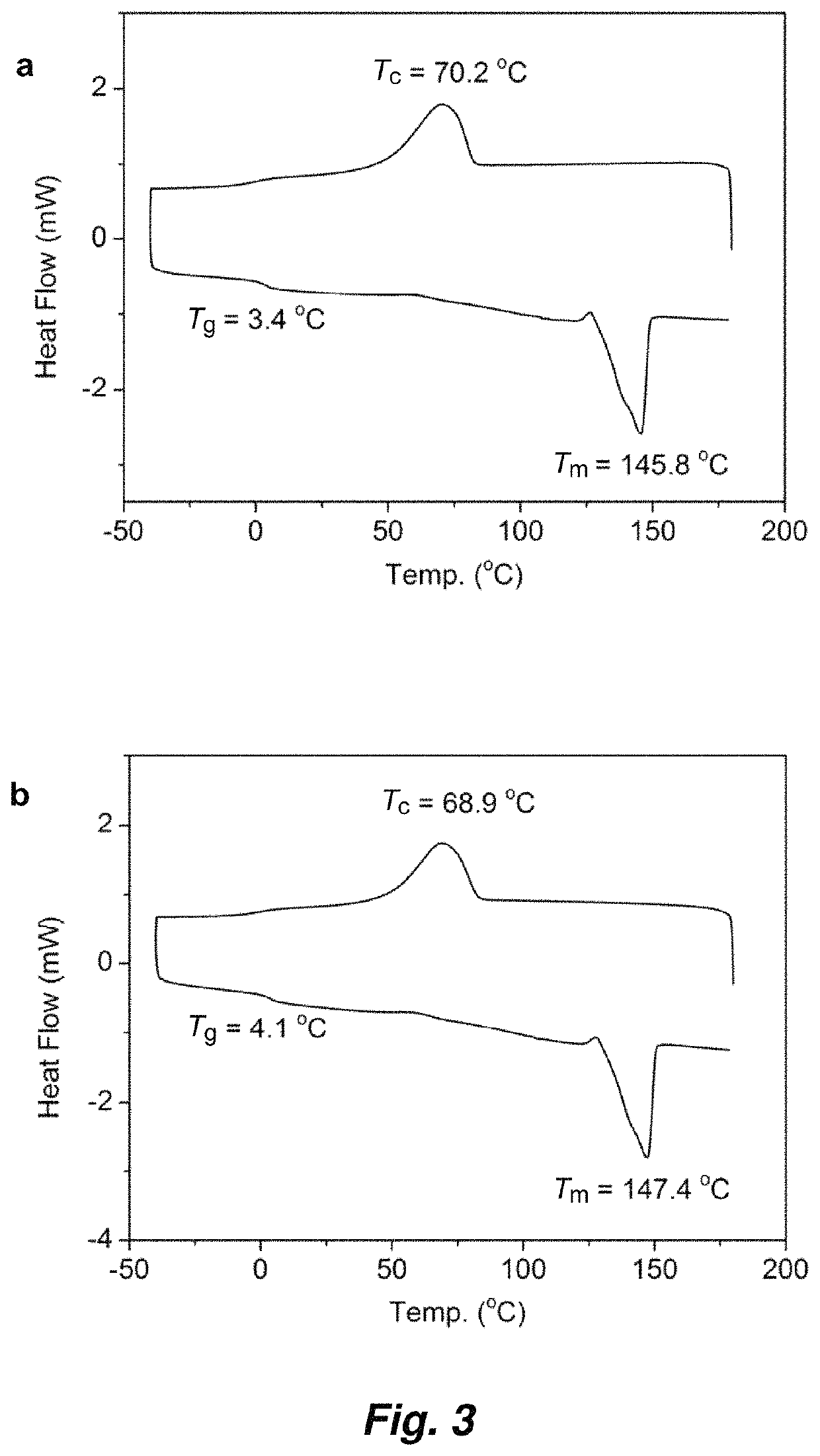
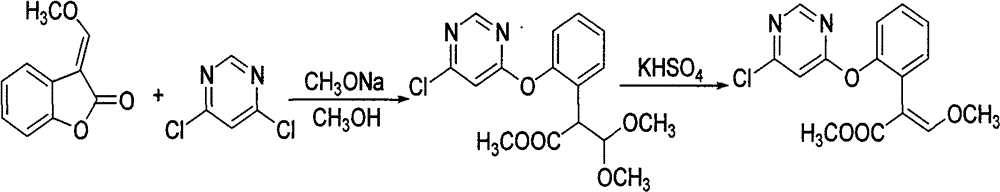
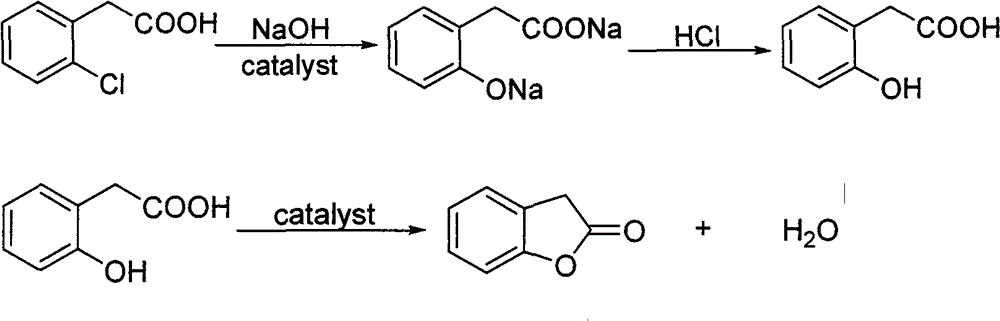
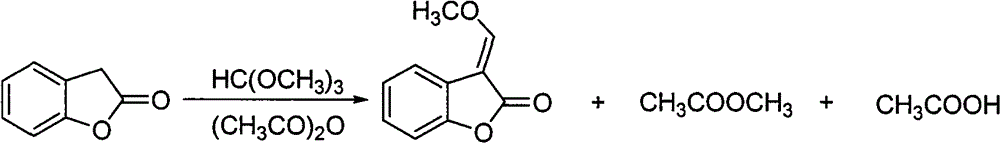
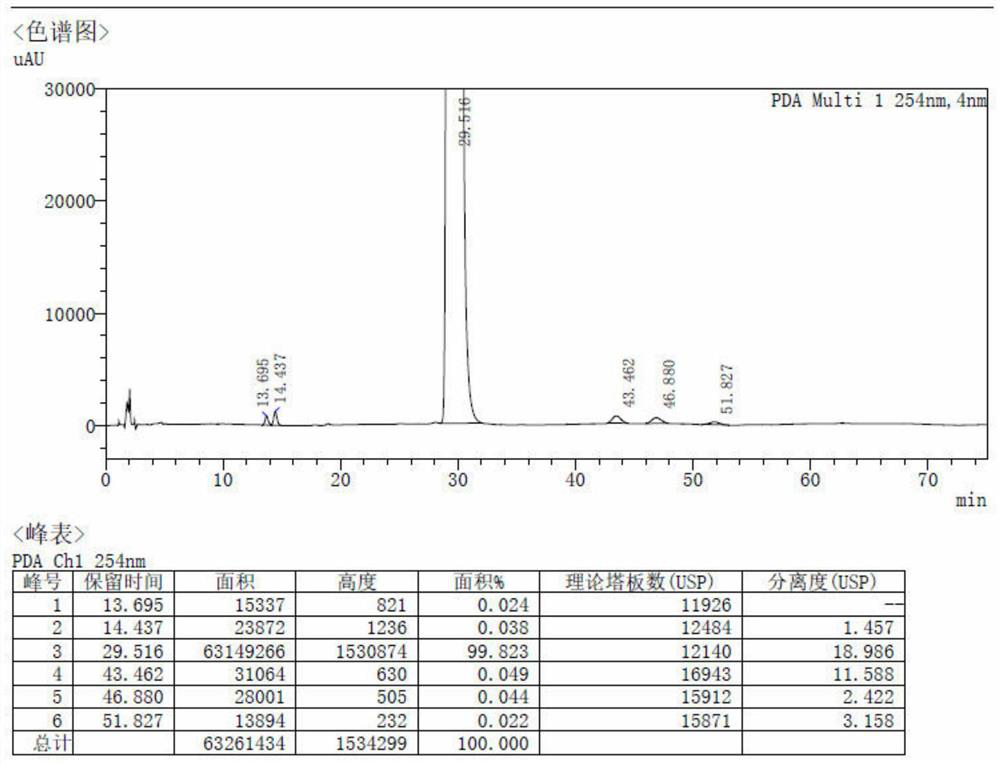
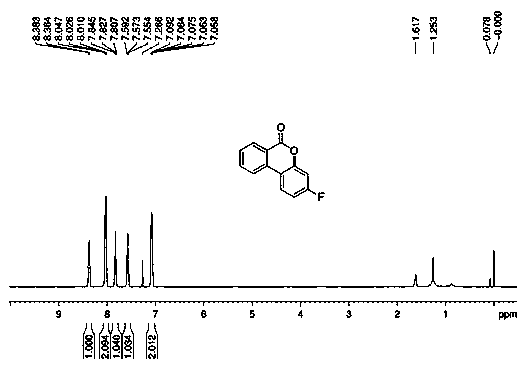
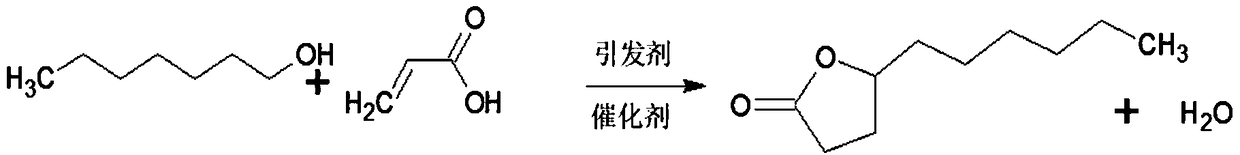
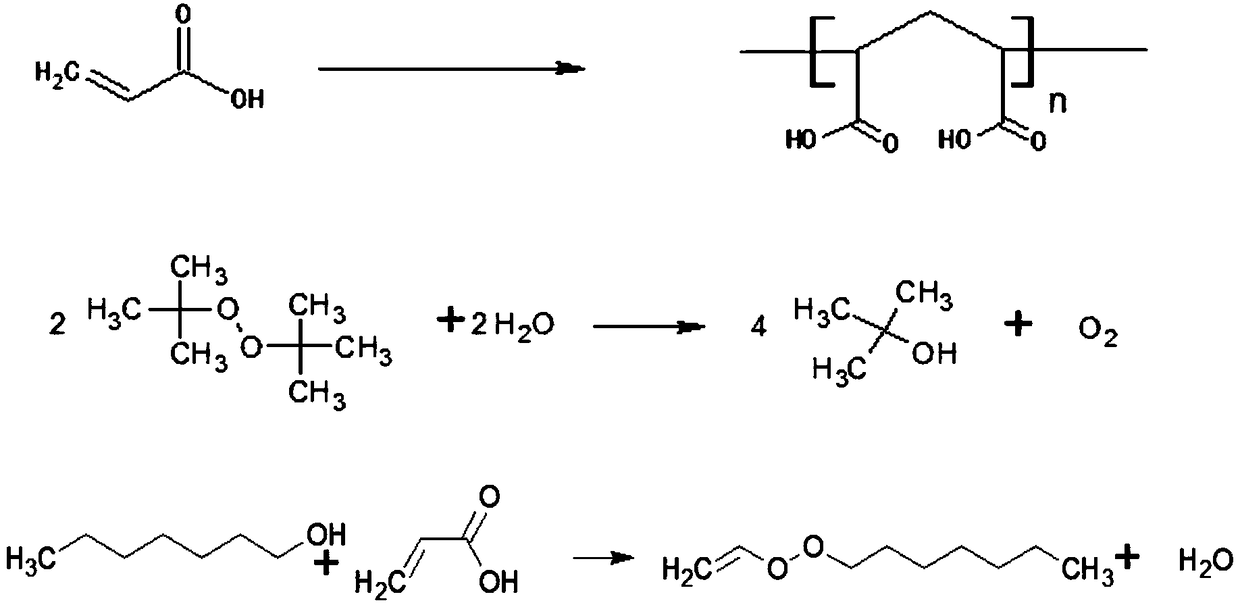
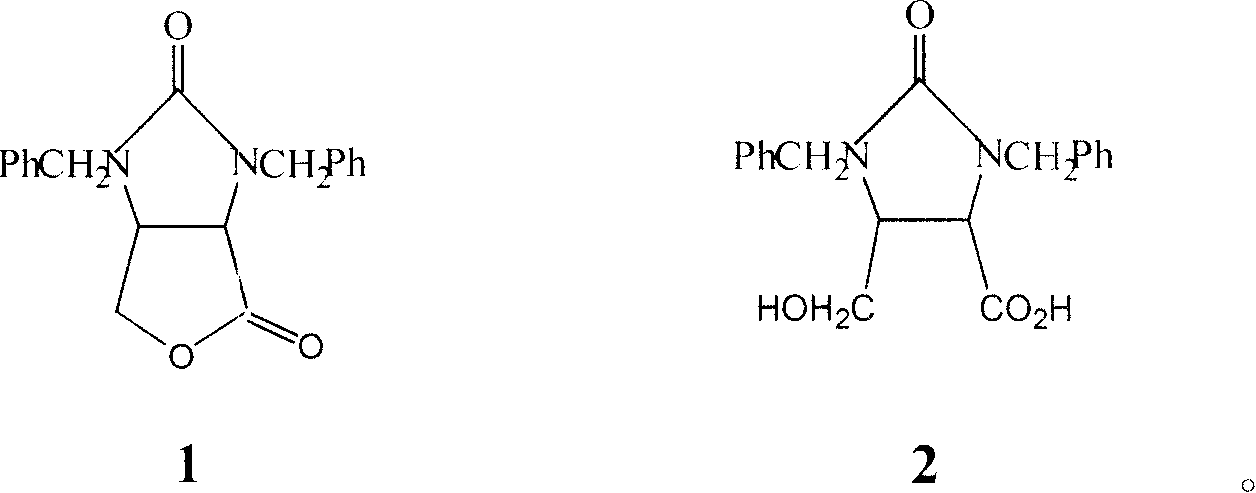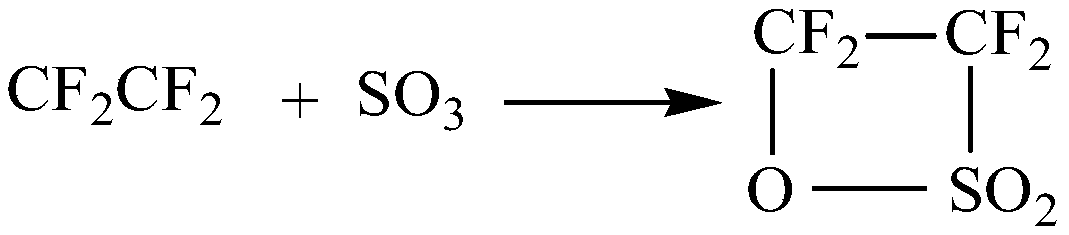Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Lactone synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
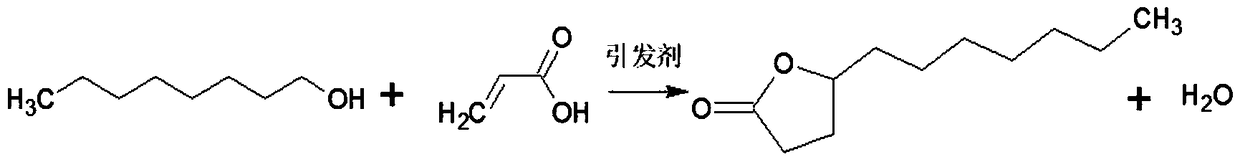
Many methods adopted for ester synthesis can be applied for Lactone synthesis. Methods include Shiina macro-lactonization, nucleophilic abstraction and Yamaguchi esterification. Lactones like γ-nonalactone,γ-octalactone, γ-undecalactone, γ-decalactone can be synthesized in a single step process.
Method for preparing dextral biotin intermediate lactone
ActiveCN101186938ALow yieldReduce pollutionHydrolasesMicroorganism based processesASPERGILLUS NIGER LIPASEYeast
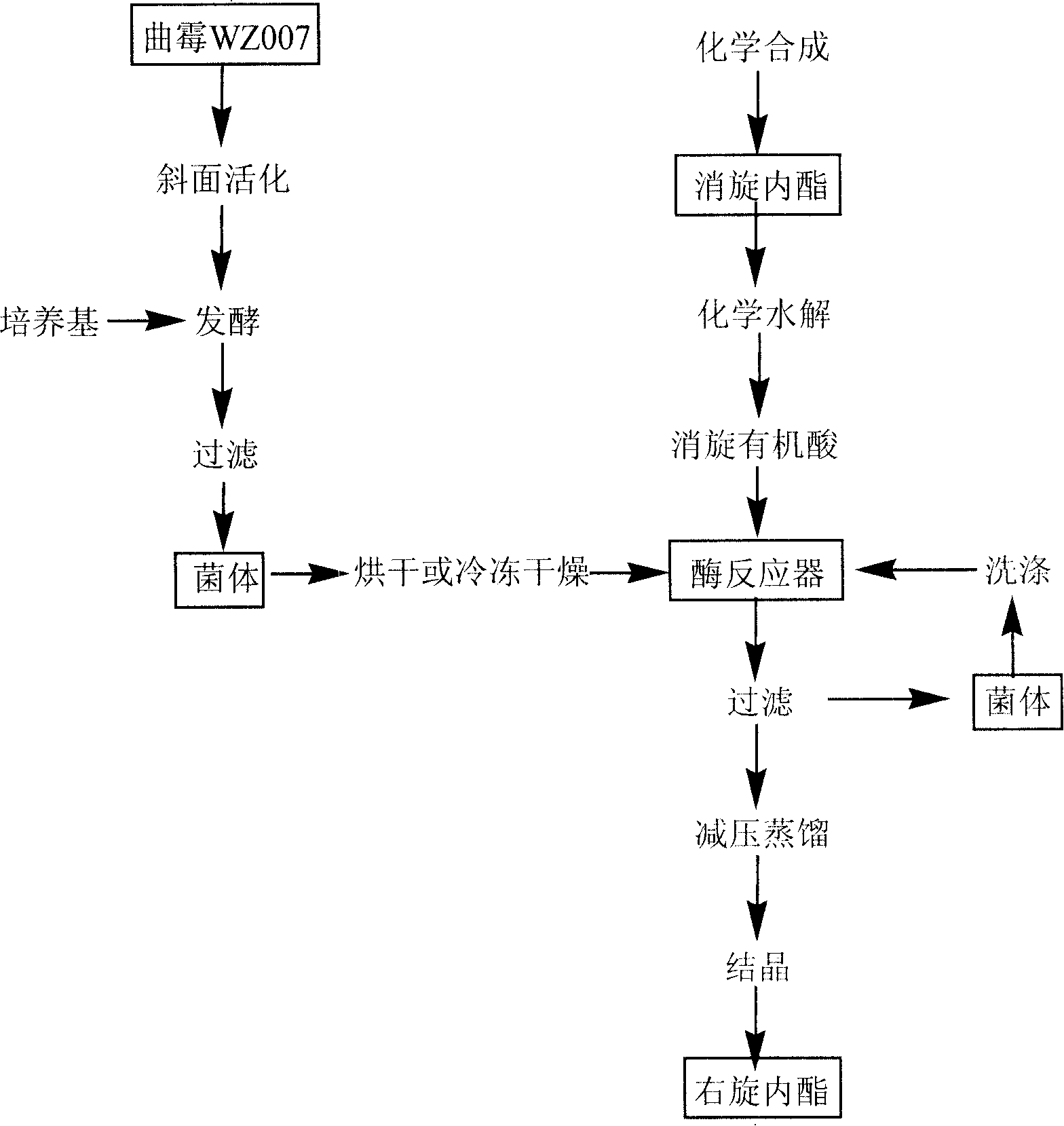
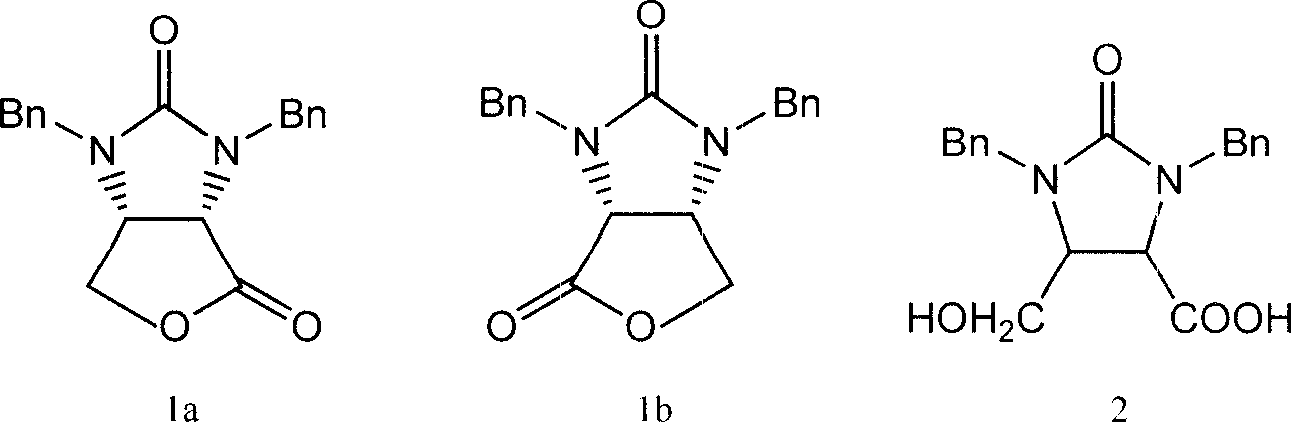
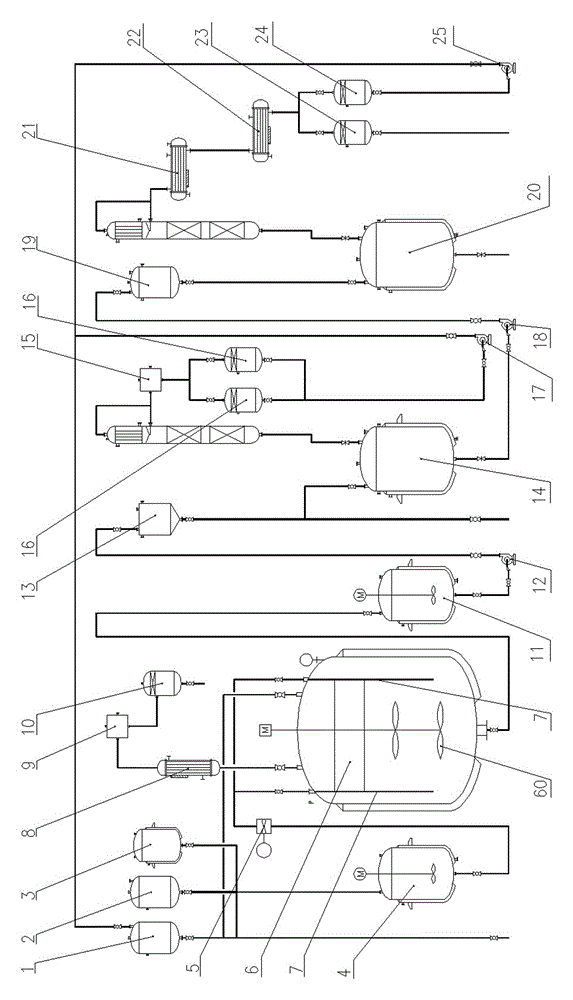
The invention provides a process for preparing the lactone of the intermediate of dextral biotin. The invention employs racemic acid 2 (1, 3-dibenzyl-5(hydroxymethyl)-2-oxo-4-Imidazolidinecarboxylic aid) which is achieved by the hydrolyzing the lactone 1 of the intermediate of racemization biotin 1 (tetrahydro-1, 3-bis (phenylylmethyl)-1 H-Furo (3, 4-d) imidazole-2, 4-dione) is utilized as reaction substrate, and the lactone of the intermediate of dextral biotin 9 is selectively synthesized in the function of lactone synthase. Further, the lactone synthase is one of the following materials, including (1) pig liver esterase, (2) pancreatin, (3) aspergillus oryzae lipase, (4) aspergillus niger lipase, (5) aspergillus flavus lipase, (6) blue mold lipase, (7) rhizopus chinensis lipase, and (8) yeast lipase. The beneficial effects of the process for preparing the lactone of the intermediate of dextral biotin include that resolution specificity is high, the resolution efficiency is high, the manufacture cost is greatly reduced, the operation is simple, the environmental pollution is low, and the invention is adaptable for industrial production.
Owner:ZHEJIANG UNIV OF TECH
Synthesis for weak hydrophobic framework amide resin and application of the same in purifying gingko total lactone
InactiveCN101100495AGood use strengthGood synergyGinkgophyta medical ingredientsLactone synthesisFlavones
Synthesis of amide adsorptive resin with weak hydrophobic skeleton is carried out by co-polymerizing methyl acrylate with vinyl benzene to obtain ester-based resin, aminolyzing for ester base, acidylating to obtain amide adsorptive resin with weak hydrophobic skeleton, adsorbing flavone compound from Ginkgo extract onto resin by the resin and hydrogen bond synergistic function, flowing ginkgo-lactone, re-crystallizing and eluting to obtain final product. The purity is above 90%, it's simple and non-toxic, it has friendly environment and can be used to supply experimental samples for development of ginkgo lactone.
Owner:NANKAI UNIV
Constant-pressure production device for methyl decalactone synthesized spice and production method
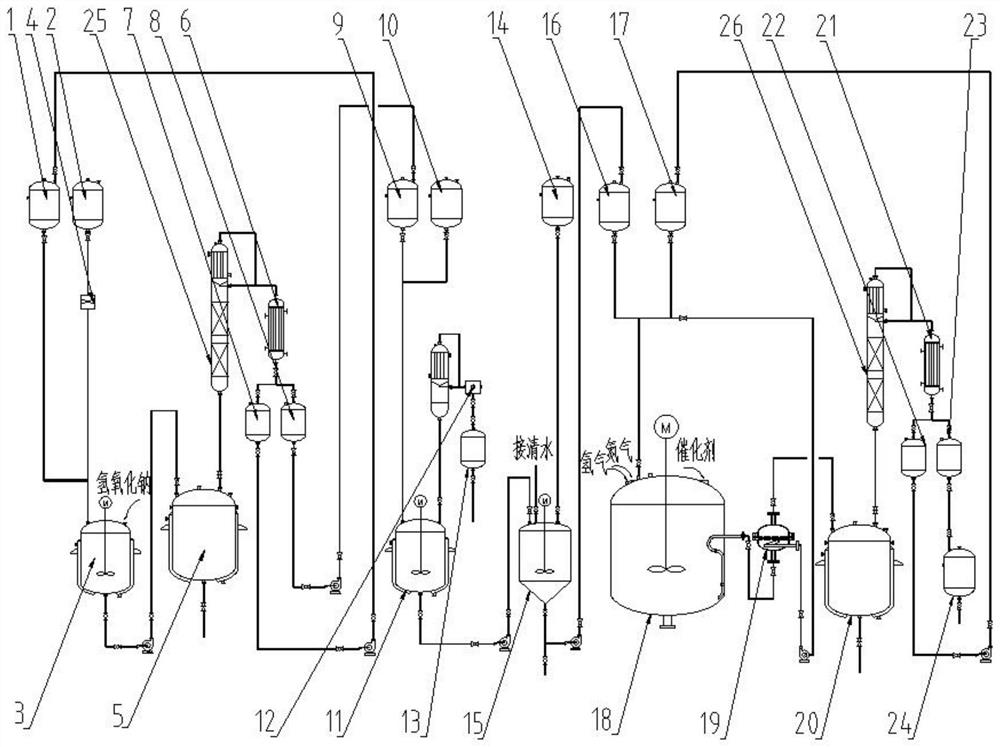
The invention provides a constant-pressure production device for methyl decalactone synthesized spice. The constant-pressure production device comprises a DL-2-Octanol high-level tank, a di-tert-butyl peroxide high-level tank, and an acrylic or methyl acrylate high-level tank and further comprises a kettle type rectifying tower, wherein the DL-2-Octanol high-level tank, the di-tert-butyl peroxide high-level tank and the acrylic or methyl acrylate high-level tank are in pipeline connection with an ingredient pan; the ingredient pan is in pipeline connection with a dropwise adding pump, one or more dropwise adding pipes are arranged in a reaction kettle, and the dropwise adding pump is connected with the dropwise adding pipes; the bottom of the reaction kettle is in pipeline connection with a rinsing pan, the bottom of the rinsing pan is in pipeline connection with a standing high-level tank through a pump A, the standing high-level tank is in pipeline connection with a kettle type distillation tower, and the bottom of the kettle type distillation tower is in pipeline connection with a material high-level temporary storage groove through a pump C; the material high-level temporary storage groove is in pipeline connection with the kettle type rectifying tower, and the kettle type rectifying tower is connected with a product vacuum holding tank through a first-class condenser and a second-class condenser. According to the constant-pressure production device disclosed by the invention, the methyl decalactone synthesized spice can be synthesized in a constant-pressure manner, the product yield is high, the technological conditions are mild, the production cost is relatively low, and environmental pollution is avoided in a production process.
Owner:ANHUI HYEA AROMAS
Method for synthesizing delta-decalactone
InactiveCN102382090ASolve complex, low-yield problemsHigh yieldOrganic chemistryDistillationRetention time
The invention discloses a method for synthesizing delta-decalactone, which comprises the following steps of: (1) performing aldol condensation reaction on cyclopentanone and valeraldehyde which are taken as initial raw materials by taking polyethylene glycol 400 (PEG-400) as a phase transfer catalyst under the alkaline condition, and dehydrating under the action of an acid catalyst to obtain 2-pentene cyclopentanone; (2) performing normal pressure catalytic hydrogenation on the 2-pentene cyclopentanone by taking palladium-carried ion exchange resin as a catalyst and methanol as a solvent to obtain 2-pentyl cyclopentanone; and (3) performing Baeyer-villiger rearrangement reaction on the 2-pentyl cyclopentanone by taking methanol as a solvent and acid as a catalyst in a new reaction system adopting hydrogen peroxide for direct oxidation to synthesize crude delta-decalactone, and treating the crude delta-decalactone by using a vacuum thin-film distillation device with shorter retention time to obtain the high-purity product. The method is feasible, and is easy and convenient to implement; the raw materials are readily available, and wide in sources; the yield is high; the yield of the product of delta-decalactone is increased, and the purity of the delta-decalactone is greatly improved; and the catalysts can be repeatedly used, and have great application value.
Owner:ANHUI UNIV OF SCI & TECH
Synthetic method of perfluoroalkyl sultone
InactiveCN111072627ALarge specific surface areaAvoid the problem of uneven heat transfer effect and potential safety hazardsOrganic chemistryChemical/physical/physico-chemical microreactorsMicroreactorReaction temperature
The invention discloses a synthetic method of perfluoroalkyl sultone. The preparation method comprises the following steps: continuously introducing sulfur trioxide and perfluoroolefin into a micro-mixer for mixing; entering into a microreactor for reaction, wherein the molar ratio of sulfur trioxide to perfluoroolefin is 1: (1-1.5), the reaction temperature is 30-80 DEG C, the reaction pressure is 0.1-0.7 MPa, the retention time is 1.5-15 minutes, cooling after the reaction is finished and performing gas-liquid separation, and distilling and purifying the obtained liquid productto obtain theperfluoroalkyl sultone product. By utilizing the efficient mass transfer and heat transfer characteristics of the micro-mixer and the micro-reactor, the operation process of perfluoroalkyl sultone synthesis is simplified, the reaction time is shortened, the continuous reaction is realized, and the safety of the equipment in the operation process is improved; meanwhile, the method has the advantages of high reaction yield, few by-products and easiness in subsequent enlarged production.
Owner:JUHUA GROUP TECH CENT
Method for electrochemically synthesizing lactone
InactiveCN107699917ASimple processEasy to controlElectrolysis componentsElectrolytic organic productionCarboxylic acidElectrochemistry
The invention discloses a method for electrochemically synthesizing lactone. According to the method, carboxylic acid serves as a raw material, and electrochemical oxidation is carried out for C-H / O-Hcross coupling so as to directly obtain the lactone with various structures, such as biaryl lactone, coumarin lactone, benzofuran lactone. The synthetic method is simple in process, high in stability, green and environment-friendly and easy to control and produce in large scale, the raw materials are directly oxidized into lactone products under the electrochemical condition, and thus the methodis more efficient and rapid. In addition, the raw material substrate is wide in application range and easy to obtain, a transition metal catalyst, a photocatalyst or an organic small molecular catalyst do not need to be added in the preparation process, thus the universality of lactone synthesis is further increased, and the yield of lactone can also be improved.
Owner:NANYANG NORMAL UNIV
Methyl decyl lactone synthetic perfume normal pressure production method
The present invention provides a methyl decyl lactone synthetic perfume normal pressure production method, raw materials are easy to obtain, product yield is high, process conditions are mild, production cost is low, the process of production is free of environmental pollution, and the raw materials cause no production equipment corrosion. The method includes the following steps: a) stirring and mixing evenly acrylic acid, sec-octyl alcohol, initiator di-tertiary butyl peroxide and catalyst boric acid; b) adding the sec-octyl alcohol into a reaction kettle according to the dosage; c) dropwise adding the mixture obtained by the step a) into the reaction kettle for atmospheric pressure reaction; d) stirring at 100-200 DEG C for reaction for 2-5 hours; e) transferring reaction products into a water washing pot, adding clear water for layering, and separating the lower layer water phase; f) transferring upper layer organic phase reaction products to a kettle type distillation tower, heating, recycling the sec-octyl alcohol under vacuum; and g) transferring surplus reaction products into a kettle type rectification tower for fractionation to obtain methyl decyl lactone with the content of 99%.
Owner:ANHUI HYEA AROMAS
Method for producing epsilon-decalactone synthetic perfume
InactiveCN109748900AReduce separation and purification processHigh reaction yieldOrganic chemistryCyclohexanoneAlkaline water
The invention belongs to the technical field of perfume production and in particular relates to a method for producing an epsilon-decalactone synthetic perfume. The method comprises the following steps: (1) preparing alkaline water; (2) preparing a mixture A of cyclohexanone and n-butanal; (3) putting the alkaline water into a reaction kettle, and uniformly mixing a bottom material, namely the cyclohexanone, in the reaction kettle; (4) dropping the mixture A into the reaction kettle, dropping and stirring simultaneously, continuously stirring after dropping is completed, and performing thermalreaction till the content of n-butanal is less than 1%; and (5) purifying the product of the step (4), and carrying out hydrogenation, separation and purification, oxidation and separation and purification in sequence, thereby obtaining the epsilon-decalactone synthetic perfume. By adopting the n-butanal and the cyclohexanone as initial raw materials, an aldol condensation reaction is carried out, hydrogenation is further carried out, a peracetic acid oxidation ring enlargement reaction is performed to synthesize the epsilon-decalactone synthetic perfume, the excessive cyclohexanone is adopted as a reaction solvent which is recycled and applied mechanically, a reaction solvent separation and purification procedure is reduced, and in addition, the method is easy in raw material obtaining and high in reaction yield.
Owner:安徽华业香料合肥有限公司
Device and method for treating lactone synthetic perfume organic waste gas through combined process
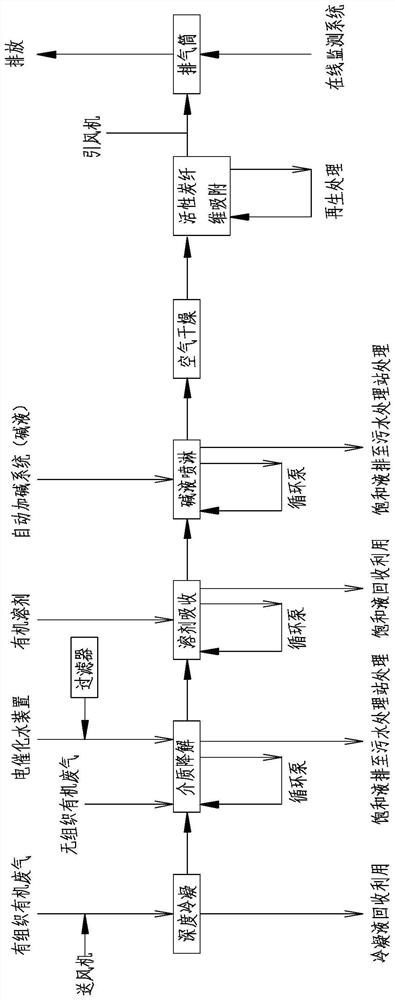
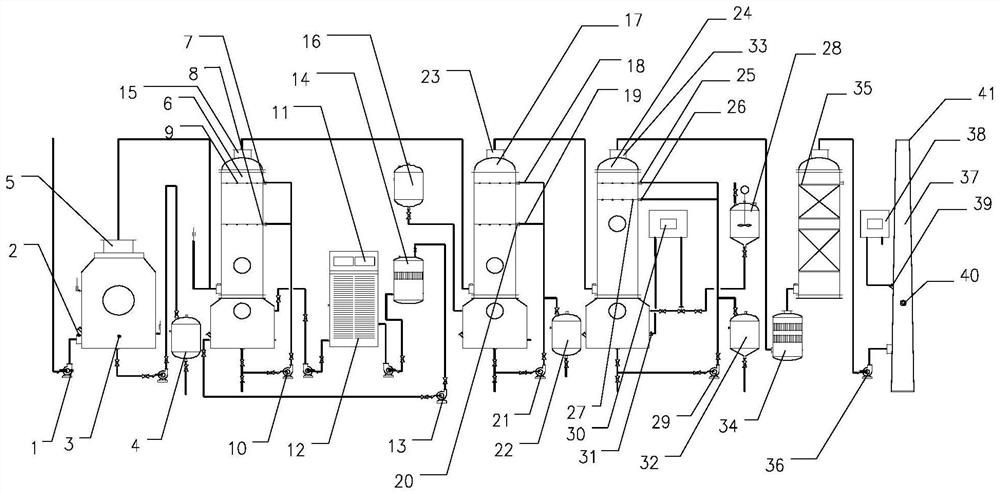
PendingCN114522503AImprove processing efficiencyMeet the requirements of emission standardsGas treatmentDispersed particle separationActivated carbonOrganic solvent
The invention discloses a device and method for treating lactone synthetic perfume organic waste gas through a combined process, and belongs to the technical field of organic waste gas treatment.The device comprises an air feeder, a deep condenser is arranged on one side of the air feeder, a waste gas main inlet is formed in one side of the deep condenser, and the output end of the air feeder communicates with the waste gas main inlet; the condensate temporary storage tank is connected with the deep condenser through a pump; the medium degradation tower is connected with a condensed tail gas outlet of the deep condenser through a pipeline; a medium degradation first spraying pipe and a medium degradation second spraying pipe are arranged on the medium degradation tower, and the medium degradation tower is connected with a water tank at the bottom of the medium degradation tower through a medium circulating pump. After the lactone perfume organic waste gas is subjected to deep condensation, medium degradation, organic solvent absorption, alkali liquor spraying and activated carbon fiber adsorption treatment, the factory odor concentration and organized waste gas emission can meet the up-to-standard emission requirements.
Owner:安徽华业香料合肥有限公司
Gingko cell culture system-based study for biosynthesizing ginkgo terpene lactones from levopimaradiene
The invention relates to an application of ginkgo suspension cell biotransformation of levopimaradiene. It is found that the levopimaradiene can significantly promote the synthesis of the ginkgo terpene lactones in gingko cells, and the ginkgo terpene lactones in stem cells have more kinds and higher content than ginkgo terpene lactones in dedifferentiated cells; and the levopimaradiene has stronger influences on the synthesis of the ginkgo terpene lactones in the stem cell system than the dedifferentiated cells. Additionally, the levopimaradiene can promote the synthesis of bilobalide in the stem cell system and the dedifferentiated cell systems, so the bilobalide can be metabolized by ginkgolides in an MEP pathway. A PCR result shows that the levopimaradiene affects the biosynthesis of the ginkgo terpene lactones through regulating the expression level of key enzymes in the MEP pathway.
Owner:于荣敏 +2
Synthesis process of natural delta-decalactone
ActiveCN113861146AHigh yieldSolve complex, low-yield problemsOrganic chemistryChemical recyclingFood additivePtru catalyst
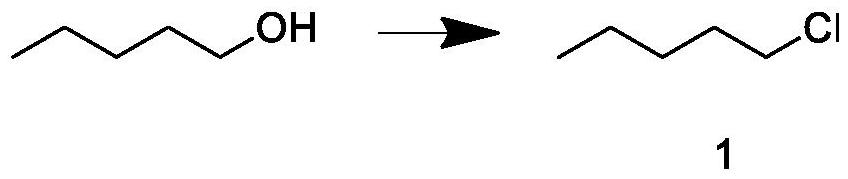
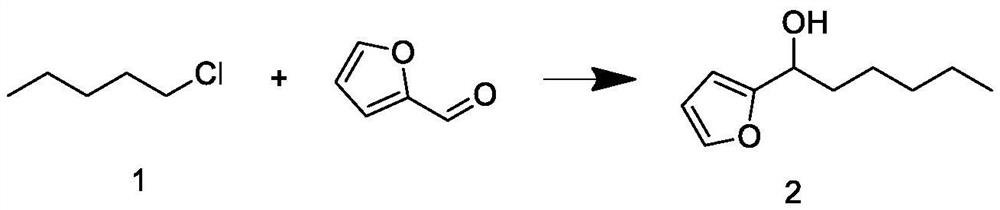
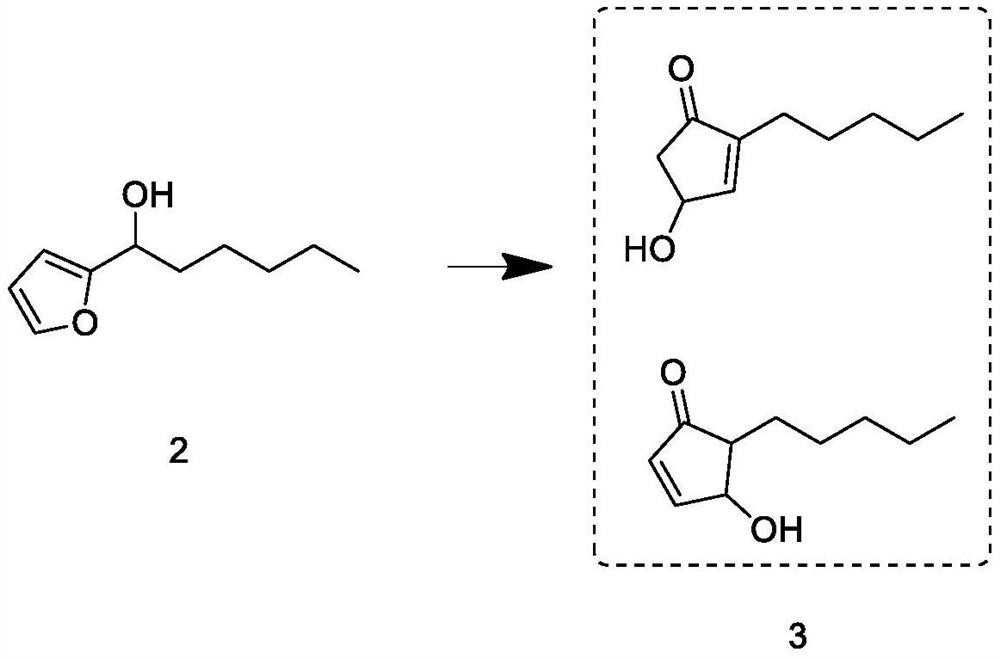
The invention relates to a synthesis process of natural delta-decalactone, and belongs to the technical field of food additives, and the natural delta-decalactone is obtained by taking furfural and n-amyl alcohol as raw materials through chlorination, Grignard coupling, Piancatelli rearrangement, hydrogenation and oxidation. The purity of the delta-decalactone prepared in the synthesis process of the natural delta-decalactone can reach 98% or above, the natural degree detected by isotope mass spectrometry C14 is 95% or above, and the problems that the synthesis route of the delta-decalactone is complex and the yield is low are solved; and the experimental method is simple, convenient and feasible, the reaction condition is mild, the experimental condition is easy to realize and control, and the method has the characteristics that the raw materials are easy to obtain and rich in source, the yield is relatively high, the used catalyst can be repeatedly used and the like.
Owner:安徽华业香料合肥有限公司
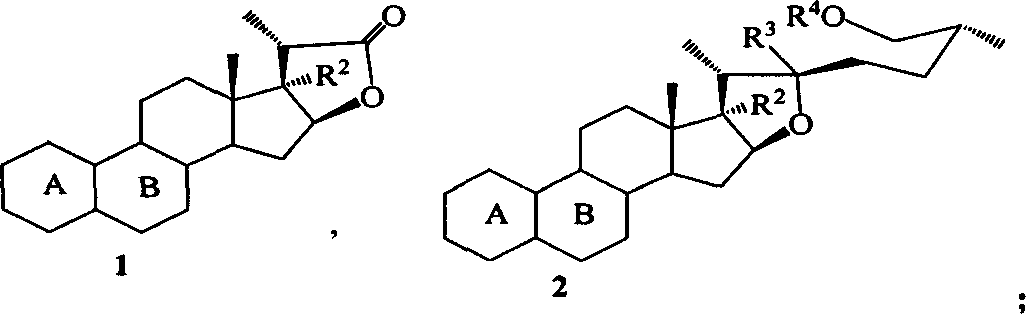
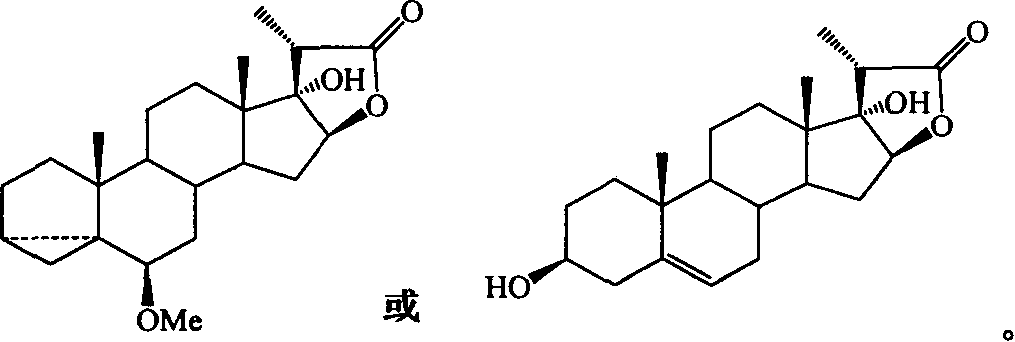
Process for synthesizing pennosapogenin by 17-hydroxy-steroid internal ester
The invention relates to a method to form spraying brain sapogenin by 17-hydroxy-steroid lactone. The key process includes inducing side chain, and closing of steroid E / F loop. R1, R4 and R5 are H, MOM, Me, Et, Bn, THP, Ac, Ms, Ts, Bz, Piv, TMS, TES, TBS or TPS. R2 is OH, OMe, OEt, OMOM or OTMS. R3 is OH, OMe, OEt, OMOM, OTMS or ether linkage of R3 and R4. The invention is easy to operate, and the raw material is easy to gain.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
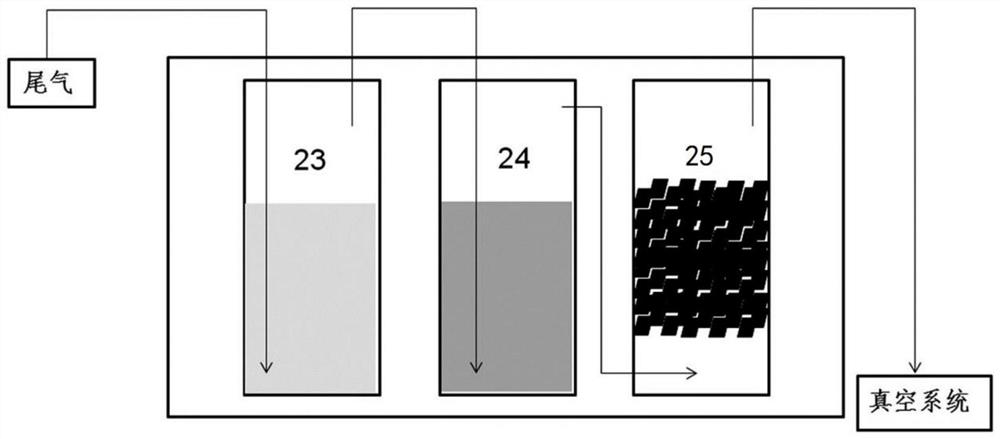
Solution preparation device of solid material, and method and system for removing peroxide in epsilon-caprolactone synthesis system
ActiveCN111841403AAvoid it happening againReduce health damageOrganic chemistryTransportation and packagingProcess engineeringCaprolactone
The invention provides a solution preparation system for a solid material, which can prevent dust from being generated in the preparation process of a reductive solid material solution, reduce the labor intensity and reduce the health injury of operators, and is suitable for continuous and automatic production. One vacuum system can be utilized to realize automatic feeding and stirring of variousmaterials and a plurality of mixing equipment, so that the equipment purchase and operation cost can be reduced while the mixing efficiency is improved. By a peroxide reduction equipment module, residual peroxide is removed, meanwhile, epsilon-caprolactone product loss is reduced, pollution gas emission is reduced through a volatile material recycling system, and the peroxide reduction equipment module is more economical and environmentally friendly. The invention also provides a method and device for removing peroxide in an epsilon-caprolactone synthesis system.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
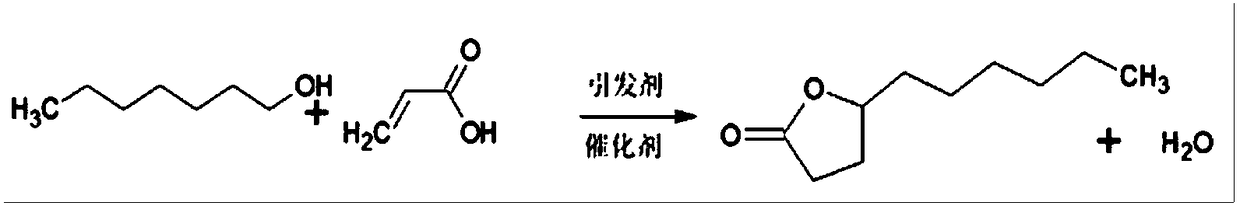
Method for synthesizing gamma-undecalactone synthetic perfume by reactive distillation
InactiveCN108997272AShort reaction timeQuick responseOrganic chemistryChemical industryRotary evaporatorDi-tert-butyl peroxide
The invention belongs to the technical field of the fine chemical industry, and specifically relates to a method for synthesizing gamma-undecalactone synthetic perfume by reactive distillation. The method comprises the following steps: (a) mixing octanol, acrylic acid and di-tert-butyl peroxide to obtain a mixture of ingredients; (b) weighing octanol, adding octanol to a heater of a reactive distillation column, and feeding the mixture of ingredients in the step (a) into a reaction zone of the reaction distillation column; (c) opening condensate water at the top of the reaction distillation column, and separating a crude product of gamma-undecalactone from the bottom of the column; and (d) transferring the crude product of gamma-undecalactone into a rotary evaporator, controlling the vacuum degree, temperature and rotating speed and collecting gamma-undecalactone. The method provided by the present invention adopts a reactive distillation technology to separate by-products from the reaction system in time, the reaction is carried out in two directions through promoting the separation by the reaction and promoting the reaction by the separation, the reaction efficiency is improved,and the reaction yield is ensured.
Owner:ANHUI HYEA AROMAS
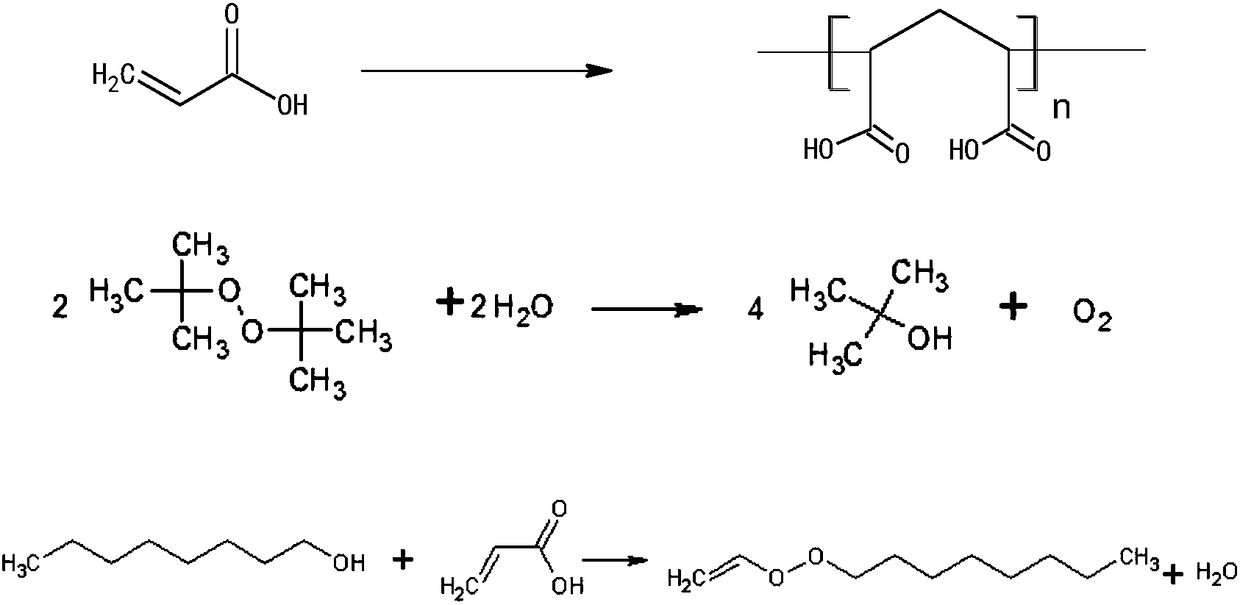
Synthesis of crystalline polymers from cyclic diolides
ActiveUS10954335B2Organic-compounds/hydrides/coordination-complexes catalystsChemical synthesisPolymer science
Owner:COLORADO STATE UNIVERSITY
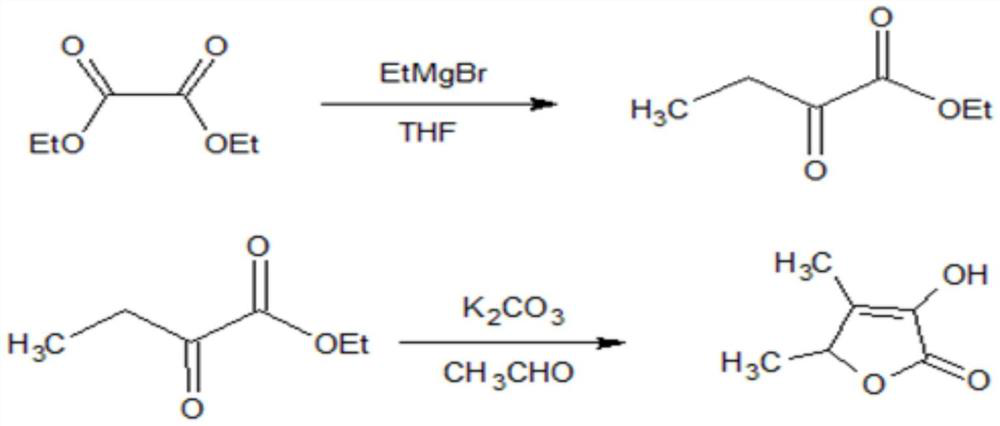
A kind of production method of trigonelline synthetic perfume
ActiveCN110372645BHigh yieldHigh purityOrganic chemistryChemical industryDiethyl oxalateGrignard reaction
The invention discloses a production method for synthesizing spices from trigonelline, which relates to the technical field of spice production. Anhydrous tetrahydrofuran is used as a solvent, and diethyl oxalate undergoes a Grignard reaction under nitrogen protection conditions, extracted with dichloromethane, washed, After drying over anhydrous sodium sulfate, reclaim dichloromethane for mechanical application, and collect 2-oxobutanoic acid ethyl ester by distillation under reduced pressure; then use absolute ethanol as solvent, under the effect of potassium carbonate and acetaldehyde, 2-oxobutyric acid Carry out cyclization of ethyl ester, recover ethanol under normal pressure, extract and wash with dichloromethane, dry with anhydrous sodium sulfate, recover dichloromethane and apply mechanically, and distill under reduced pressure to obtain trigonelline fragrance product; the process control parameters of the present invention are clear Moreover, the process has good repeatability, the solvent is reasonably recovered and reused, the environmental pollution is reduced and the production cost is reduced at the same time, and the yield and purity of the obtained product trigonelactone are high, which meets the requirements of spices.
Owner:ANHUI HYEA AROMAS
A solution preparation device for solid materials, method and system for removing peroxide in ε-caprolactone synthesis system
ActiveCN111841403BAvoid it happening againReduce health damageOrganic chemistryTransportation and packagingProcess engineeringCaprolactone
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
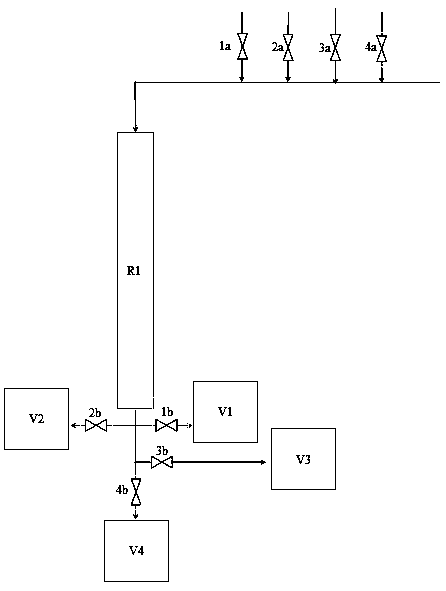
Cyclic oxidation method and cyclic oxidation device for preparing epsilon-caprolactone
InactiveCN110878076AImprove stabilityReduce difficultyOrganic chemistryChemical/physical processesCyclohexanonePtru catalyst
The invention provides a cyclic oxidation method and a cyclic oxidation device for preparing epsilon-caprolactone. A reactor adopts a fixed bed with a jacket or adopts a tubular reactor, and is filledwith a solid acid. A hydrogen peroxide reaction liquid, a cleaning liquid and a cyclohexanone solution sequentially flows through the reactor in sequence to complete a synthesis reaction of epsilon-caprolactone, the cleaning liquid flows through the reactor finally to complete regeneration, and a next cycle is started. A novel solid peroxy acid oxidation technology used in the invention makes residual water and a sulfuric acid catalyst simply washed off by using an anhydrous solvent, and the used solid organic peroxy acid can be recycled after being washed with a simple solvent washing, so peroxy acid and water residues are avoided, the energy consumption and danger are reduced, and the product quality can be guaranteed.
Owner:河南能源化工集团研究总院有限公司
A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process
ActiveCN109400555BEliminate useLower synthesis costOrganic chemistryAcetamidine hydrochlorideSodium salt
The invention relates to the technical field of vitamin B synthesis intermediates, in particular to a process for dissociating acetamidine hydrochloride with alpha-acetyl-gamma-butyrolactone sodium salt. The process comprises the following steps: making alpha-acetyl-gamma-butyrolactone sodium salt react with the acetamidine hydrochloride and separating products to obtain acetamidine and alpha-acetyl-gamma-butyrolactone. According to the process, the intermediate product alpha-acetyl-gamma-butyrolactone sodium salt in the synthesis step of the alpha-acetyl-gamma-butyrolactone reacts with the acetamidine hydrochloride, the alpha-acetyl-gamma-butyrolactone sodium salt utilize hydrochloric acid coordinated in the acetamidine hydrochloride to achieve the effect that the alpha-acetyl-gamma-butyrolactone sodium salt produces the alpha-acetyl-gamma-butyrolactone, and the hydrochloric acid in the acetamidine hydrochloride is removed to form acetamidine. Synchronous production of two target products is achieved, the steps are saved, and the process is environmentally friendly and increases the revenue.
Owner:江苏兄弟维生素有限公司
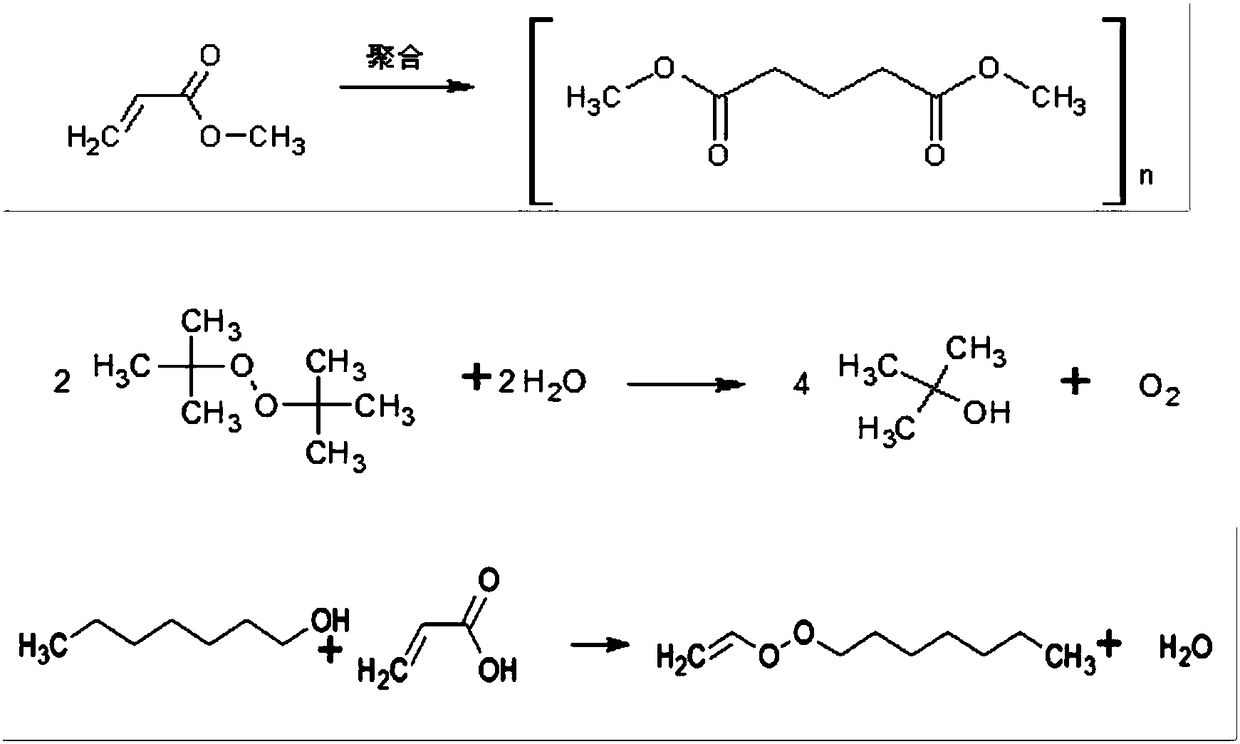
Method for compounding gamma-decalactone synthetic perfume through reactive distillation
InactiveCN109180616AFacilitate positive reactionsShort reaction timeOrganic chemistryChemical industryRotary evaporatorFine chemical
The invention belongs to the technical field of fine chemical engineering and specifically relates to a method for compounding a gamma-decalactone synthetic perfume through reactive distillation. Themethod comprises the following steps: (a) mixing heptanol, methyl acrylate, sodium borohydride and bi-tertiary butyl peroxide, thereby acquiring a mixture; (b) weighting heptanol, adding into a heaterof a reactive distillation column and using a dropwise adding pump for feeding the mixture acquired in the step (a) into a reaction zone of the reactive distillation column; (c) opening condensate water on the top of the reactive distillation column and separating a crude product of gamma-decalactone from column bottom; (d) transferring the crude product of gamma-decalactone into a rotary evaporator, controlling vacuum degree, temperature and rotational speed, and collecting gamma-decalactone. According to the invention, a reactive distillation technology is adopted, by-products are timely separated from a reaction system, separation is boosted by reaction, reaction is boosted by separation and the reaction yield is guaranteed.
Owner:ANHUI HYEA AROMAS
Synthesis of 3-(α-methoxy)methenylbenzofuran-2(3h)-one
InactiveCN102417498BEmission reductionHigh yieldOrganic compound preparationCarboxylic acid esters preparationFuranAcetic acid
Owner:CHONGQING UNISPLENDOUR CHEM
Method for preparing spirolactone by chemical-enzymatic method
ActiveCN113528607AStrong specificityIncrease catalytic rateLactone steroidsFermentationOrganic synthesisDehydrogenation
The invention discloses a method for preparing spirolactone by a chemical-enzyme method, and belongs to the technical field of organic synthesis. According to the method for preparing the spirolactone through the chemical-enzymatic method, 4AD serves as a raw material, and the spirolactone is obtained through enol etherification, epoxidation, lactonization, enzymatic dehydrogenation and sulfo-reaction. The enzymatic dehydrogenation method adopted by the spirolactone synthesis process route is good in specificity, mild in condition, free of special equipment and high in catalytic rate; the reaction product in each step involved in the method is easy to purify, the total mass yield of the final product is higher than 92%, and the HPLC purity is higher than 99.8%; the method is low in cost and suitable for industrial large-scale production, and has good economic benefits.
Owner:ZHEJIANG SHENZHOU PHARMA
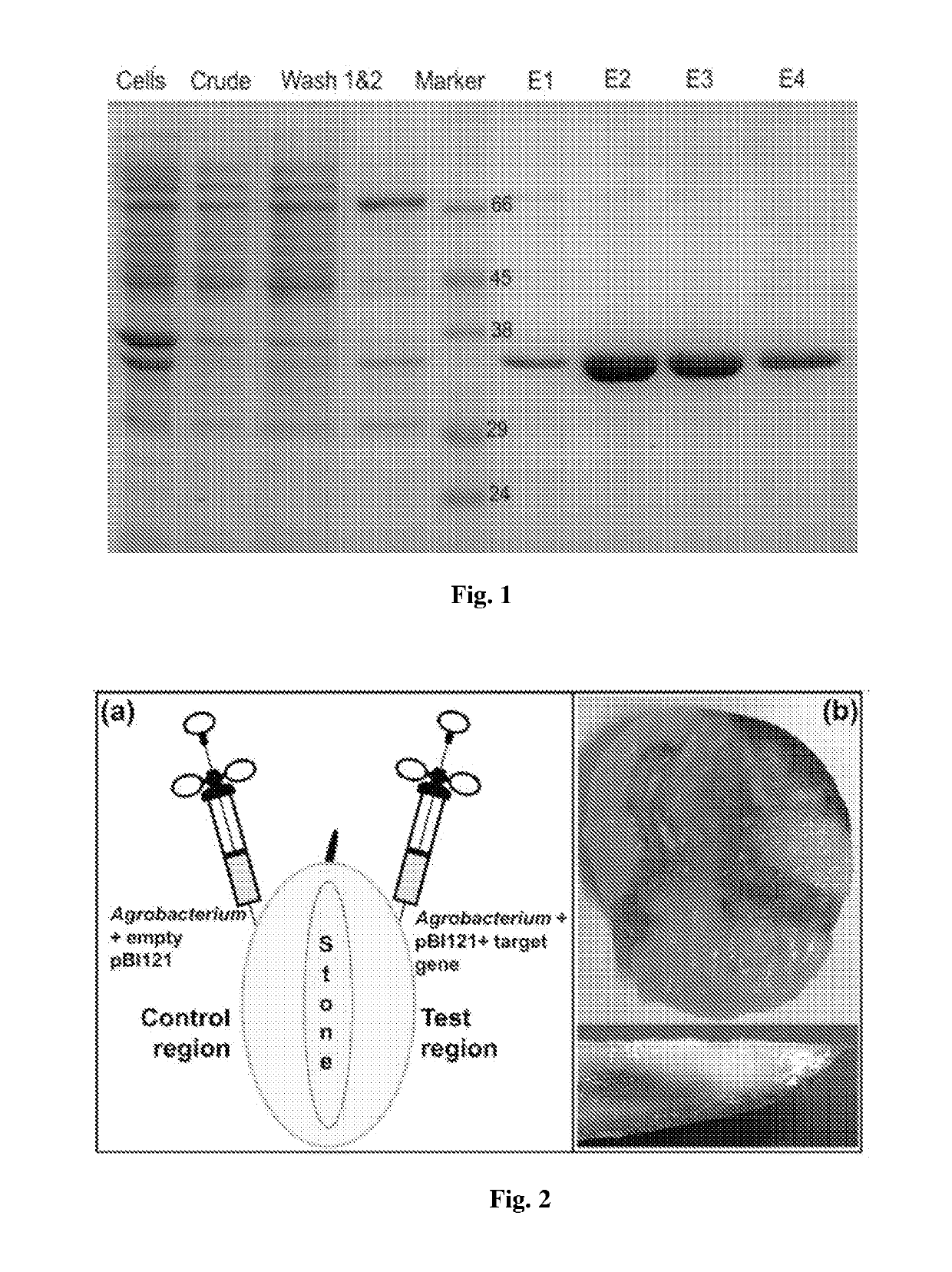
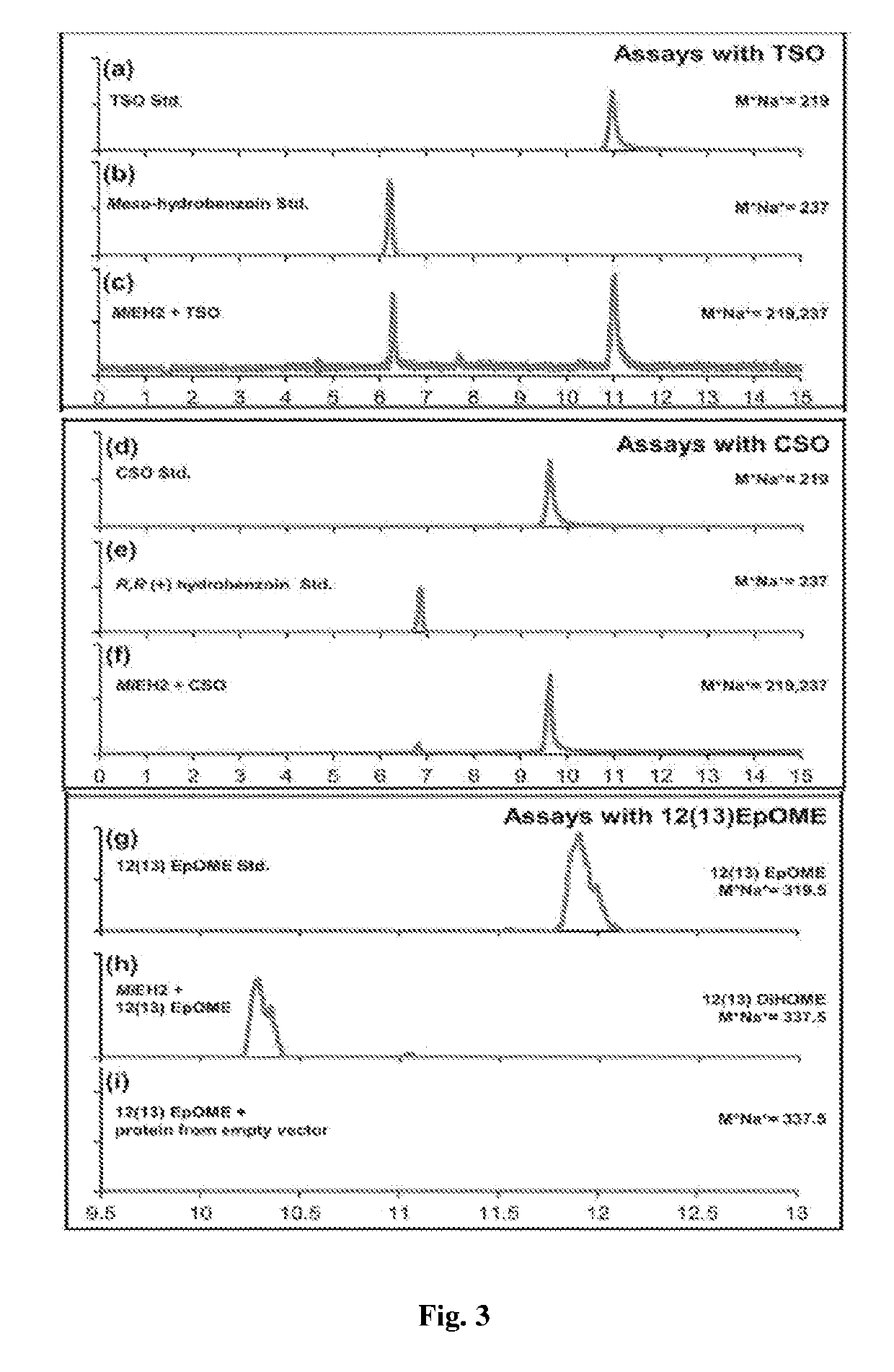
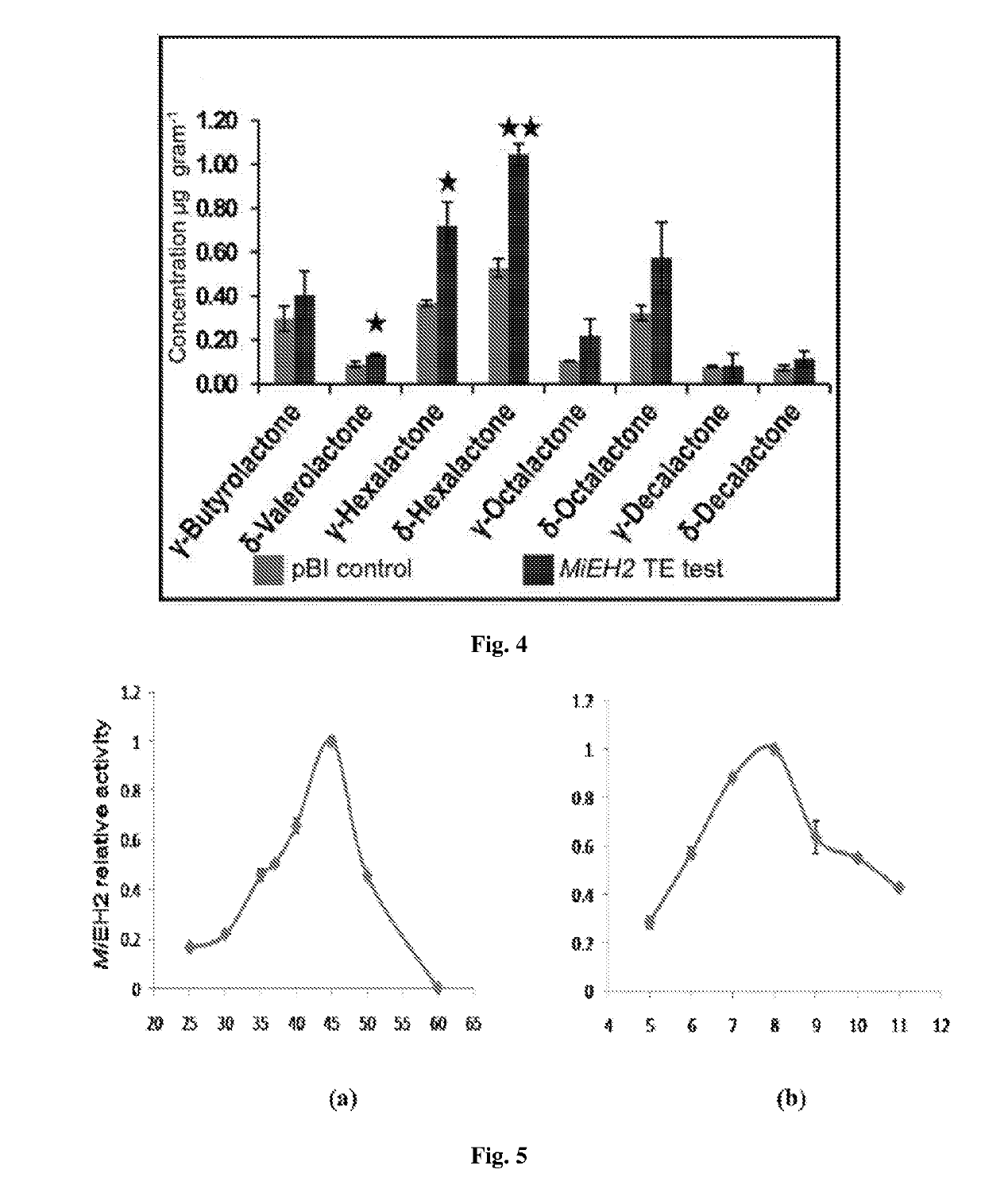
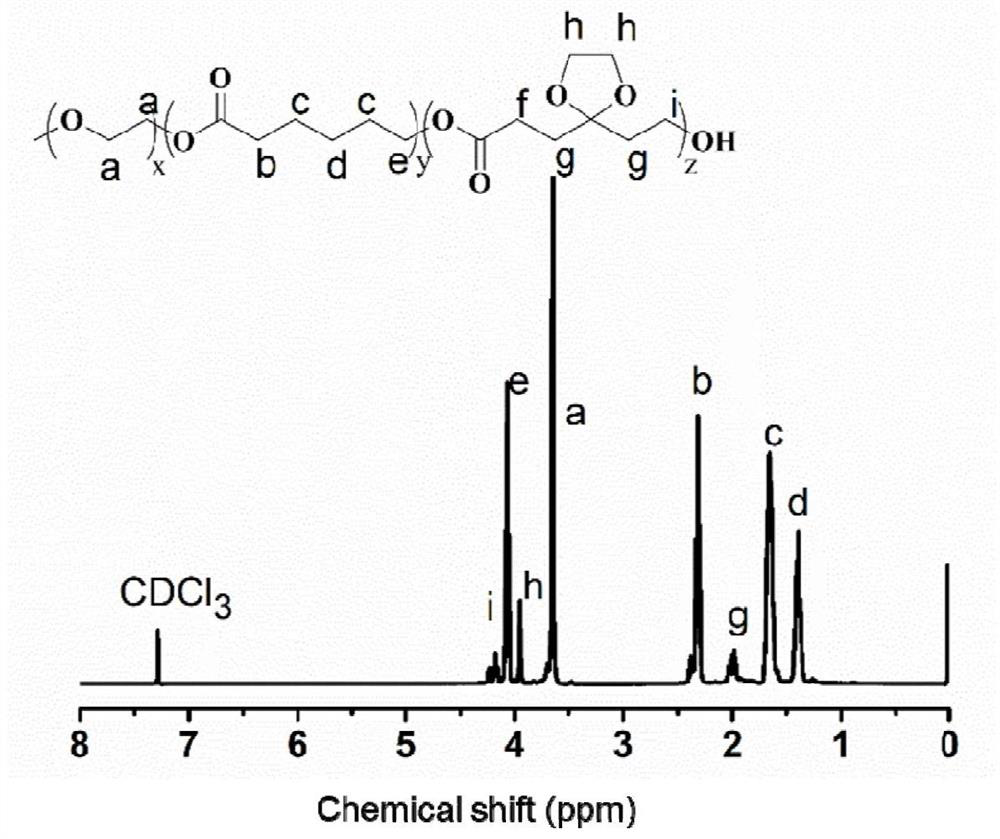
Recombinant polynucleotide involved in lactone synthesis and process for synthesis of lactones thereof
ActiveUS10801033B2Increase productionHydrolasesVector-based foreign material introductionNucleotidePolynucleotide
Owner:COUNCIL OF SCI & IND RES
Recombinant polynucleotide involved in lactone synthesis and process for synthesis of lactones thereof
ActiveUS20190153459A1Increased lactone productionIncrease productionHydrolasesVector-based foreign material introductionNucleotideADAMTS Proteins
The present invention provides the polynucleotide encoding enzyme involved in lactone synthesis and a process for synthesis of lactones using said polynucleotides. The invention also provides recombinant plasmid expression vector comprising said polynucleotide sequence. The recombinant protein encoded by said polynucleotides leads to synthesis of lactones having flavour peculiar to Alphonso mangoes.
Owner:COUNCIL OF SCI & IND RES
Preparation method and application of gel for in-situ delivery of nano-micelles
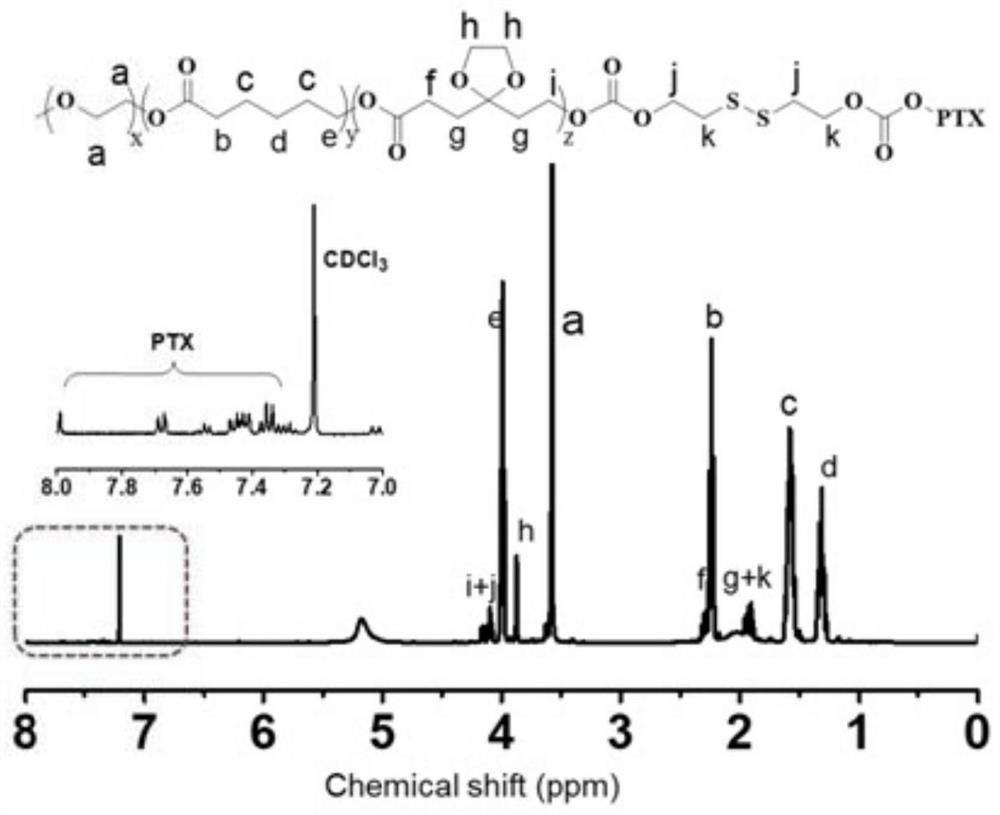
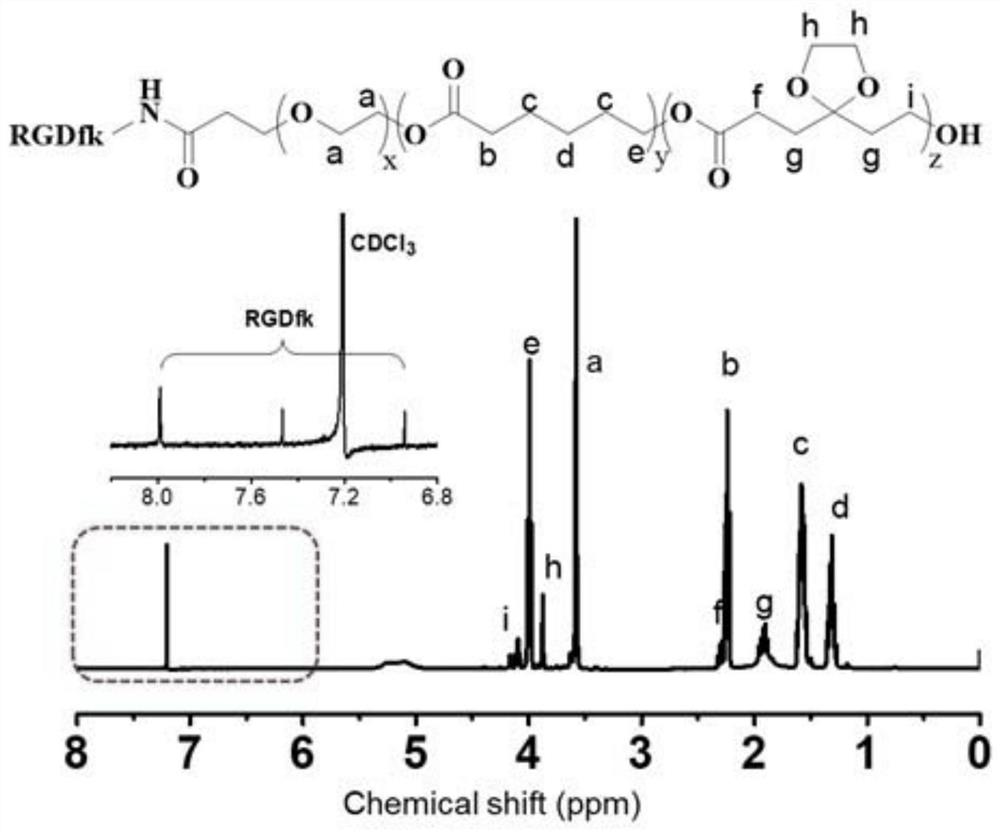
ActiveCN114522140AAvoid toxicityInduced deathOrganic active ingredientsAerosol deliveryCyclic peptidePolymer science
The invention discloses a preparation method of a gel system. Comprising the following steps: synthesis of poly (5-ethylene ketal-epsilon-caprolactone-epsilon-caprolactone)-polyethylene glycol monomethyl ether, synthesis of poly (5-ethylene ketal-epsilon-caprolactone-epsilon-caprolactone)-polyethylene glycol monomethyl ether bonded with paclitaxel molecules, synthesis of RGD cyclopeptide-polyethylene glycol-poly (5-ethylene ketal-epsilon-caprolactone-epsilon-caprolactone), synthesis of poly (5-ethylene ketal-epsilon-caprolactone), synthesis of poly (5-ethylene ketal-epsilon-caprolactone), synthesis of poly (5-ethylene ketal-epsilon-caprolactone) and synthesis of poly (5-ethylene ketal-epsilon-caprolactone). The preparation method comprises the following steps: preparing a paclitaxel drug, preparing a nano-micelle, synthesizing and the like, the gel prepared on the basis of the method has injectability and can release the functional nano-micelle in situ, and the released nano-micelle can specifically target tumor cells, can respond to glutathione in the tumor cells to release the paclitaxel drug and promote death of the tumor cells.
Owner:济南国科医工科技发展有限公司
A method for electrochemically synthesizing lactone
InactiveCN107699917BSimple processEasy to controlElectrolysis componentsElectrolytic organic productionCarboxylic acidElectrochemistry
The invention discloses a method for electrochemically synthesizing lactones. The method uses carboxylic acids as raw materials, and directly obtains lactones with various structures through electrochemically oxidized C-H / O-H cross-coupling, such as biaryl lactones. Esters, coumarin lactones, benzofuran lactones, etc. The synthesis method has the advantages of simple process, strong stability, environmental protection, easy control and large-scale production, and the raw materials are directly oxidized into lactone products under electrochemical conditions, which is more efficient and quicker. In addition, the raw material substrate has a wide range of applications and is easy to obtain. At the same time, there is no need to add transition metal catalysts, photocatalysts or organic small molecule catalysts during the preparation process, which increases the versatility of lactone synthesis and is also conducive to improving the yield of lactone. .
Owner:NANYANG NORMAL UNIV
Synthesis for weak hydrophobic framework amide resin and application of the same in purifying gingko total lactone
InactiveCN100480289CGood use strengthGood synergyGinkgophyta medical ingredientsGinkgolideOrganosolv
Owner:NANKAI UNIV
Production method of synthesizing gamma-decalactone synthetic perfume by reactive distillation
InactiveCN108997270AShort reaction timeQuick responseOrganic chemistryChemical industryBoiling pointGamma-decalactone
The invention belongs to the technical field of fine chemical production, and specifically relates to a production method of synthesizing gamma-decalactone synthetic perfume by reactive distillation.The method includes the following steps: (a) mixing materials through pipelines to obtain a mixture of ingredients; (b) discharging materials from a heptanol elevated tank into a preheater for preheating; (c) pumping heptanol into a high-boiling-point feed section of a reaction zone of a reactive distillation column, and pumping the mixture of ingredients into a low-boiling-point feed section of the reaction zone of the reactive distillation column; (d) separating out a crude product of gamma-decalactone; (e) transferring the separated heptanol to the preheater through a material pump to continue to participate in the reaction; and (f) further separating and purifying the crude product of gamma-decalactone. According to the production method provided by the present invention, the reactionis carried out in two directions through promoting the separation by the reaction and promoting the reaction by the separation, the reaction time is shortened, the reaction speed is improved, the reaction efficiency is improved, the energy is saved by using reaction heat, and equipment investment is saved.
Owner:ANHUI HYEA AROMAS
Method for separating neopentyl glycol from DL-pantoic acid lactone synthesis feed liquid
ActiveCN114478192AFully removedHigh purityOrganic compound preparationHydroxy compound preparationTowerLactone synthesis
The invention provides a method for separating neopentyl glycol from a DL-pantoic acid lactone synthesis feed liquid. The method comprises the steps that the DL-pantoic acid lactone synthetic feed liquid is dewatered and then added into a rectifying tower to be rectified, the rectifying tower sequentially comprises a tower bottom, a tower lower part, a tower middle part and a tower top from bottom to top, the temperature of the tower bottom is controlled to range from 170 DEG C to 175 DEG C, the temperature of the tower lower part is controlled to range from 160 DEG C to 165 DEG C, the temperature of the tower middle part is controlled to range from 145 DEG C to 150 DEG C, and the temperature of the tower top is controlled to range from 115 DEG C to 120 DEG C; the vacuum pressure in the rectifying tower is-0.093 to-0.099 MPa, and collecting neopentyl glycol from the top of the tower. The method provided by the invention has a relatively high removal rate on the neopentyl glycol in the DL-pantoic acid lactone synthesis feed liquid, and the separated neopentyl glycol has relatively high purity.
Owner:安徽泰格生物科技有限公司
Production device and method of delta-caprolactone synthetic perfume
PendingCN112266372AAchieve separationReduce wasteOrganic chemistryChemical industryPtru catalystDistillation
The invention discloses a production device and method of delta-caprolactone synthetic perfume, which comprises the following steps: proportionally adding ethyl acetoacetate and sodium hydroxide intoa reaction kettle, uniformly stirring, heating, dropwisely adding methyl acrylate, and recovering excessive ethyl acetoacetate to obtain acetyl succinate; adding acetyl succinate and sulfuric acid into a decarboxylation kettle, heating and stirring for reaction, collecting a byproduct ethanol to obtain acetylbutyric acid, transferring the acetylbutyric acid into a water washing kettle for alkali washing and water washing, adding the acetylbutyric acid and methanol into a hydrogenation kettle, and introducing hydrogen for hydrogenation reaction; after the reaction is finished, carrying out solid-liquid separation through an automatic backwash precision filter, pressing a solid-phase catalyst back into the reaction kettle, continuing to apply the next batch of reaction, transferring a liquidphase into a rectifying kettle, and carrying out reduced pressure distillation to collect a delta-caprolactone product after methanol recovery at normal pressure is finished; according to the method,the problems of low delta caprolactone synthesis yield, high cost and complex process flow are solved.
Owner:安徽华业香料合肥有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com