Method for preparing 1,2-pentanediol by furfuryl alcohol liquid phase selectivity and hydrogenolysis
A technology for pentanediol and furfuryl alcohol, applied in the field of selective hydrogenolysis of furfuryl alcohol to prepare 1,2-pentanediol, can solve the problems of industrial production limitation, large environmental pollution, poor results, etc., achieve excellent catalytic effect and reduce energy consumption , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
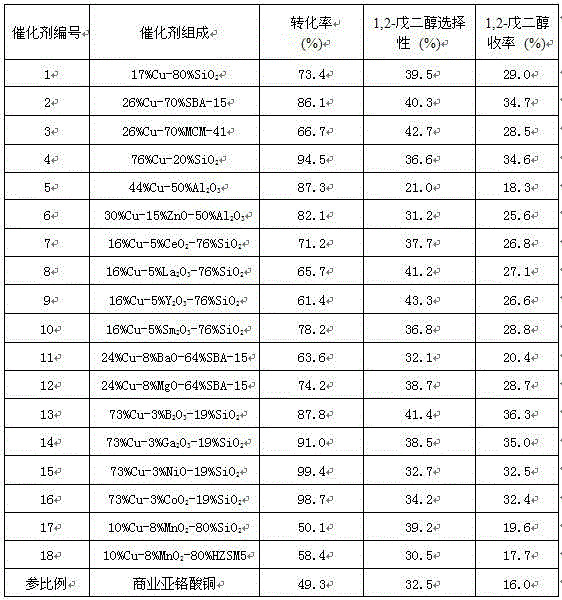
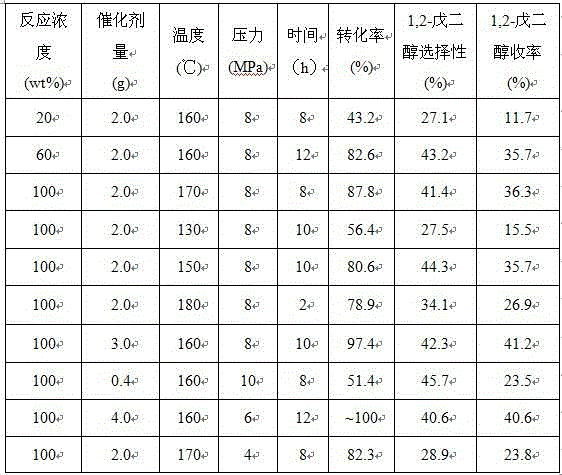
Image
Examples
Embodiment 1
[0028] Weigh 4.83 g of copper nitrate trihydrate, 32.00 g of 20 wt% acidic silica sol and 12.00 g of urea into the autoclave, add 100 mL of distilled water, seal the autoclave, stir and heat up to 100 °C, and precipitate for 2 h. After cooling down to room temperature, it was suction-filtered, washed three times with distilled water and absolute ethanol, dried at 120 °C for 12 h, roasted in a muffle furnace at 400 °C for 3 h, pressed into tablets, ground and sieved (80-100 mesh). The sieved calcined sample was reductively activated in a hydrogen atmosphere at 300 °C for 4 h to obtain the active catalyst 1 provided by the present invention.
Embodiment 2
[0030] The operation was the same as in Example 1, except that 32.00 g of 20 wt% acidic silica sol was replaced by 3.71 g of SBA-15 molecular sieve powder, and after reduction and activation, the active catalyst 2 provided by the present invention was obtained.
Embodiment 3
[0032] The operation was the same as in Example 1, except that 32.00 g of 20 wt% acidic silica sol was replaced by 3.71 g of MCM-41 molecular sieve powder, and after reduction and activation, the active catalyst 3 provided by the present invention was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com