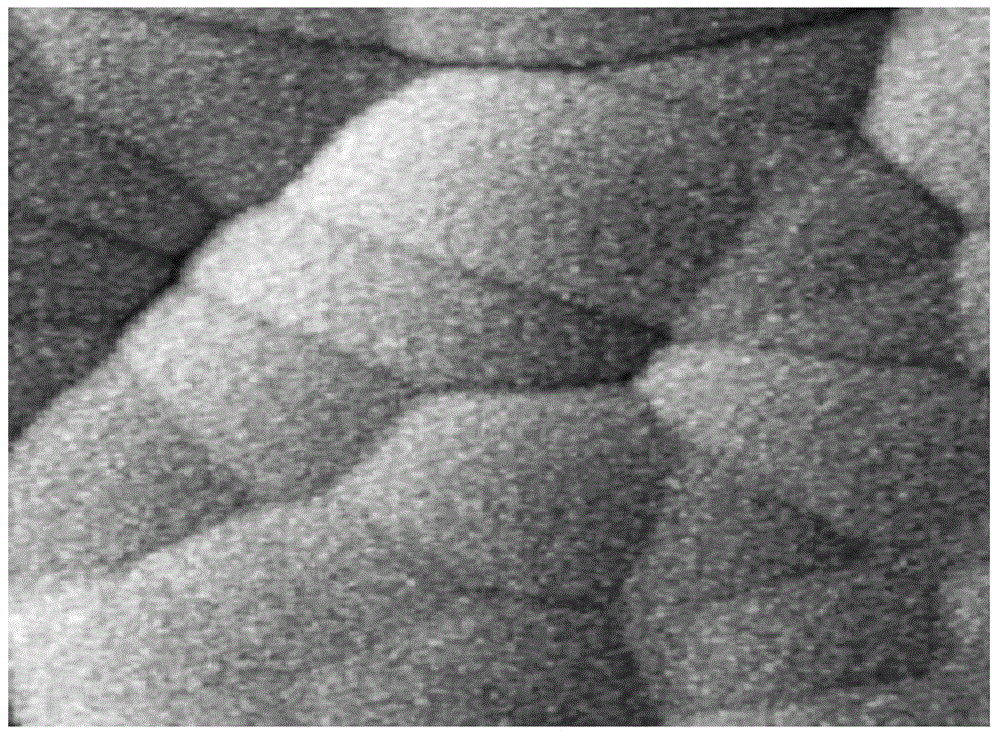
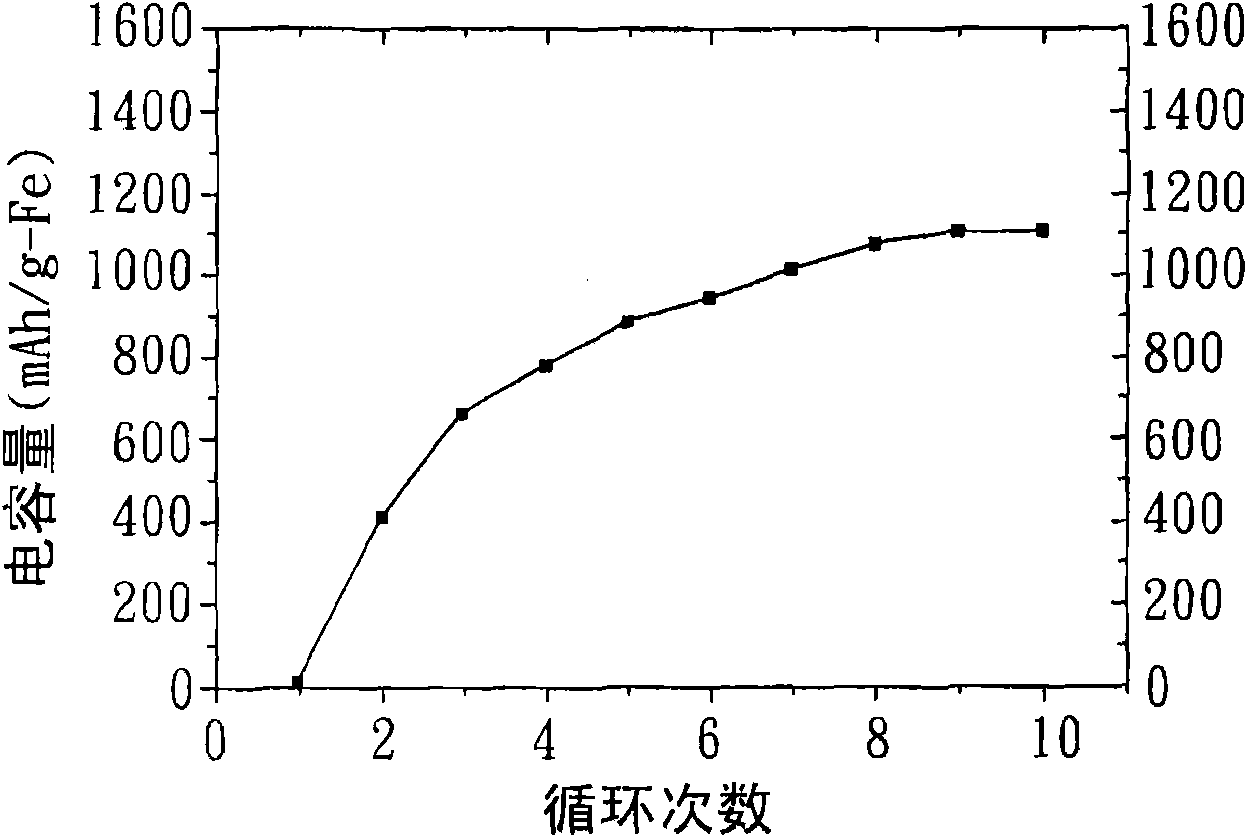
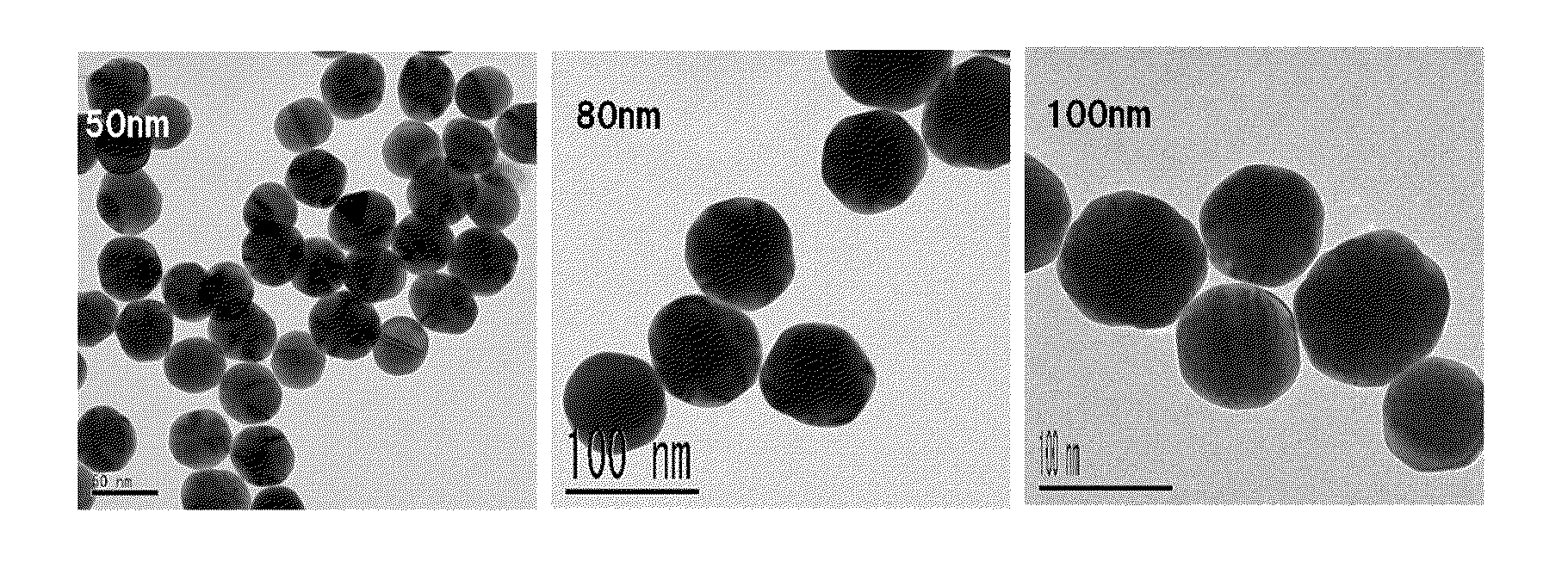
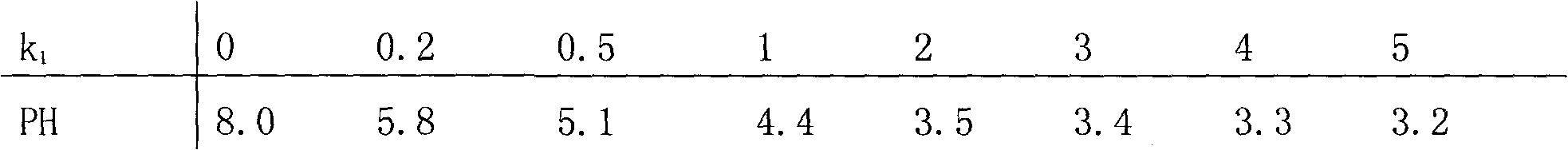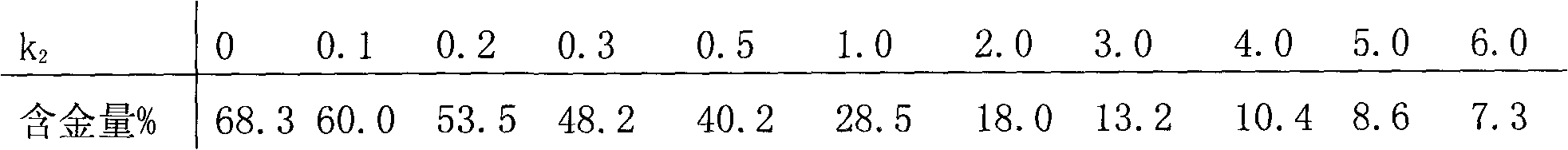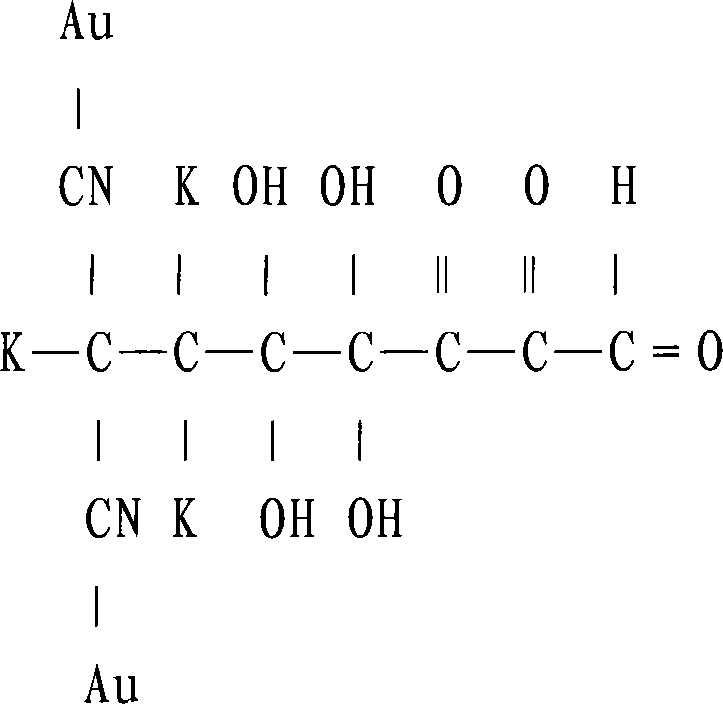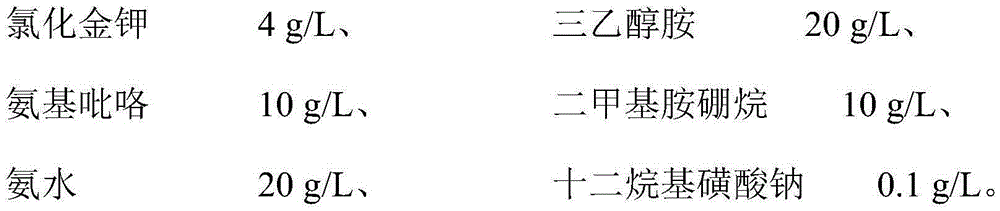Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
200 results about "Gold salts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gold salts are ionic chemical compounds of gold. The term, "gold salts" is a misnomer, and is the term for the gold compounds used in medicine. "Chrysotherapy" and "aurotherapy" are the applications of gold compounds to medicine. Contemporary research on the effect of gold salts treatment began in 1935, primarily to reduce inflammation and to slow disease progression in patients with rheumatoid arthritis. The use of gold compounds has decreased since the 1980s because of numerous side effects and monitoring requirements, limited efficacy, and very slow onset of action. Most chemical compounds of gold, including some of the drugs discussed below, are not salts, but are examples of metal thiolate complexes.
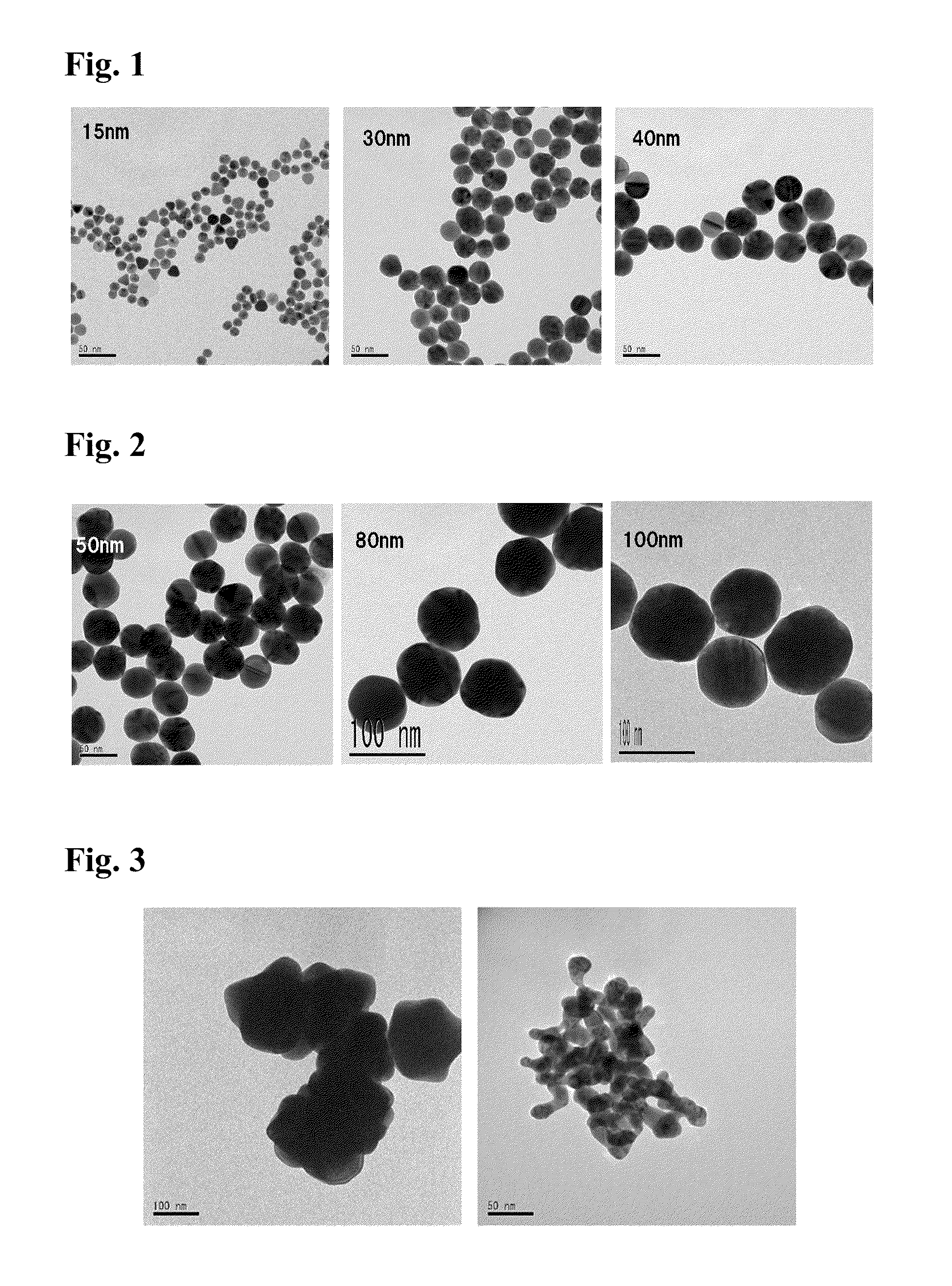
Method of producing gold nanoparticle
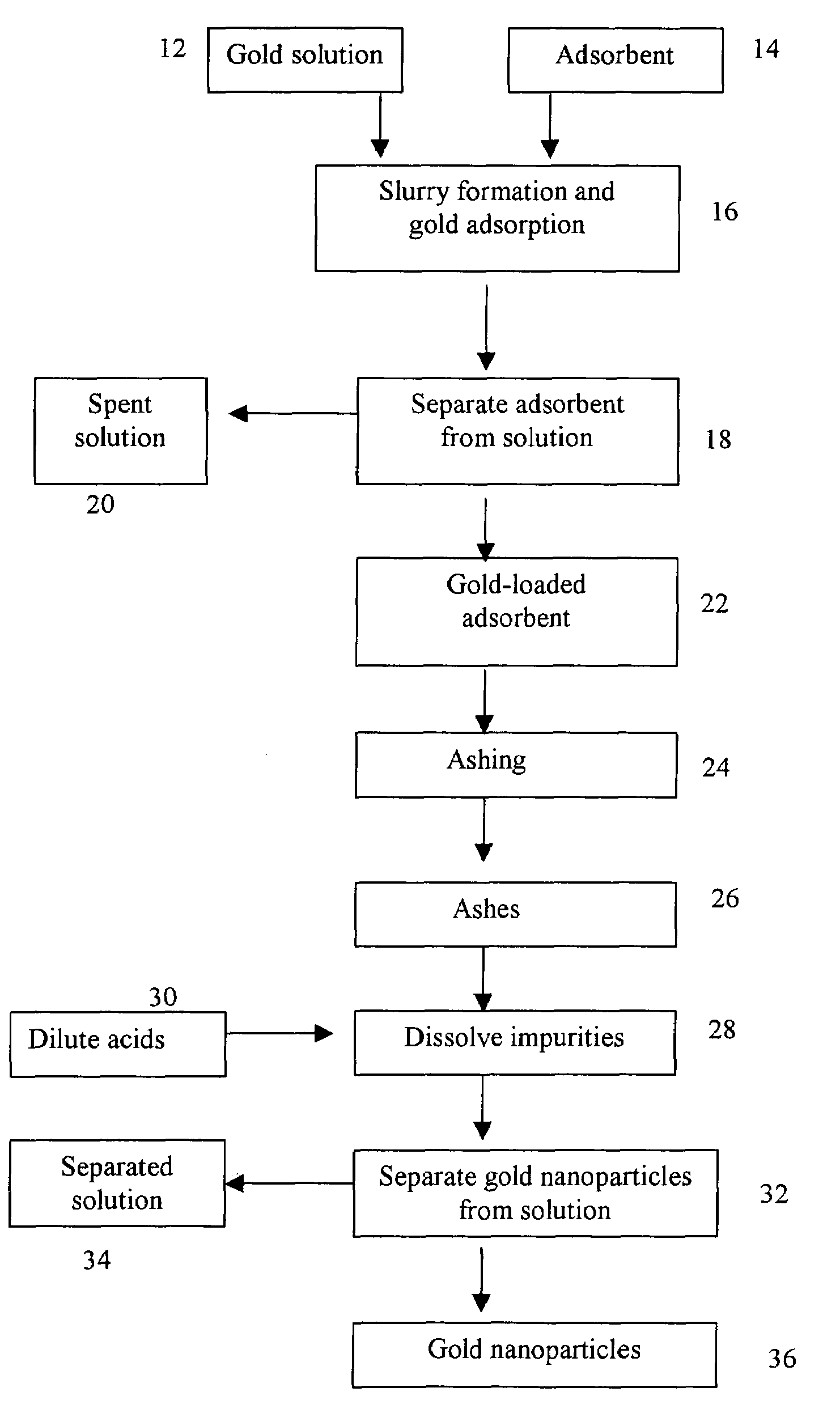
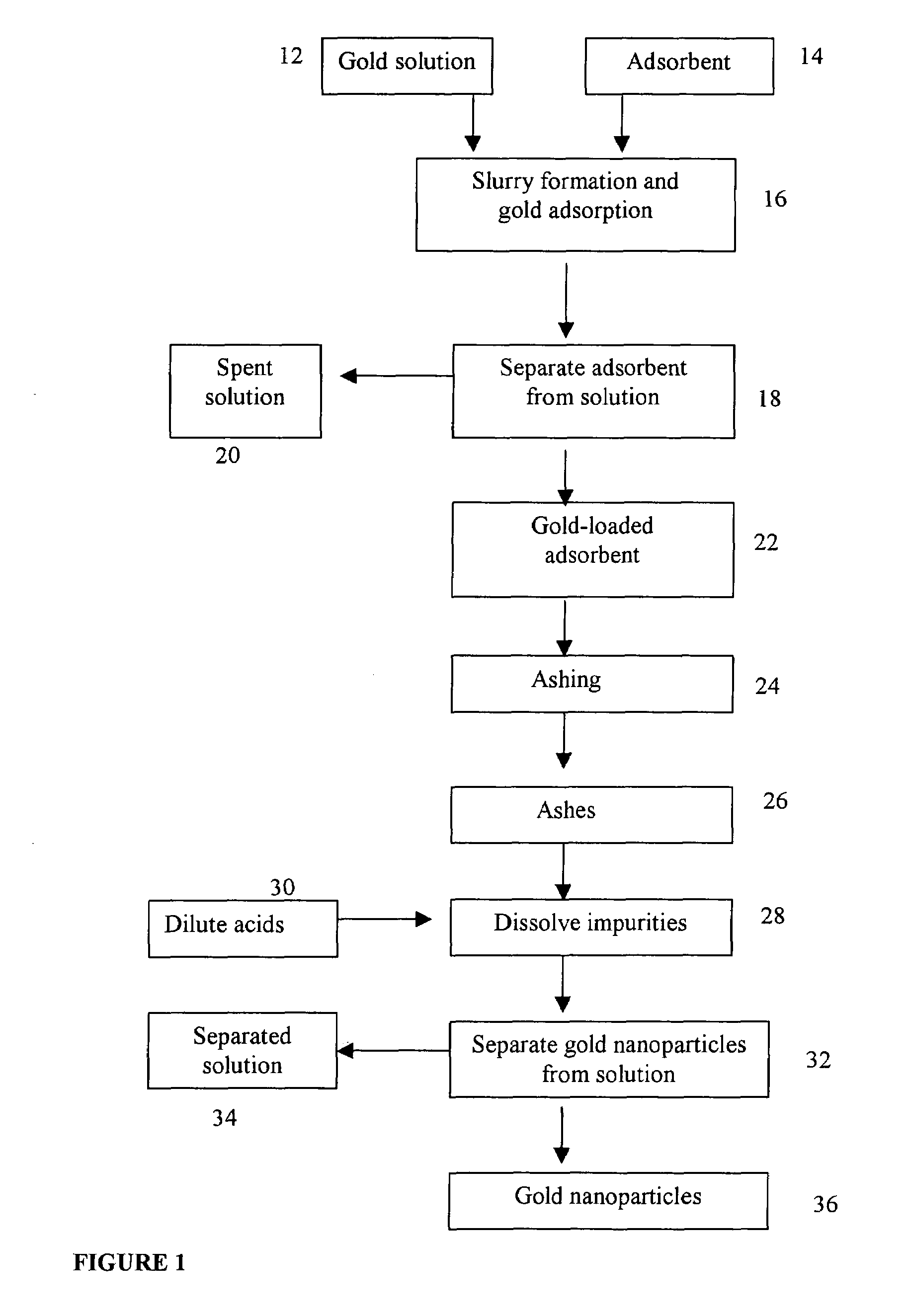
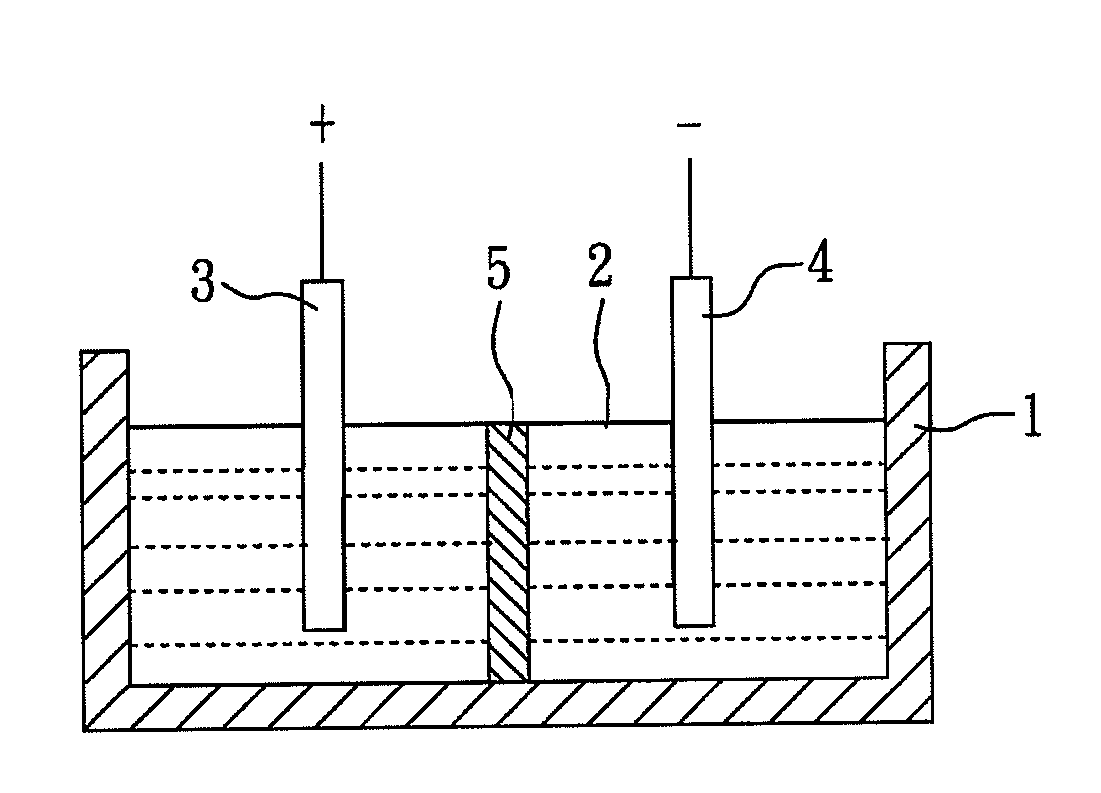
A method for producing gold nanoparticles is disclosed. When gold salt solution is mixed with an adsorbent, gold in the form of complexes is adsorbed onto the surface of the adsorbent. The gold-loaded adsorbent, after being separated from the solution by screening, filtration, settling or other methods, is ashed to form ashes. The ashes contain gold nanoparticles and impurities such as oxides of sodium, potassium and calcium. The impurities can be removed by dissolution using dilute acids. The relatively pure gold nanoparticles are obtained after the impurities are removed. Activated carbon or gold-adsorbing resin can be used as the adsorbent. Silver or platinum group metal nanoparticles can also be produced by this method.
Owner:LIN HSING KUANG +1
Non-mercuric catalyst used in hydrochlorination of acetylene and method for preparing vinyl chloride by using catalyst
InactiveCN102029189AGood stability in continuous useSolution to short lifePreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsPhosphatePotassium
The invention relates to a non-mercuric catalyst used in hydrochlorination of acetylene and a method for preparing vinyl chloride by using the catalyst. The non-mercuric catalyst used in hydrochlorination of acetylene for preparing vinyl chloride comprises main active ingredients-gold salts, auxiliary active ingredients-non precious metal salts and a carrier, wherein the main active ingredients are gold salts and can be halides, complexes and the like of gold; gold in the gold salts accounts for 0.1-10% by weight of the catalyst; the auxiliary active ingredients are non precious metal salts and can be halides, acetates, phosphates, complexes and the like of potassium, barium, lanthanum and copper; the non precious metal salts account for 0.1-10% by weight of the catalyst; and the carrier is activated carbon, comprising coconut shell carbon, coal carbon, nutshell carbon or silica gel. The non-mercuric catalyst can be prepared by the conventional impregnation method, has simple preparation method, is environment-friendly and has the advantages of less by-products, good stability and long service life. The conversion rate of acetylene can be 90-99% and the selectivity of vinyl chloride is not lower than 99%.
Owner:EAST CHINA UNIV OF SCI & TECH
Nanogold solution and method for detecting Co<2+> by using same
ActiveCN102416482AModification method is simpleGood repeatabilityMaterial nanotechnologyMaterial analysis by observing effect on chemical indicatorIon clustersPotassium borohydride
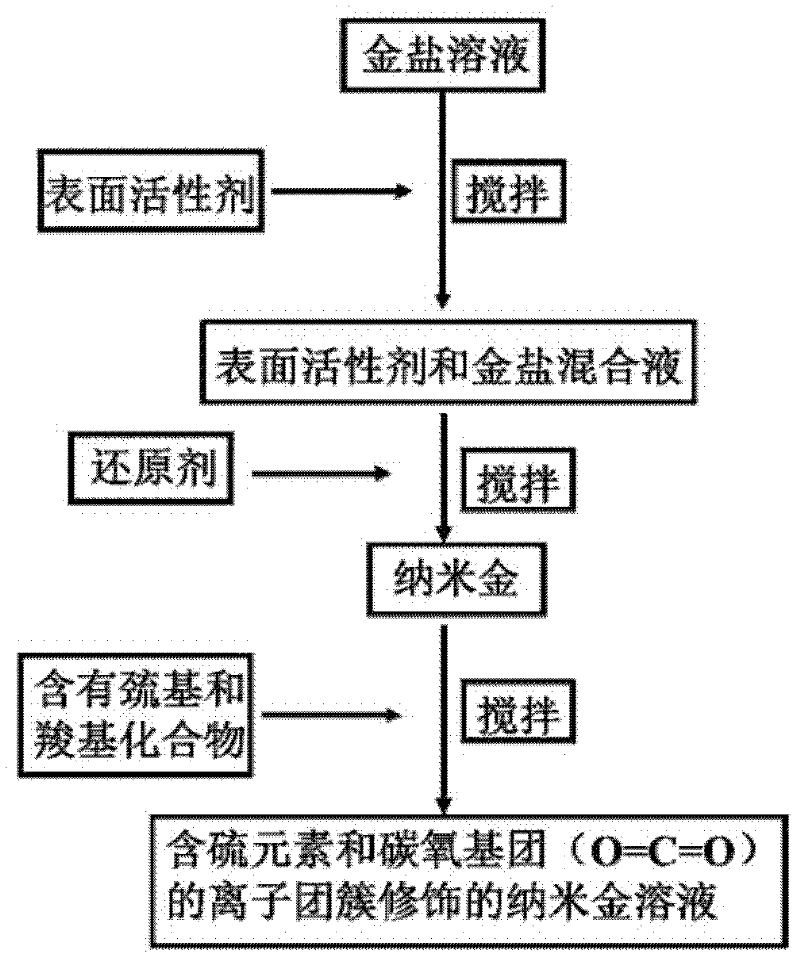
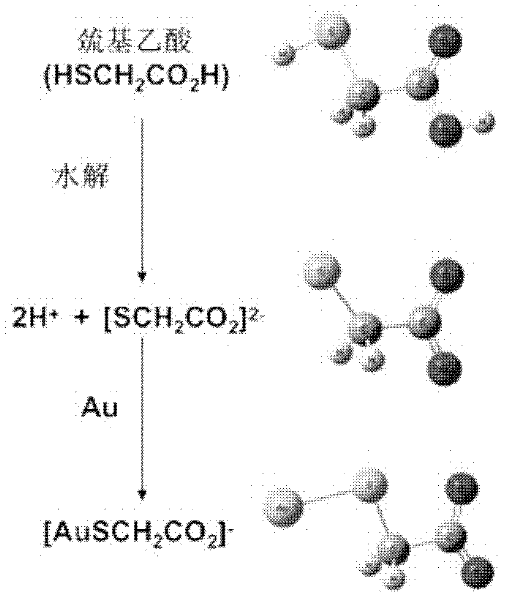
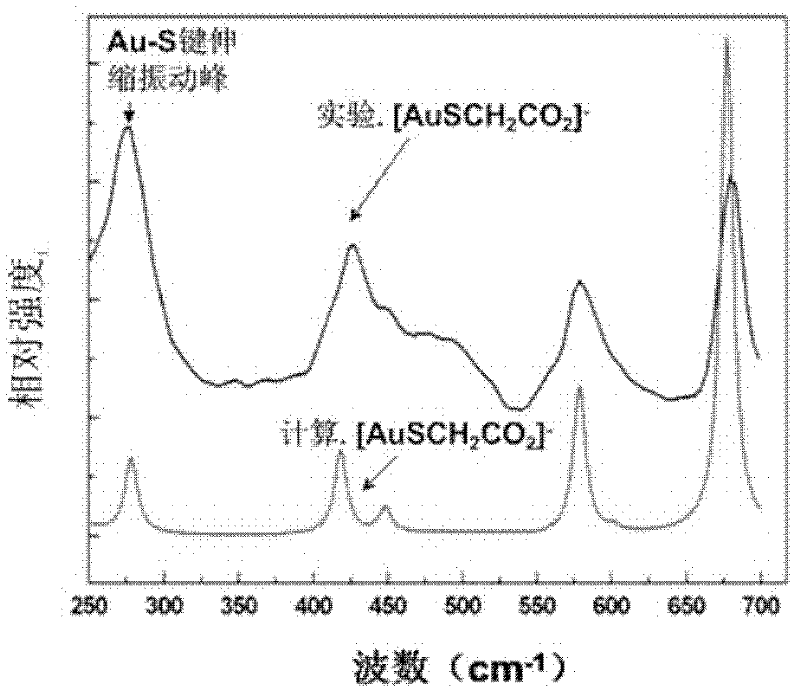
The invention discloses a nanogold solution and a method for detecting Co<2+> by using the same. The water soluble functional nanogold solution is prepared by using a gold salt as the resource of gold nanoparticles, a quaternary ammonium salt as a surfactant, ion clusters containing sulfur elements and carbon oxygen groups (O=C=O) as ligands, sodium borohydride, potassium borohydride or ascorbic acid as a reducing agent, and deionized water as a synthesis medium and by modifying the surface of the nanogold with the ion clusters containing sulfur elements and carbon oxygen groups (O=C=O) under the action of gold-sulfur bonds (Au-S). The nanogold solution has high speed, selectivity, sensitivity and practicality for detection of trace amount of Co<2+> in the water solution system, and therefore can be used in combination of a colorimetric process for detecting trace amount of Co<2+>; the detection concentration is lower than 8.5*10<-7>M; the detection threshold value may reach 5*10<-10>M; the detection speed is high, the selectivity is high, the cost is low, and the carrying is convenient; and the detection method has a bright application prospect in fields of environmental science, detection chemistry and analysis chemistry, and the like.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Gold potassium citrate for gold plating and preparation method thereof
InactiveCN101781784ALow toxicityPH value is stableLiquid/solution decomposition chemical coatingChemical compositionCITRATE ESTER
The invention relates to gold potassium citrate for gold plating and a preparation method thereof. The gold potassium citrate is used to substitute for gold potassium cyanide in acidic and neutral gold plating technology. The problem that the product coverage area is too wide when gold citrate is used in the gold plating technology is solved. The technical scheme is that the gold potassium citrate is the mixed crystal of gold potassium cyanide and citric acid potassium salt and the chemical composition of the gold potassium citrate is KAu (CN)2 (K3C6H5O7. k1C6H8O7) k2, wherein KAu (CN)2 is gold potassium cyanide, K3C6H5O7.k1C6H8O7 is citric acid potassium salt which is mixed by potassium citrate K3C6H5O7 and citric acid C6H8O7, k1 is the molar ratio of potassium citrate to citric acid, is more than or equal to zero and is less than or equal to three, and k2 is the molar ratio of citric acid potassium salt to gold potassium cyanide, is more than or equal to one point three and is less than five. The method is mainly suitable for the acidic and neutral gold plating technology.
Owner:张东山
Cyanide-free gold plating solution
The invention discloses a cyanide-free gold plating solution. The cyanide-free gold plating solution comprises sulphurous acid gold salt, a main complexing agent, an auxiliary complexing agent, conducting salt, a stabilizing agent and a pH buffering agent. Under the synthetic action of the sulphurous acid gold salt, the stabilizing agent and the other components, stable composite complex ions are formed through the cyanide-free gold plating solution, the plating solution is more stable, the storage period of the plating solution is long, and the sealed storage period at the normal temperature can reach over 6 months.
Owner:SHENZHEN RUN SUN CHEM TECH
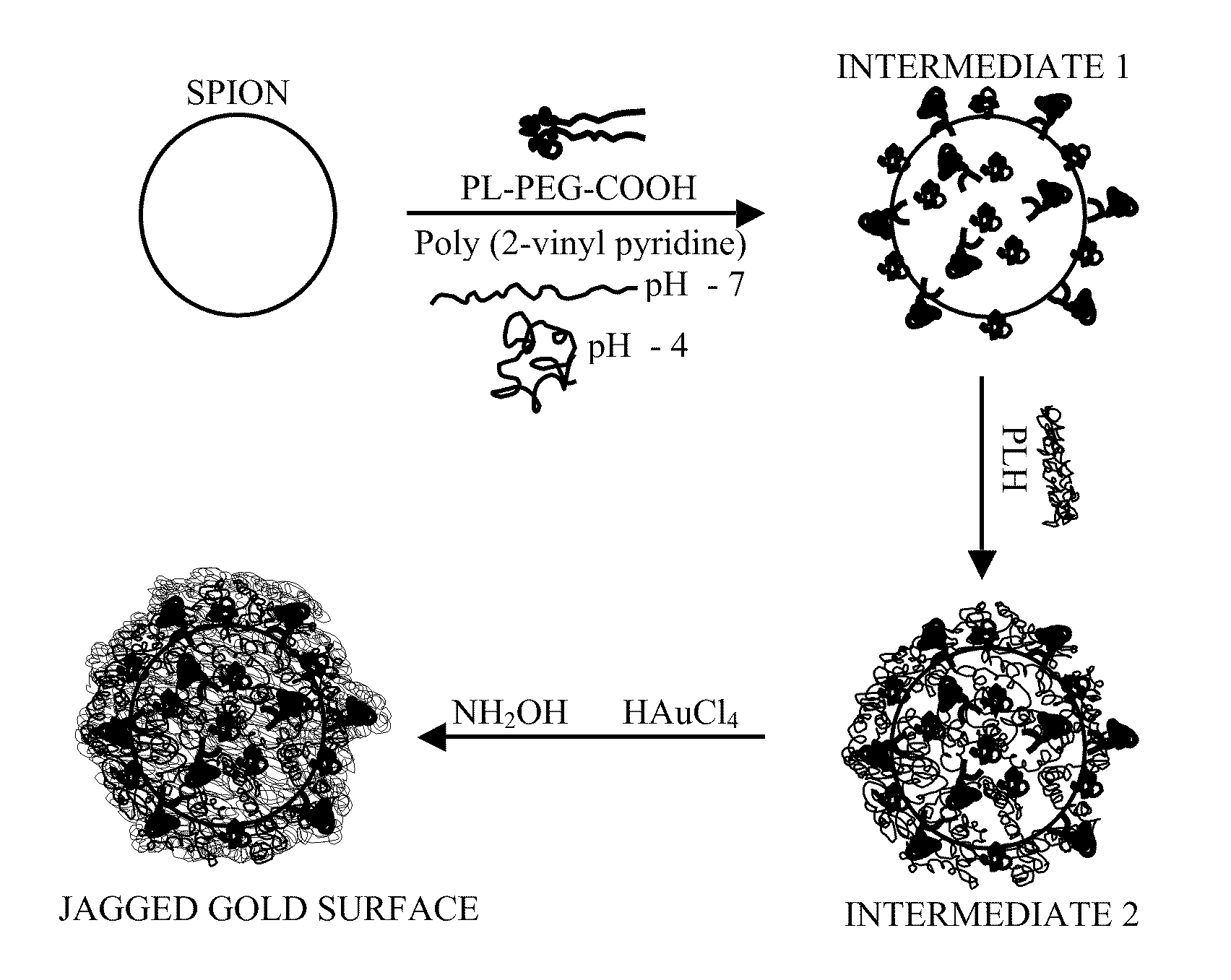
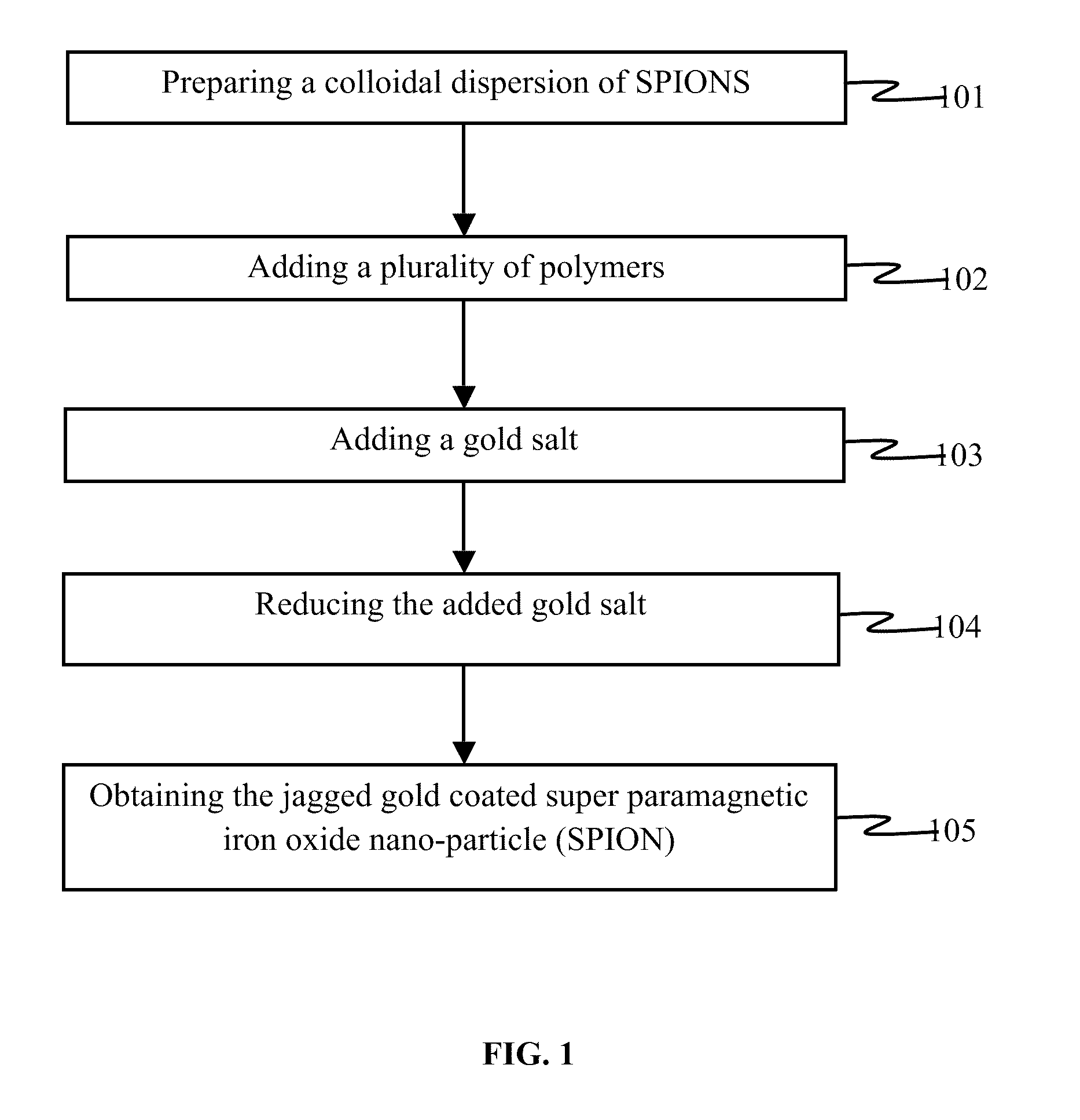
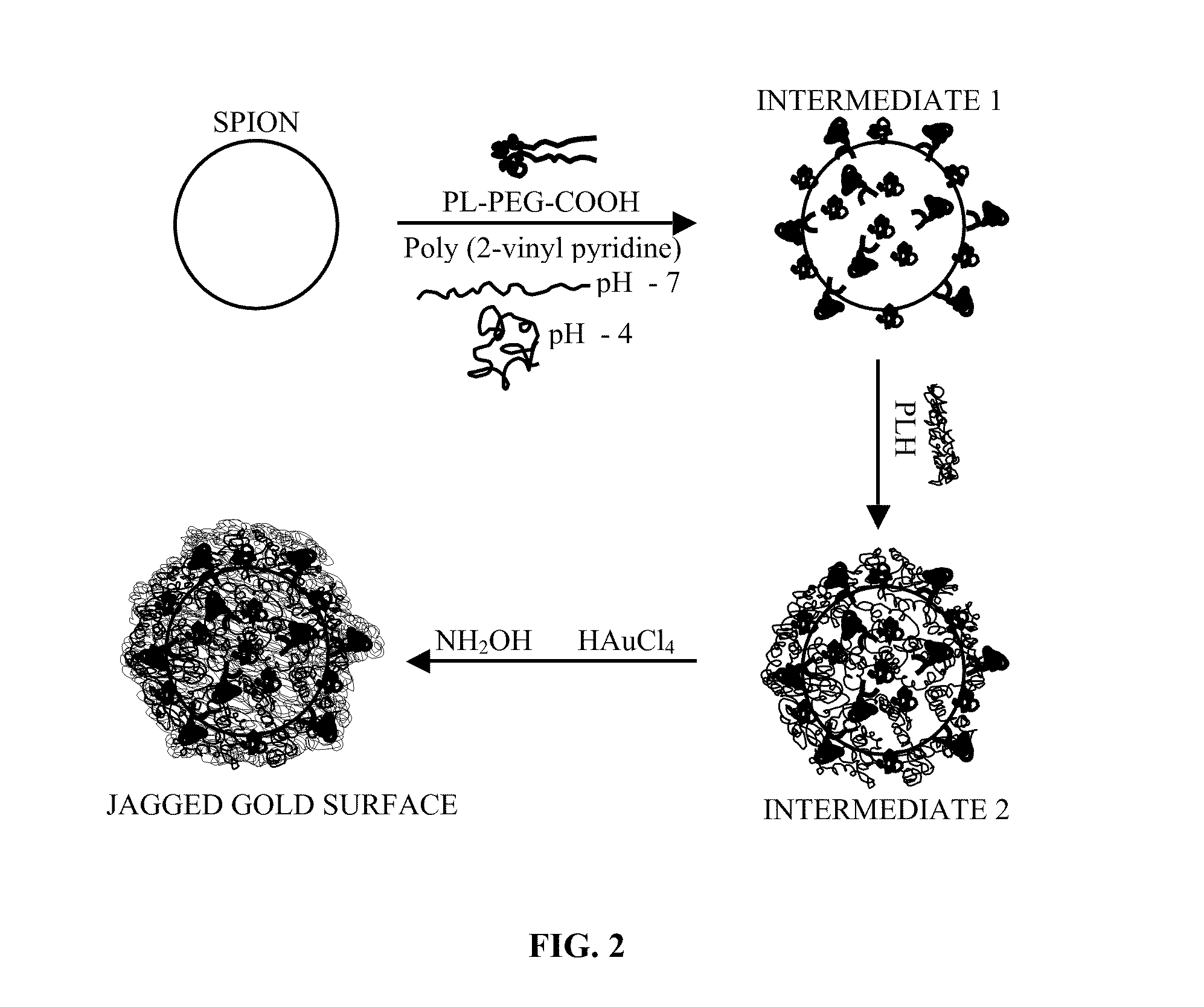
Gold coated super paramagnetic iron oxide nano-particles (spions) and a method of synthesizing the same
InactiveUS20110206619A1Non-uniform gapImprove abilitiesPowder deliveryMaterial nanotechnologySynthesis methodsPH-sensitive polymers
The various embodiments herein provide a gold coated SPIONs with jagged surface. The gold coated SPIONs have a core and a shell. The core is a SPION molecule and the shell is a jagged gold layer. A non-uniform polymeric gap exists between the core and the shell. The embodiments also provide a method of producing the jagged gold coated SPIONs by mixing a colloidal dispersion of SPIONs with pH sensitive polymers. Adding a gold salt to the above mixture and reducing the gold salt to form jagged gold coated SPIONs.
Owner:MAHMOUDI MORTEZA +1
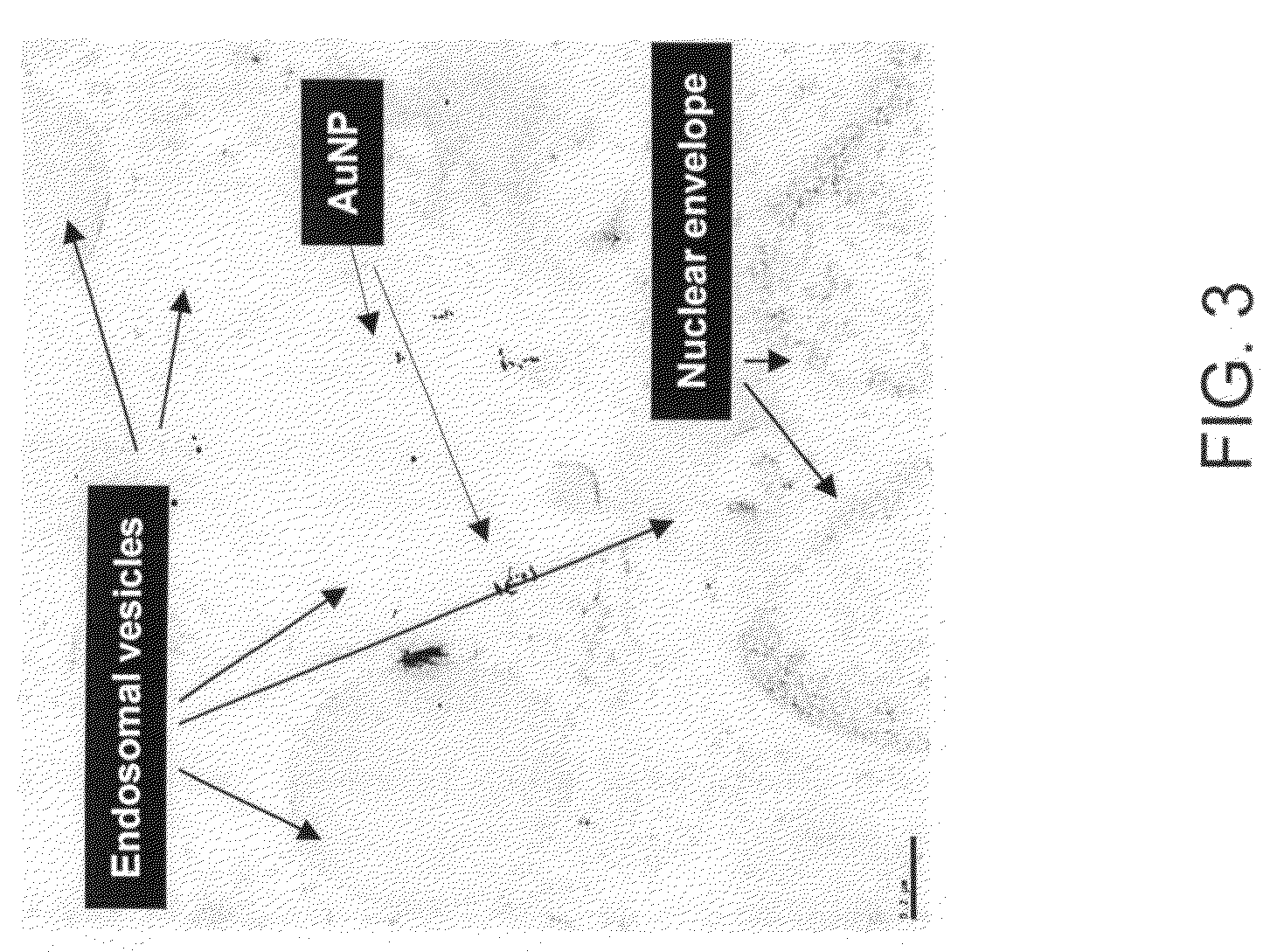
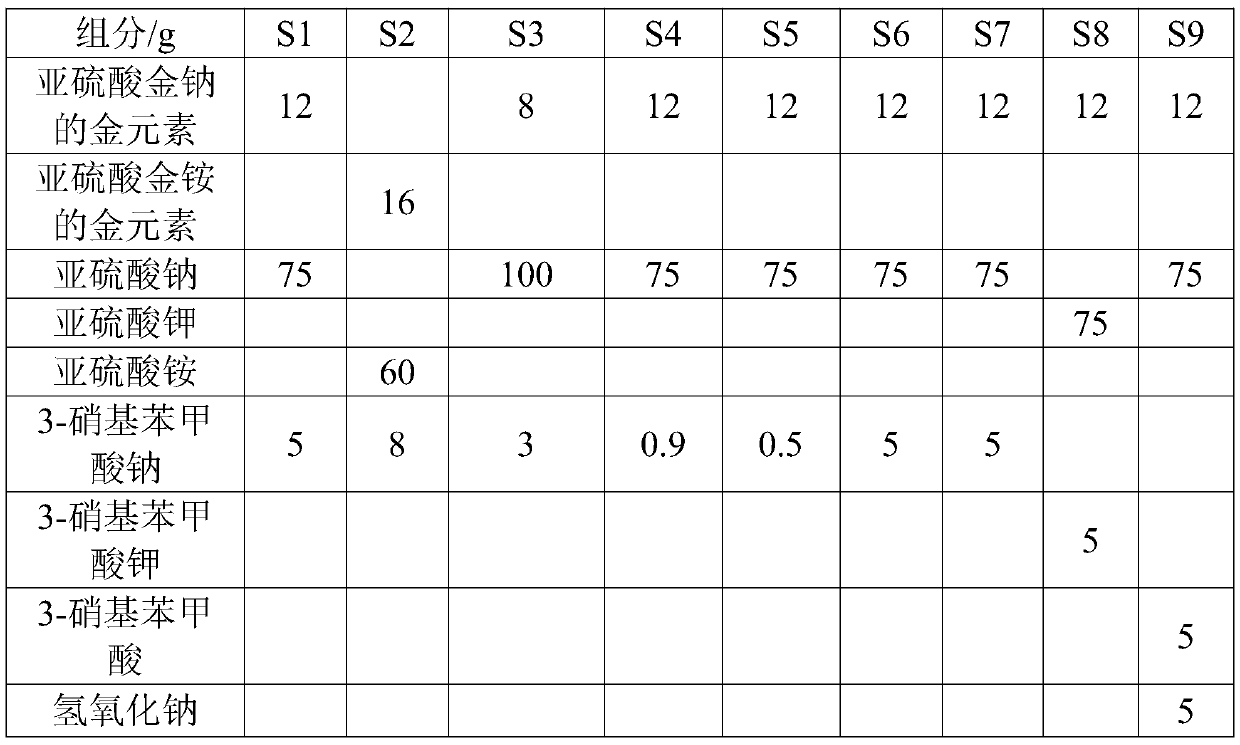
Stabilized, biocompatible gold nanoparticles and enviro-friendly method for making same
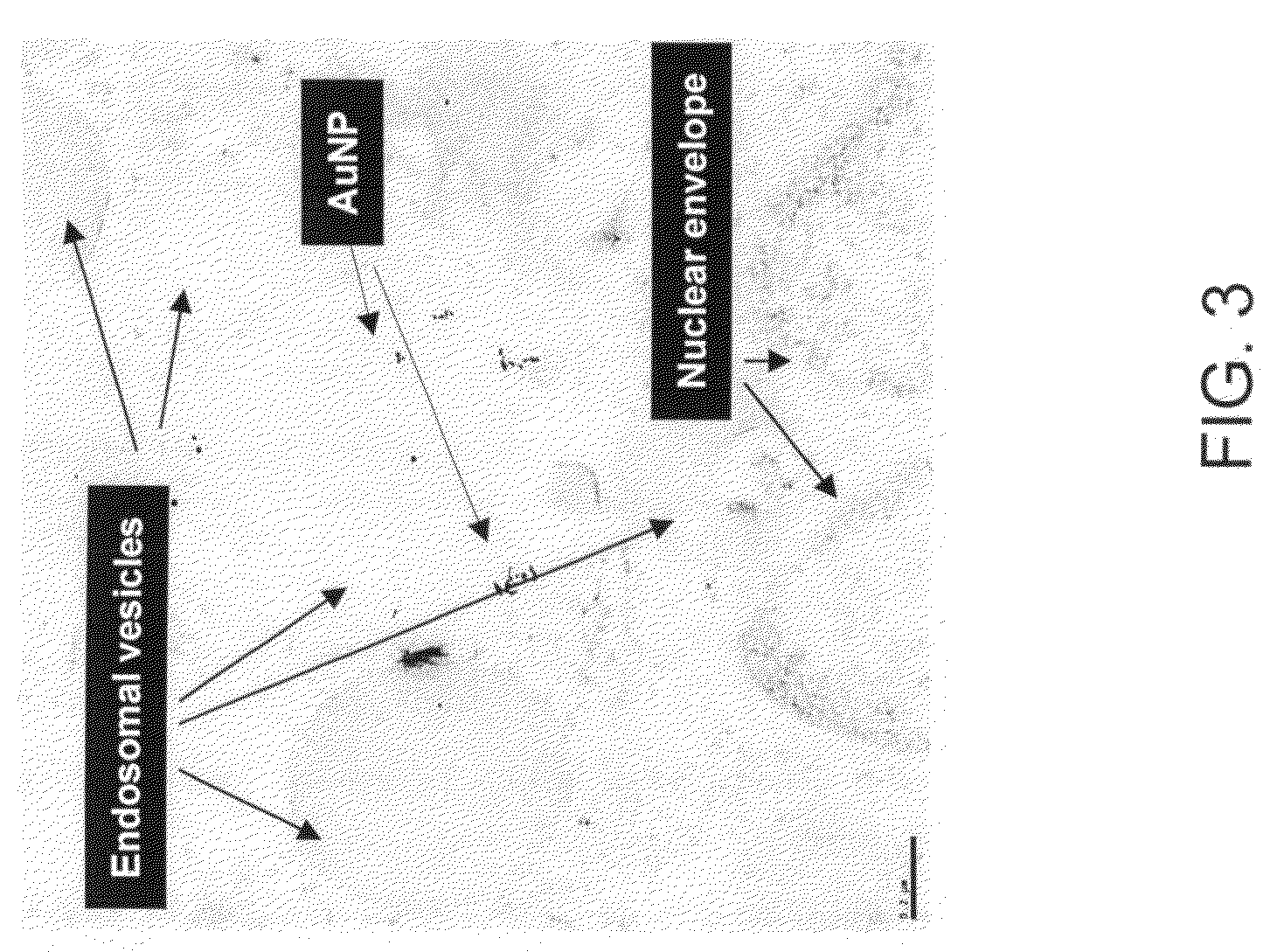
The invention provides stabilized, biocompatible gold nanoparticles that are stabilized with material from polyphenols- or flavanoids-rich plant material or reactive phytochemical components of the plant material. The gold nanoparticles of the invention can be fabricated with an environmentally friendly method for making biocompatible stabilized gold nanoparticles. In methods of the invention, an aqueous solution containing gold salts is mixed with polyphenols- or flavanoids-rich plant material. In preferred embodiment methods of making, an aqueous solution containing gold salts is provided. The aqueous solution is mixed with black tea, turmeric, curcumin or cinnamon or a similar naturally occurring polyphenols- or flavanoids-rich plant material. The gold salts react to form biocompatible gold nanoparticles that are stabilized with a coating of the polyphenols- or flavanoids-rich plant material. The black tea, turmeric, curcumin or cinnamon or similar naturally occurring polyphenols- or flavanoids-rich plant material can be a powder or can be in its root or bark form.
Owner:UNIVERSITY OF MISSOURI
Gold nanoparticle-halloysite nanotube and method of forming the same
InactiveUS20090092836A1Wider surface areaLiquid surface applicatorsGlass/slag layered productsHalloysiteNanoparticle
A gold nanoparticle-halloysite nanotube, on a surface of which a gold nanoparticle is formed, and a method for forming the same are disclosed. In order to form the gold nanoparticle on a surface of the halloysite nanotube, a gold salt is added to an agitated suspension solution. By the gold salt, a gold ion is formed on the surface of the halloysite nanotube. If the reducing agent is added to the halloysite nanotube on which the gold ion is formed, the gold ion is reduced into the gold nanoparticles. The formed gold nanoparticle has the very small size, and distributed on the surface of the halloysite nanotube. Accordingly, without the separate protective agent or the surface reformation, the gold nanoparticle may be easily formed.
Owner:GWANGJU INST OF SCI & TECH
Soy or lentil stabilized gold nanoparticles and method for making same
The invention provides stabilized, biocompatible gold nanoparticles that are stabilized with material from soy or lentil plant material or a reactive extract thereof of the plant material. The gold nanoparticles of the invention can be fabricated with an environmentally friendly method for making biocompatible stabilized gold nanoparticles. In methods of the invention, an aqueous solution containing gold salts is mixed with soy or lentil plant material or a reactive extract thereof. In preferred embodiment methods of making, an aqueous solution containing gold salts is provided. The aqueous solution is mixed with soy or lentil plant material or a reactive extract thereof. The gold salts react to form biocompatible gold nanoparticles that are stabilized with a robust coating derived of the soy or lentil plant material or a reactive extract thereof.
Owner:UNIVERSITY OF MISSOURI
Method for producing metal nanoparticles
InactiveUS20090239280A1High reliability of resultsLow costAntibacterial agentsBiocidePlatinumColloidal nanoparticles
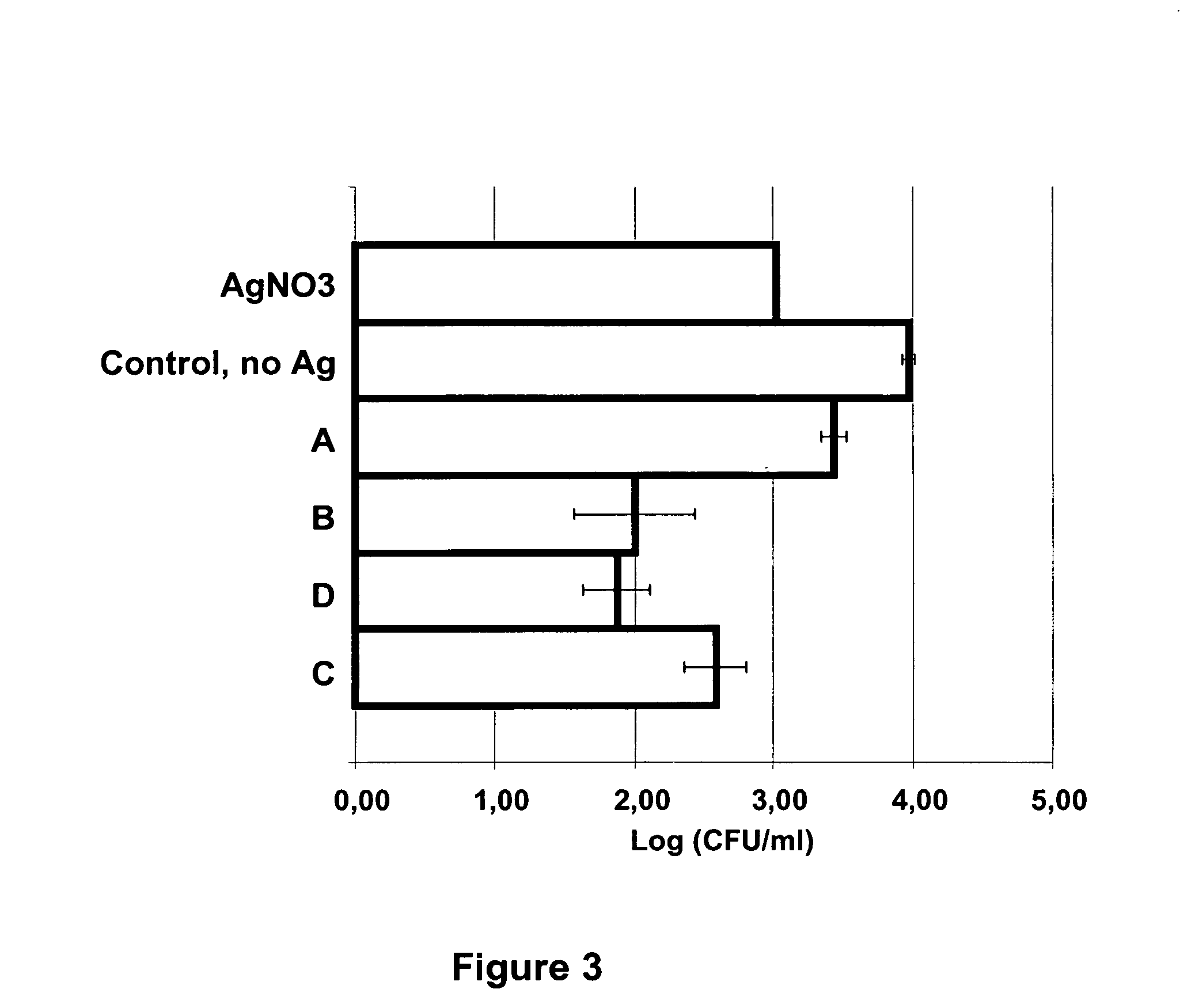
This invention provides a method for producing a composition comprising colloidal nanoparticles of metals including silver, gold, zinc, mercury, copper, palladium, platinum, or bismuth, by contacting a metal or metal compound with bacteria. An embodiment of the method comprises a step of incubating probiotic bacteria with an aqueous solution comprising at least 4 mM of a silver or gold salt. A resulting nanosilver-containing composition is useful as a highly efficient antimicrobial agent, for instance when impregnated onto a carrier, or an algicide agent or a herbicide agent.
Owner:JANSSEN PHARMA NV
Gold potassium lemon acid for gold plating and method for producing the same
ActiveCN101172946BReduce processing costsReduce pollutionCarboxylic acid salt preparationGold contentPotassium
The invention discloses citric acid gold potassium used for gold plating and the preparation method thereof. The molecular formular of the citric acid gold potassium is K3Au2C9H5O7N2. The method comprises the steps as follows: gold trichloride is dissolved in water of certain temperature, is concentrated and diluted under the temperature for multiple times, and is reacted with potassium citrate, ethylene diamine tetraacetic acid and malononitrile in a reactor under the certain temperature and the certain time to produce the organic gold salt which has low toxicity and contains no free cyanogen. When the product has the same gold content with the potassium gold cyanide, the total CN- is 5 to 6 percent, being 50 percent lower than that of the CN- in the potassium gold cyanide. The waste liquid after gold plating contains free the CN- in small amount. The invention reduces the cost of waste water treatment for the electroplating plants.
Owner:HENGSHENG TECH R&D CO LTD
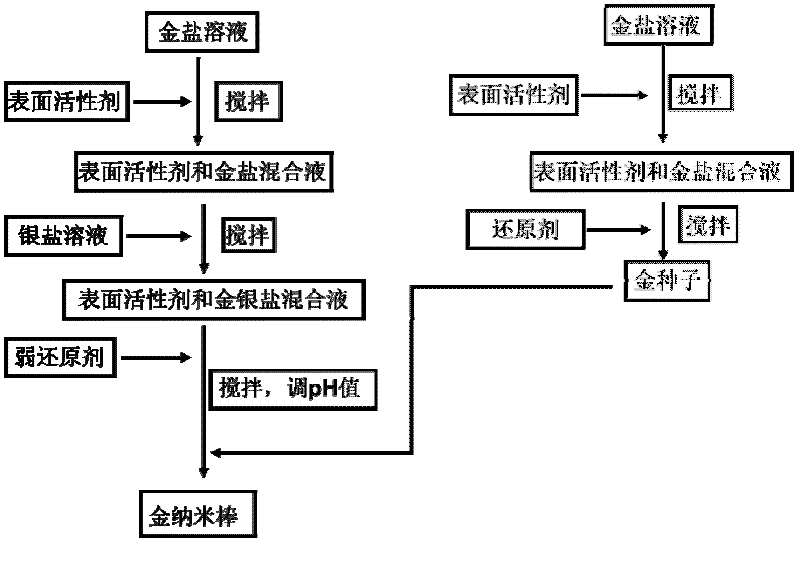
Preparation method of nanometer gold bar mainly made of (200) crystal face
The invention discloses a preparation method of a nanometer gold bar mainly made of (200) crystal face. The method is: adding certain amount of surface active agent, silver salt solution and weak reductant into gold salt solution so as to synthesize growth solution, thus while gold salt is under protection of surface active agent, Au+ or Au3+ is reduced to gold atom by the weak reductant in the growth solution; then adding gold seed into the growth solution, wherein the gold seed is synthesized by adding certain amount of surface active agent and reductant into the gold salt solution, under the induction of silver ion, the surface active agent carrying gold atoms is absorbed on the gold seed which is taken as a growing point, so that a nanometer gold bar mainly made of (200) crystal face is obtained. The method needs temperate condition and is easy to operate, capable of producing nanometer gold bar mainly made of (200) crystal face with controllable shape and size, good water-solubility and sound dispersibility with a high yield. It can be applied into fields of biological analysis, biomedical diagnosis and imaging, biomedical cure, environmental science and analytical science.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
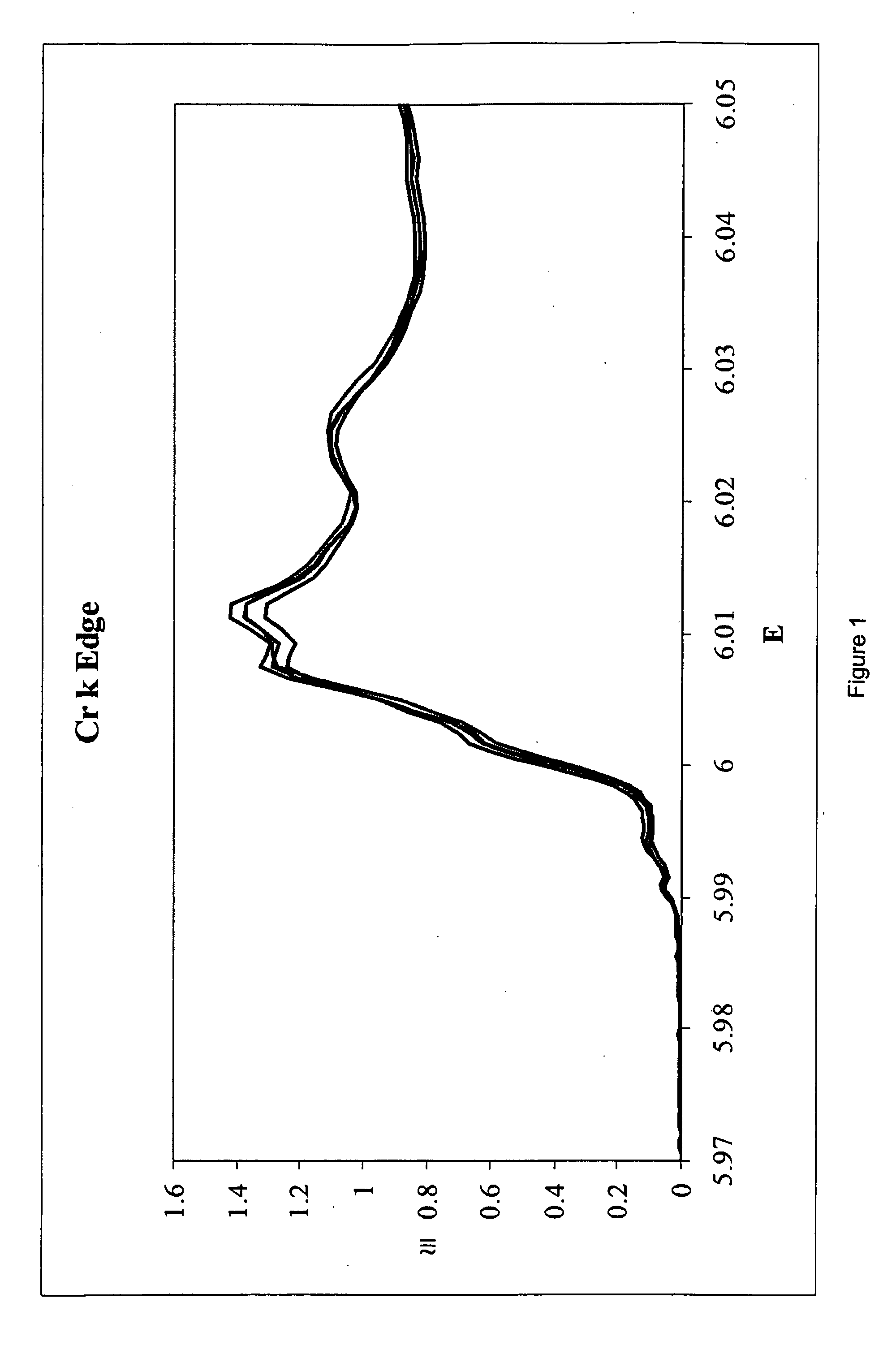
Compositions containing chromium, oxygen and gold, their preparation, and their use as catalysts and catalyst precursors
InactiveUS20080207964A1Preparation by dehalogenationPreparation by hydrogen halide split-offGold contentOxygen
A catalyst composition is disclosed that includes chromium, oxygen, and gold as essential constituent elements. The amount of gold in the composition is from about 0.05 atom % to about 10 atom % based on the total amount of chromium and gold. Also disclosed is a process for changing the fluorine distribution (i.e., content and / or arrangement) in a hydrocarbon or halogenated hydrocarbon in the presence of the catalyst composition; and methods for preparing said catalyst composition. One preparation method involves; (a) co-precipitating a solid by adding ammonium hydroxide (aqueous ammonia) to an aqueous solution of a soluble gold salt and a soluble chromium salt that contains at least three moles of nitrate per mole of chromium in the solution and has a gold content of from about 0.05 atom % to about 10 atom % of the total content of gold and chromium in the solution to form an aqueous mixture containing co-precipitated solid; (b) drying the co-precipitated solid formed in (a); and (c) calcining the dried solid formed in (b) in an atmosphere containing at least 10% oxygen by volume. Another preparation method involves (a) impregnating solid chromium oxide with a solution of a soluble gold salt, (b) drying the impregnated chromium oxide prepared in (a); and optionally, (c) calcining the dried solid. A third preparation method involves (a) evaporating an aqueous solution of chromium(VI) oxide and a soluble gold salt to form a solid; (b) drying the solid formed in (a); and (c) calcining the dried solid formed in (b) in an atmosphere containing at least 10% oxygen by volume.
Owner:EI DU PONT DE NEMOURS & CO
Manufacturing method of chemical nickel palladium gold plating plated with thick palladium
InactiveCN104419916AReduce manufacturing costHigh hardnessLiquid/solution decomposition chemical coatingEtchingChemical plating
The invention relates to the technical field of chemical nickel palladium gold platings, and discloses a manufacturing method of a chemical nickel palladium gold plating plated with thick palladium. The method is used for forming a nickel palladium gold plating on a surface of a circuit board, and comprises the following steps: 1) acidic oil removing; 2) micro-etching; 3) acid washing; 4) acid pre-pickling; 5) palladium activation; 6) acid post-pickling; 7) chemical nickel plating; 8) chemical plating of thick palladium, that is, chemical deposition of a palladium layer on a nickel layer of a circuit board by using organic acid and palladous sulfate; 9) chemical gold dipping. The palladium layer is formed on the nickel layer by using organic acid and palladous sulfate. The palladium layer is a palladium-storing system, and has a high thickness, so only a thin gold layer is needed to be formed on the palladium layer. Therefore, the production cost of the plating is reduced, the use of gold salt with severe toxicity is reduced, and the safety is greatly improved; figure 2 shows the surface nanometer morphology of the palladium layer; the palladium layer has low hardness and internal stress, and has excellent wetting performance; therefore, the gold wire bonding performance and the tin soldering performance of the plating are excellent.
Owner:SHENZHEN SUNTAK MULTILAYER PCB
Gold potassium lemon acid for gold plating and method for producing the same
ActiveCN101172946AReduce processing costsReduce pollutionCarboxylic acid salt preparationGold contentPotassium
The invention discloses citric acid gold potassium used for gold plating and the preparation method thereof. The molecular formular of the citric acid gold potassium is K3Au2C9H5O7N2. The method comprises the steps as follows: gold trichloride is dissolved in water of certain temperature, is concentrated and diluted under the temperature for multiple times, and is reacted with potassium citrate, ethylene diamine tetraacetic acid and malononitrile in a reactor under the certain temperature and the certain time to produce the organic gold salt which has low toxicity and contains no free cyanogen. When the product has the same gold content with the potassium gold cyanide, the total CN- is 5 to 6 percent, being 50 percent lower than that of the CN- in the potassium gold cyanide. The waste liquid after gold plating contains free the CN- in small amount. The invention reduces the cost of waste water treatment for the electroplating plants.
Owner:HENGSHENG TECH R&D CO LTD
Cathode electrode composite material and preparation method thereof and electrochemical device applying same
InactiveCN102214822ASimple processReduce manufacturing costAlkaline accumulator electrodesNickel accumulatorsIndiumNickel salt
The invention discloses a cathode electrode composite material, which comprises a plurality of ferriferous oxide particles and a conductive aid which is selected from copper, cobalt, nickel, tin, antimony, bismuth, indium, silver, gold, lead, cadmium, carbon black, graphite, cupric salt, cobalt salt, nickel salt, tin salt, antimonic salt, bismuth salt, indium salt, silver salt, gold salt, lead salt, cadmium salt, cupric hydroxide, cobalt hydroxide, nickel hydroxide, tin hydroxide, stibine hydroxide, bismuth hydroxide, indium hydroxide, silver hydroxide, gold hydroxide, lead hydroxide, cadmium hydroxide and a group consisting of the combinations. When applied to an electrochemical device, the cathode electrode composite material can show high charging and discharging characteristics and high electric capacity. Moreover, the invention provides a preparation method of the cathode electrode composite material and an electrochemical device applying the same.
Owner:NATIONAL TSING HUA UNIVERSITY
Reduced-form composite complexing non-cyanide chemical gold plating liquid and method
InactiveCN105349972AHigh bath stabilityLiquid/solution decomposition chemical coatingCyanideWeather resistance
The invention discloses reduced-form composite complexing non-cyanide chemical gold plating liquid and a method. The reduced-form composite complexing non-cyanide chemical gold plating liquid is characterized by being preparing from 0.2-10 g / L of non-cyanide gold salt, 10-100 g / L of composition complexing agent, 5-30 g / L of reducing agent, 5-30 g / L of buffering agent and 0.01-10 g / L of stabilizing agent. The composition complexing agent used in the liquid can improve the stability of gold ions and enables a plated layer to be compact and smooth; and the reduced-form composite complexing non-cyanide chemical gold plating liquid has the beneficial effects that process stability is good, a long continuous production cycle is achieved, and the plated layer is bright in color, good in corrosion resistance and weather resistance and the like.
Owner:广东致卓环保科技有限公司
Process for production of colloidal gold and colloidal gold
ActiveUS20100159471A1Suitable for useSharp particle size distributionMaterial nanotechnologyTransportation and packagingCITRATE ESTERPrill
It is an object of the present invention to provide a method for producing gold colloid having a targeted particle size, a sharp particle size distribution and a uniform and perfect spherical shape. The present invention relates to a method for producing gold colloid including a nucleation step of forming nuclear colloidal particles by adding a first reducing agent to a first gold salt solution; and a growth step of growing nuclear colloid by adding a second gold salt and a second reducing agent to the solution of the nuclear colloidal particles, characterized in that the growth step is performed at least once; a citrate is used as the first reducing agent and an ascorbate is used as the second reducing agent; and the addition of the ascorbate in the growth step is performed simultaneously with addition of the second gold salt. According to the method for producing gold colloid of the present invention, gold colloid having a sharp particle size distribution and a uniform and perfect spherical shape can be obtained.
Owner:TANAKA PRECIOUS METAL IND
Preparation of palladium-gold catalysts
ActiveUS20070179310A1Improve activity stabilityHigh catalytic activityOrganic compound preparationCarboxylic acid esters preparationAmmonium compoundsPalladium
A new method for preparing supported palladium-gold catalysts is disclosed. The method comprises sulfating a titanium dioxide support, calcining the sulfated support, impregnating the calcined support with a palladium salt, a gold salt, and an alkali metal or ammonium compound, calcining the impregnated support, and reducing the calcined support. The resultant supported palladium-gold catalysts have increased activity and stability in the acetoxylation.
Owner:LYONDELLBASELL ACETYLS
Method for producing nano-silver powder or nano-gold powder
The invention relates to a method for preparing nano-silver powders or nano-gold powders. In a single cell, metallic silver or an gold electrode is processed through anodic oxidation, inert conducting material is used as a cathode, room-temperature ionic liquid containing silver salt or gold salt is used as electrolysing solution, the electrolysis is executed under direct current, and the nano-silver powders or the nano-gold powders are obtained around the cathode; wherein, the silver salt is AgBF4; the gold salt is Au (PPh3) Cl or Au (PPh3) Br; the room-temperature ionic liquid is one of [bmim]BF4, [bmim]PF6, [emim]BF4, and [hmim]BF4. In the method, the metallic silver or gold is used as a sacrificial anode, the nano-silver powders and the nano-gold powders can be produced directly through electrolysis, the technology is simple, the cost is low, and the method is suitable for industrialized production. The room-temperature ionic liquid is used as an electrolytic dissolvent, the ionic liquid performs a role of not only a dissolvent but also a dispersant and a stabilizing agent, and nano-metal is easy to obtain, compared with water solution; compared with organic solvent, the room-temperature ionic liquid is safer and more environment-protecting.
Owner:SUZHOU UNIV +1
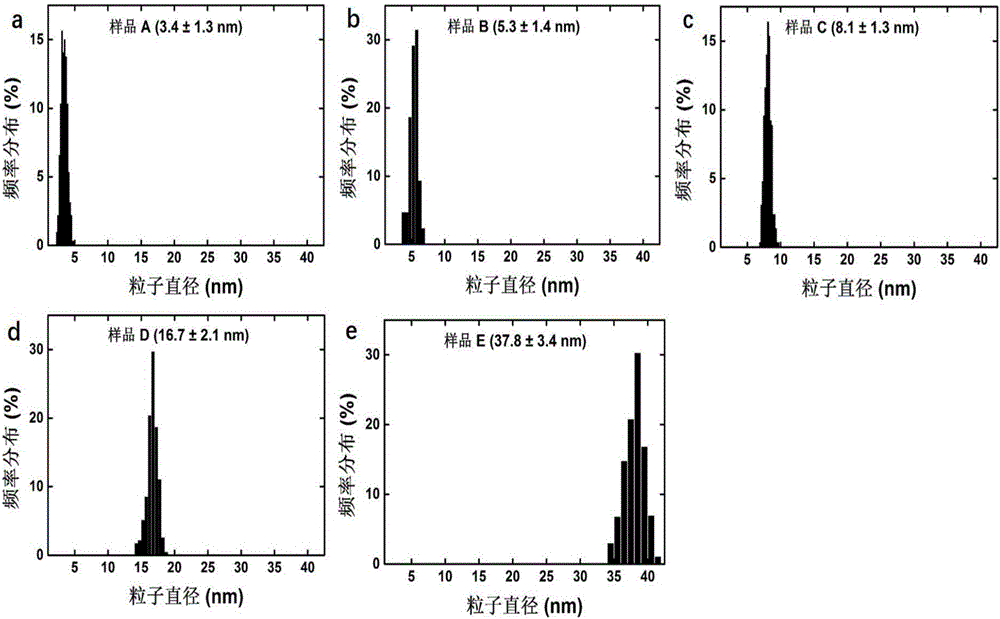
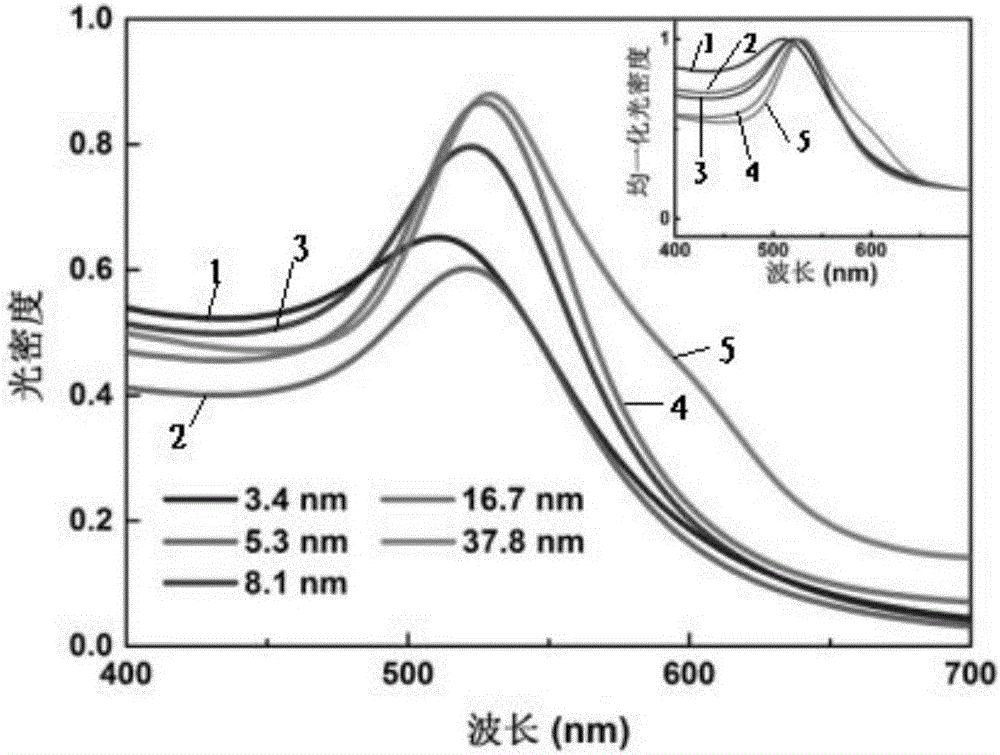
Preparation method and application of multi-sized monodisperse gold nanoparticles
The invention provides a preparation method of multi-sized monodisperse gold nanoparticles. The preparation method of the multi-sized monodisperse gold nanoparticles includes the steps of (1) subjecting gold salt to a reduction reaction in water under the joint action of citrate and a first reducing agent to obtain seed crystals of the gold nanoparticles; (2) mixing chloroauric acid, citrate and a surfactant with water to obtain seed crystal growth liquid; (3) subjecting the seed crystals of the gold nanoparticles to iterative growth in the seed crystal growth liquid under the action of a second reducing agent so as to obtain the multi-sized gold nanoparticles. The preparation method and application of the multi-sized monodisperse gold nanoparticles have the advantages that with the surfactant coating the different particle diameters of monodisperse gold nanoparticles evenly during gradual growth, particle agglomeration is improved, and the problems that the gold nanoparticles with large particle diameters are widely dispersed and poor in monodispersity are solved, so that the gold nanoparticles can give full play to a photo-thermal transformation performance under a light condition.
Owner:江苏台益纳米科技有限公司
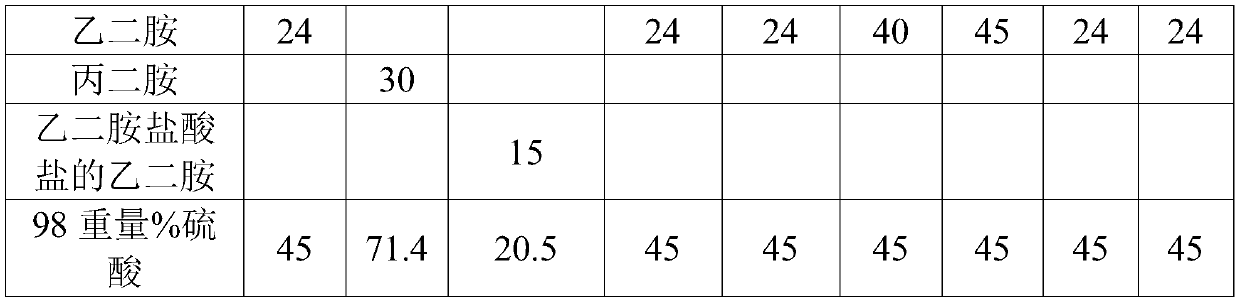
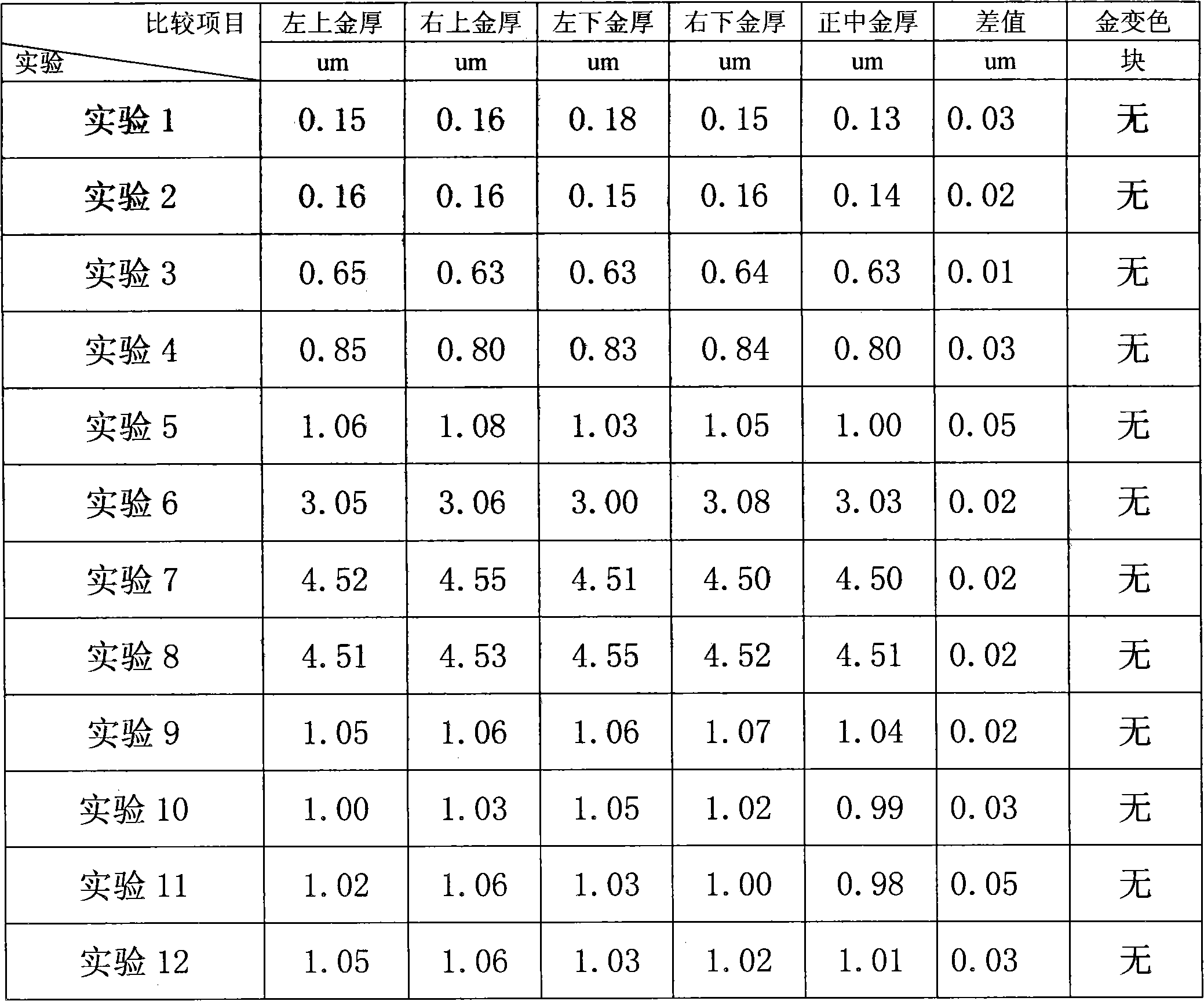
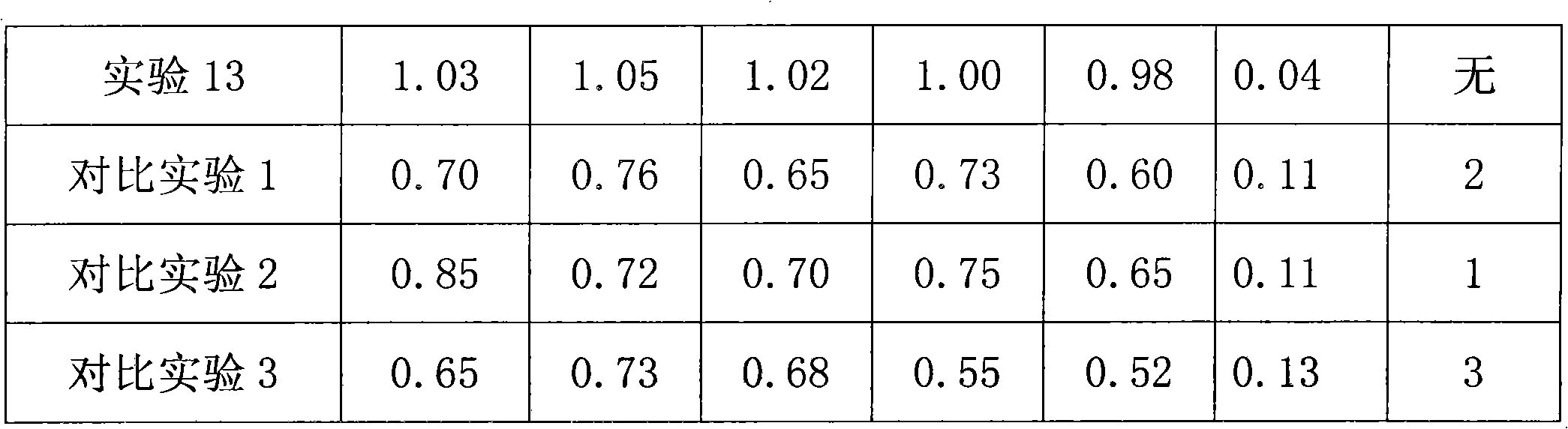
Organic amine system non-cyanide electroplating gold plating bath and method
The invention discloses an organic amine system non-cyanide electroplating gold plating bath and method. The organic amine system non-cyanide electroplating gold plating bath is characterized in that the raw materials of the plating bath comprise: gold salt of 1-20 g / L, an organic amine coordinating agent of 20-200 g / L, conducting salt of 50-150 g / L, and additives of 0.01-10 g / L, wherein the organic amine type coordinating agent is compounded a from varied components of hydramine, acidamide and nitrogen containing heterocycles. According to the organic amine system non-cyanide electroplating gold plating bath disclosed by the invention, through the compound use of the organic amine type complexing agent, the stability of the plating bath can achieve the stability of a cyanide plating bath, and a gold plating film processed by the method is compact, good in binding force, and excellent in welding property.
Owner:广东致卓环保科技有限公司
Composite material for negative electrode, method for fabricating the same and electrochemical device using the same
ActiveUS20110236747A1Large capacityLarge scaleSilver accumulatorsNon-metal conductorsIndiumNickel salt
The present invention relates to a composite material for a negative electrode, including: a plurality of iron oxide particles; and a conductivity improver, which is selected form the group consisting of copper, cobalt, nickel, tin, antimony, bismuth, indium, silver, gold, lead, cadmium, carbon black, graphite, copper salt, cobalt salt, nickel salt, tin salt, antimony salt, bismuth salt, indium salt, silver salt, gold salt, lead salt, cadmium salt, copper hydroxide, cobalt hydroxide, nickel hydroxide, stannic hydroxide, antimony hydroxide, bismuth hydroxide, indium hydroxide, silver hydroxide, gold hydroxide, lead hydroxide, cadmium hydroxide and the combination thereof. In the case of applying the composite material for a negative electrode according to the present invention in an electrochemical device, the improved charge / discharge characteristics and high capacity can be achieved. In addition, the present invention further provides a method for fabricating the above-mentioned composite material for a negative electrode and an electrochemical device using the same.
Owner:NATIONAL TSING HUA UNIVERSITY
Method of producing gold nanoparticle
A method for producing gold nanoparticles is disclosed. When gold salt solution is mixed with an absorbent, gold in the form of complexes is adsorbed onto the surface of the absorbent. The gold-loaded absorbent, after being separated from the solution by screening, filtration, settling or other methods, is ashed to form ashes. The ashes contain gold nanoparticles and impurities such as oxides of sodium, potassium and calcium. The impurities can be removed by dissolution using dilute acids. The relatively pure gold nanoparticles are obtained after the impurities are removed. Activated carbon or gold-adsorbing resin can be used as the absorbent. Silver or platinum group metal nanoparticles can also be produced by this method.
Owner:LIN HSING KUANG +1
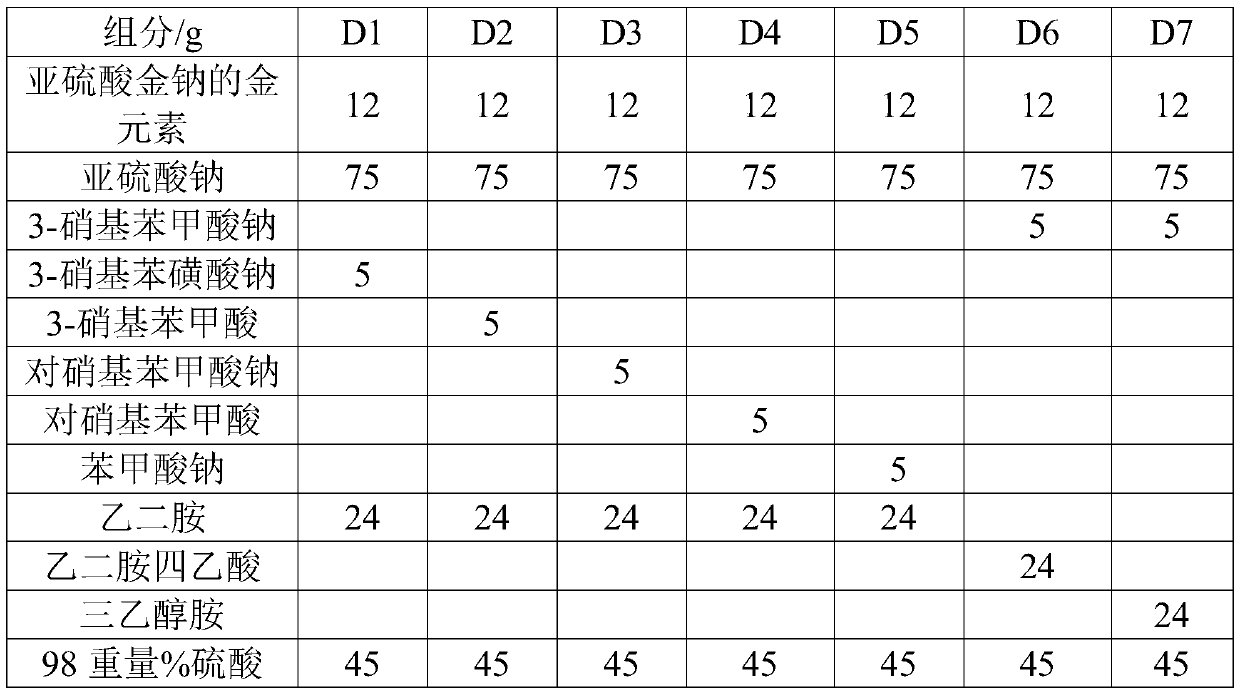
Preparation method of cyanide-free gold plating reagent sodium gold sulfite
ActiveCN105568269ASimple processProcess safetyGold compoundsLiquid/solution decomposition chemical coatingDissolutionIon
The invention relates to a preparation method of a gold salt for gold plating, particularly a preparation method of a cyanide-free gold plating reagent sodium gold sulfite, belonging to the technical field of chemistry. The preparation method comprises the following steps: gold rolling, gold bullion cleaning, gold dissolution, nitre removal, solonization, solonization, cleaning, complexing reaction, and crystallization by concentration. The method has the advantages of simple technique and high safety, can not generate dangerous intermediates in the process, can effectively lower chlorine ions, and can be used for producing the odorless green environment-friendly product.
Owner:烟台招金励福贵金属股份有限公司
Stabilized, biocompatible gold nanoparticles and enviro-friendly method for making same
Owner:UNIVERSITY OF MISSOURI
Non-cyanide gold plating bath and preparation method and application thereof
The invention relates to the field of gold plating baths, in particular to a non-cyanide gold plating bath and a preparation method and application thereof. The gold plating bath comprises a sulphurous acid gold salt, sulfite, 3-nitrobenzoic acid salt, organic amine and acid. The non-cyanide gold bath has the advantages that the plating bath stability is good, and the current efficiency stabilitywithin a certain current density range is high.
Owner:SHENZHEN UNITED BLUEOCEAN TECH DEV
New technology for plating golden yellow on automotive hub
The invention discloses new technology for plating golden yellow on an automotive hub, which comprises the technical steps: galvanizing, pre-plating copper, brightly plating copper, plating nickel, plating golden yellow, and the like. The chemical composition formula contained in each L of plating solution is that: galvanizing solution comprises 220 to 240g of potassium chloride, 40 to 55g of zinc chloride, 30 to 35g of boric acid, and 15 to 25ml of brightener; copper pre-plating solution comprises 70 to 85g of copper phosphate, 280 to 350g of phosphor copper potassium, 4 to 6ml of ammonia water, and 3 to 5ml of brightener; the bright copper plating solution comprises 200 to 220g of copper sulfate, 70 to 90g of sulfuric acid, 0.06 to 0.08g of chloride ion, and 4 to 8ml of brightener; nickel plating solution comprises 260 to 300g of nickel sulfate, 30 to 50g of nickel chloride, 30 to 40g of boric acid, 0.6 to 1ml of brightener, 8 to 12ml of softening agent, and 2 to 4ml of wetting agent; and golden yellow plating solution comprises 120 to 140g of imitation gold salt and 2 to 4ml of ammonia water. After the plating, water washing and drying are performed, and a polyurethane paint is sprayed on the surface of the automotive hub. The new technology for plating golden yellow on the automotive hub radically eliminates the damage and environmental pollution of sodium cyanide and chromic anhydride to human bodies; the plated golden yellow automotive hub is luxurious and attractive, has strong corrosion resistance, and is suitable for high-class automotive assembly.
Owner:梁新中
Method for producing gold colloid and gold colloid
ActiveUS8048193B2Control concentrationSuitable for useMaterial nanotechnologyOther chemical processesGold ColloidCITRATE ESTER
It is an object of the present invention to provide a method for producing gold colloid having a targeted particle size, a sharp particle size distribution and a uniform and perfect spherical shape. The present invention relates to a method for producing gold colloid including a nucleation step of forming nuclear colloidal particles by adding a first reducing agent to a first gold salt solution; and a growth step of growing nuclear colloid by adding a second gold salt and a second reducing agent to the solution of the nuclear colloidal particles, characterized in that the growth step is performed at least once; a citrate is used as the first reducing agent and an ascorbate is used as the second reducing agent; and the addition of the ascorbate in the growth step is performed simultaneously with addition of the second gold salt. According to the method for producing gold colloid of the present invention, gold colloid having a sharp particle size distribution and a uniform and perfect spherical shape can be obtained.
Owner:TANAKA PRECIOUS METAL IND
Gold plating solution for plating gold finger on circuit board
The invention discloses a gold plating solution for plating a gold finger on a circuit board, belonging to the filed of plating. The gold plating solution comprises gold salt the gold content of which is 1g / l to 30g / l, conductive salt, buffer solution, compounding agent, metallic brightener, organic brightener and wetting agent. The gold finger plated by the gold plating solution disclosed by the invention has the advantages of even and compact clad layer and gold solution conservation, and the plated gold is not easy to change color.
Owner:秦雅军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com