Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Acetamidine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
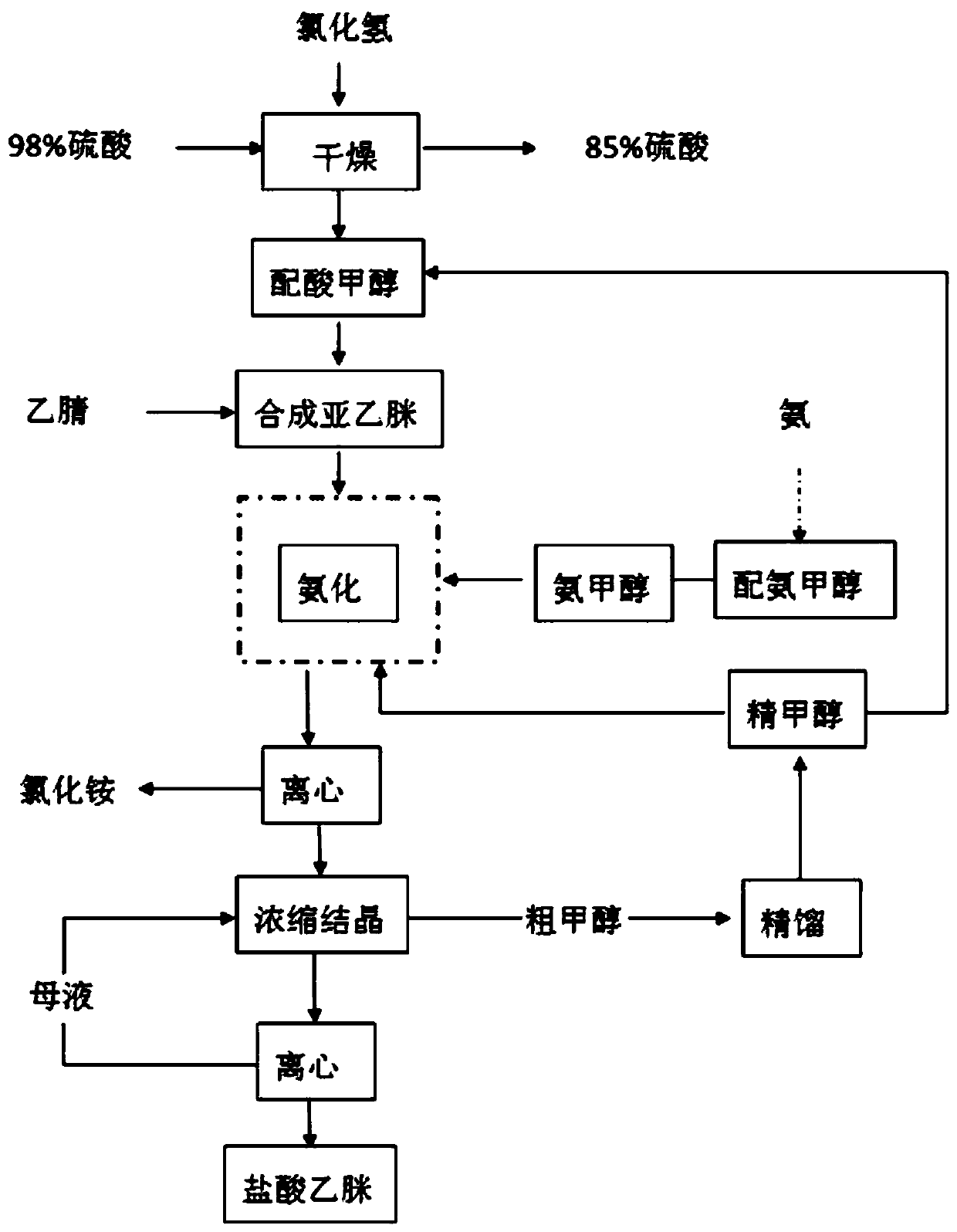
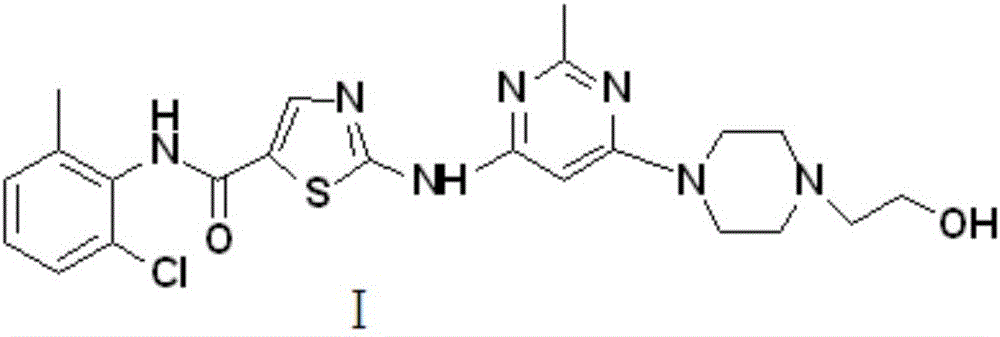
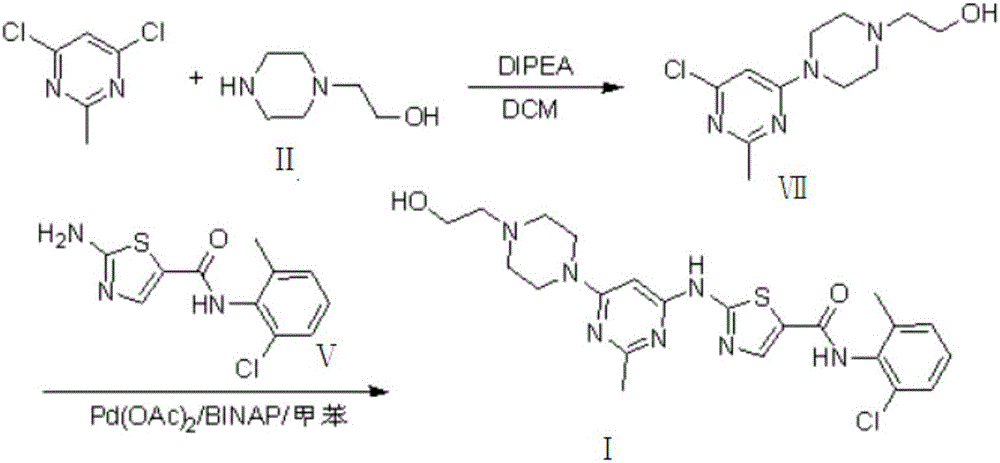
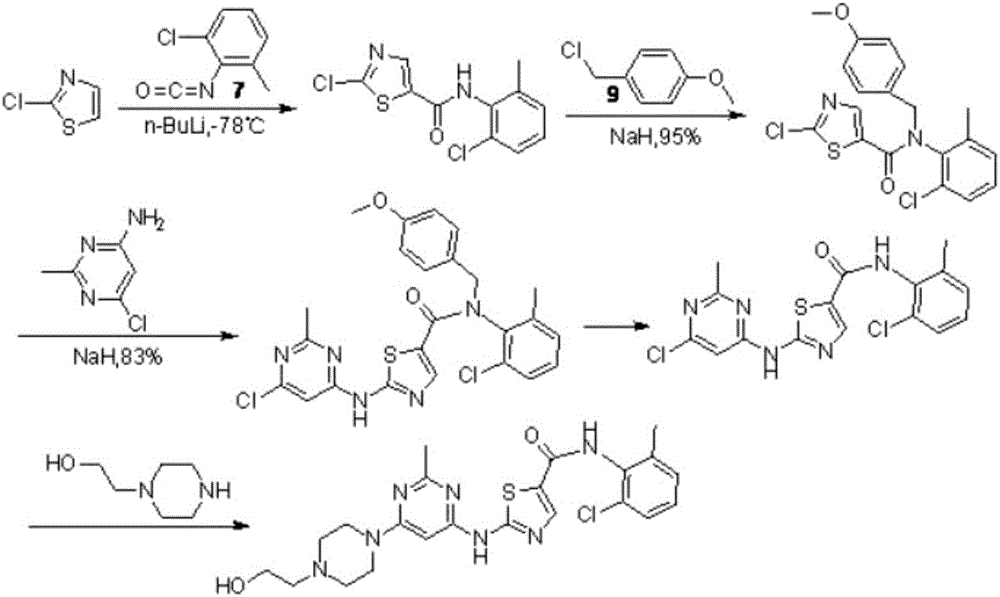
Novel process for cleanly producing acetamidine hydrochloride
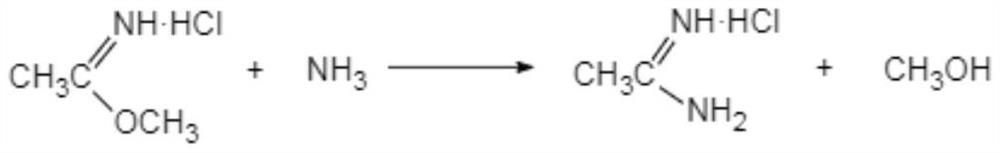
The clean production process of acetamidine hydrochloride includes the following steps: synthesis of methanol hydrochloride, synthesis of ethylidene amidine, synthesis of aminomethanol, ammonation of ethylidene amidine, concentration and centrifugation. The present invention features that hydrogen chloride gas for the synthesis of methanol hydrochloride is side product of paraffin chloride producing process, and has simple technological process, low production cost, high product yield and no environmental pollution.
Owner:LONGCHENG CHEM HAIAN COUNTY
Synthesis process of thiothiamine
The invention discloses a synthesis process of thiothiamine. The synthesis process comprises the following steps of dissociating acetamidine hydrochloride with liquid sodium methoxide and then filtering to retain the solution, placing the solution into a reactor, adding alpha-(o-chloroaniline)ylmethenyl-beta-formylaminopropionitrile (enamine), and then recovering methanol and carrying out cyclization reaction to obtain a cyclized solution; adding an aqueous phase to the cyclized solution, distilling, wherein the distillation temperature of vapor is 120 DEG C; after o-chloroaniline is completely stripped out, adding a caustic soda liquid, hydrolyzing and then adding water, adding carbon disulfide, reacting, finally adding gamma-chloro-gamma-acetyl propanol, carrying out condensation and filtering to obtain a thiothiamine crude; dissolving with hydrochloric acid, reacting, adding activated carbon, decolorizing, filtering to remain filtrate, neutralizing with the caustic soda liquid; and after a solid is precipitated, filtering and drying the solid to obtain the finished thiothiamine. The synthesis process of thiothiamine disclosed by the invention has the advantages that the process steps are simple, the yield of thiothiamine is high, the generation of wastes is reduced, the environment friendliness is achieved and the production cost is reduced.
Owner:江苏兄弟维生素有限公司
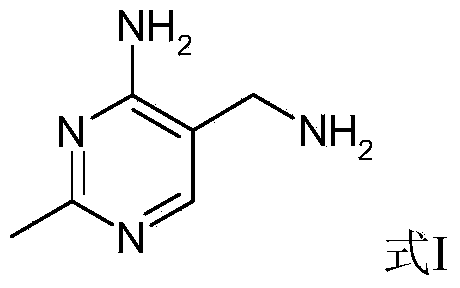
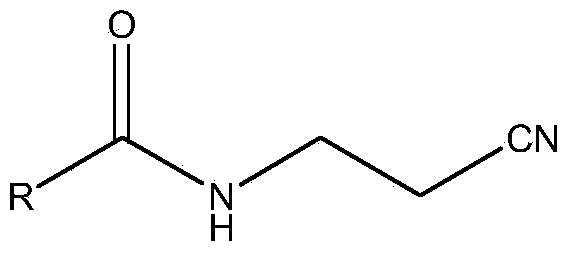
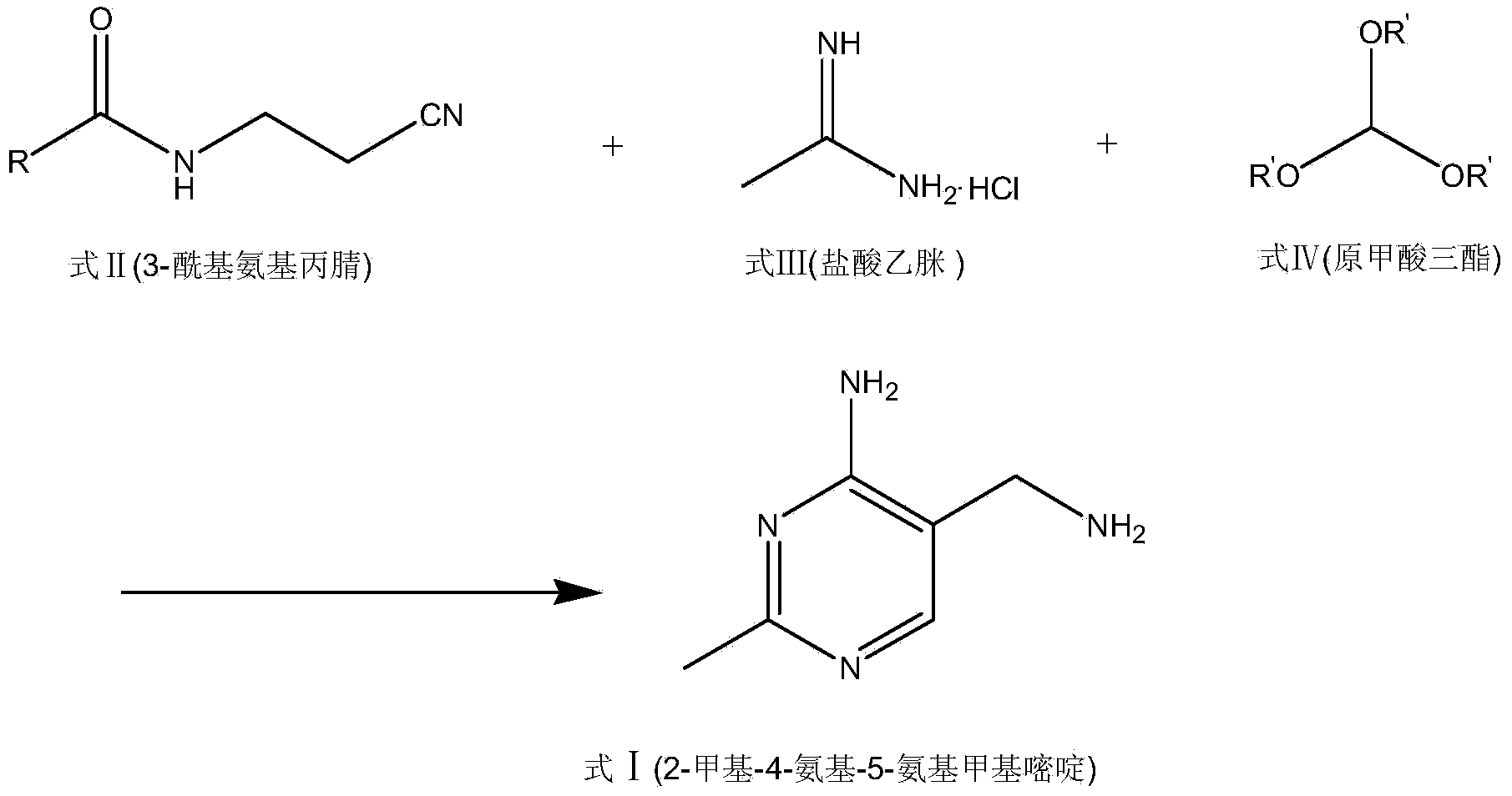
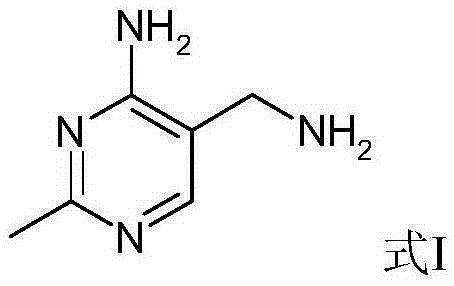
Portable synthesis method for preparing 2-methyl-4-amino-5-aminoethylpyrimidine through one-step cyclization reaction
ActiveCN103724279AGood for condensationSimple and fast operationOrganic chemistrySynthesis methodsDecomposition
The invention relates to a portable synthesis method for preparing 2-methyl-4-amino-5-aminoethylpyrimidine through one-step cyclization reaction. The method comprises the following steps: directly catalyzing acetamidine hydrochloride and 3-acyl aminopropionitrile to condense and to react with triester orthoformate for dealcoholization and cyclization by using lewis acid, and then hydrolyzing to obtain a vitamin B1 key intermediate, namely the 2-methyl-4-amino-5-aminoethylpyrimidine. According to the method disclosed by the invention, the raw materials are low in cost and easily obtained, and no sodium alcoholate needs to be used for dissociating acetamidine hydrochloride, thus the decomposition of acetamidine is reduced, and the high yield is achieved through reaction; the cyclization and hydrolysis reactions are sequentially carried out, products in all steps are not required to be separated and purified, and the synthesis method is simple and convenient to operate. Highly carcinogenic o-chloroaniline or other micromolecular aniline compounds are not used, and the portable synthesis method is environment-friendly in process, free of production of wastewater and beneficial to industrial production.
Owner:XINFA PHARMA
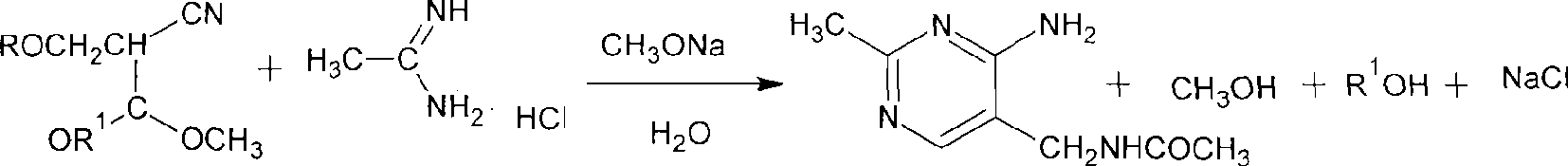
Process for preparing 2-methyl-4-amino-5-acetyl aminomethyl pyrimidine
The invention discloses a preparation method of 2-methyl-4-amino-5-acetylaminomethyl pyrimidine. The preparation method is characterized in that acetamidine hydrochloride reacts with sodium methoxide or sodium alcoholate to obtain alcoholic solution of ethanamidine after removing generated sodium chloride by filtration, and ethanamidine solution with a certain concentration is obtained after concentration; the ethanamidine solution react with acetal, methanol or ethanol is depressurized to dryness, then an obtained resultant is hydrolyzed to obtain crude acetylpyrimidine, and fixed amount of methanol or ethanol solution is added to separate refined acetylpyrimidine by crystallization. The methanol or the ethanol can be recovered from crystallization mother liquor in a distillation way, the recovered methanol or the ethanol can be applied to next batch, distillation residue is cooled and filtered after being added with a little water, and the acetylpyrimidine dissolved in a solvent is recovered. The preparation method has the advantages of high yield, good quality of the obtained product, no environmental pollution and the like.
Owner:HUAZHONG PHARMA
Preparation method of acetamidine hydrochloride
PendingCN111269145AAdjust and optimize ratioAdjust and optimize the processOrganic chemistryMethanol formationProcess engineering
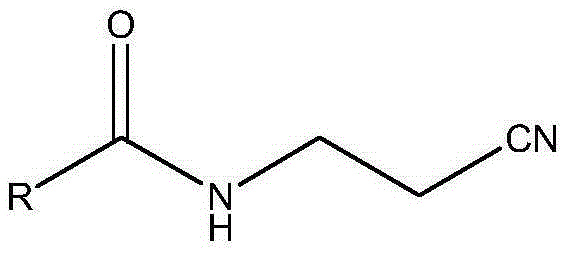
The invention discloses a preparation method of acetamidine hydrochloride. The preparation method comprises the following steps: a. introducing hydrogen chloride into a kettle in which concentrated sulfuric acid is stored, drying, and forming acid methanol with methanol; b, adding acid methanol into the reaction kettle, slowly dropwise adding acetonitrile through a metering tank, and controlling the temperature to be 9-11 DEG C; after acetonitrile is added, performing heat preservation for 5-7 hours at the temperature of about 20-28 DEG C to synthesize acetamidine; c, adding aminomethanol intothe reaction kettle, slowly adding in the earlier stage to control the temperature to be 0--5 DEG C, and quickly adding aminomethanol until the pH value is 7-8 when the adding amount is 1 / 3 and the pH value is 4-5 so as to complete ammoniation reaction; and d, after the ammoniation reaction is finished, centrifuging ammonium chloride, distilling off methanol, and centrifuging to obtain an acetamidine hydrochloride finished product. According to the method, the material ratio and process control indexes are adjusted and optimized so that the product yield is obviously improved, the product quality is obviously superior to relevant national standards, in addition, the equipment production capacity is enlarged, the methanol consumption is reduced and the operation is safe and feasible.
Owner:唐山威格化学工业有限公司
A nitrogen-modified catalyst for preparing vinyl chloride and its preparation method
ActiveCN104289254BSimple preparation processLow costPreparation by hydrogen halide split-offOrganic-compounds/hydrides/coordination-complexes catalystsBarium saltEthane Dichloride
The invention discloses a nitrogen-modified catalyst applied to preparation of vinyl chloride and a preparation method of the nitrogen-modified catalyst. According to the nitrogen-modified catalyst, active carbon is used as a carrier, wherein the nitrogen-modified catalyst comprises a metal salt loaded compound and a nitrogen-containing compound; according to the total mass of the catalyst, the mass percentage of the metal salt compound is 0.01-10%, and the mass percentage of the nitrogen-containing compound is 0.01-10%. The metal salt compound is strontium salt or barium salt or mixture of the strontium salt and the barium salt; the nitrogen-containing compound is selected from at least one of guanidine hydrochloride, acetamidine hydrochloride, acrylamide, urea, methanesulfonamide and cyanoacetamide. The nitrogen-modified catalyst is applied to reaction of preparing vinyl chloride by catalytic cracking of 1,2-dichloroethane; not only is the cracking temperature reduced, and also the nitrogen-modified catalyst is high in conversion rate of 1,2-dichloroethane and selectivity of vinyl chloride, and is efficient, and has energy-saving and environment-friendly effects.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
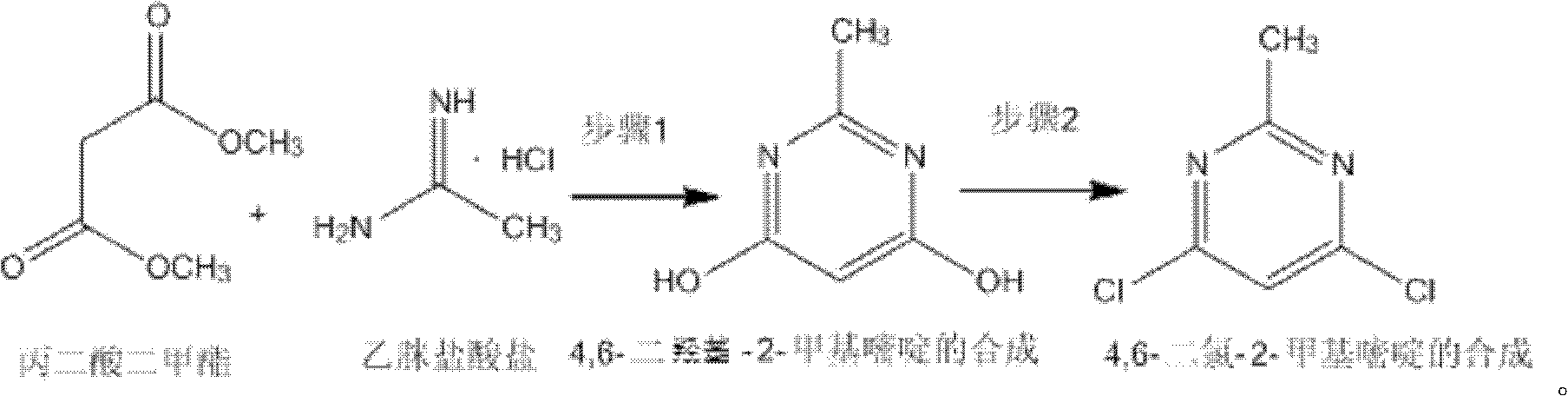
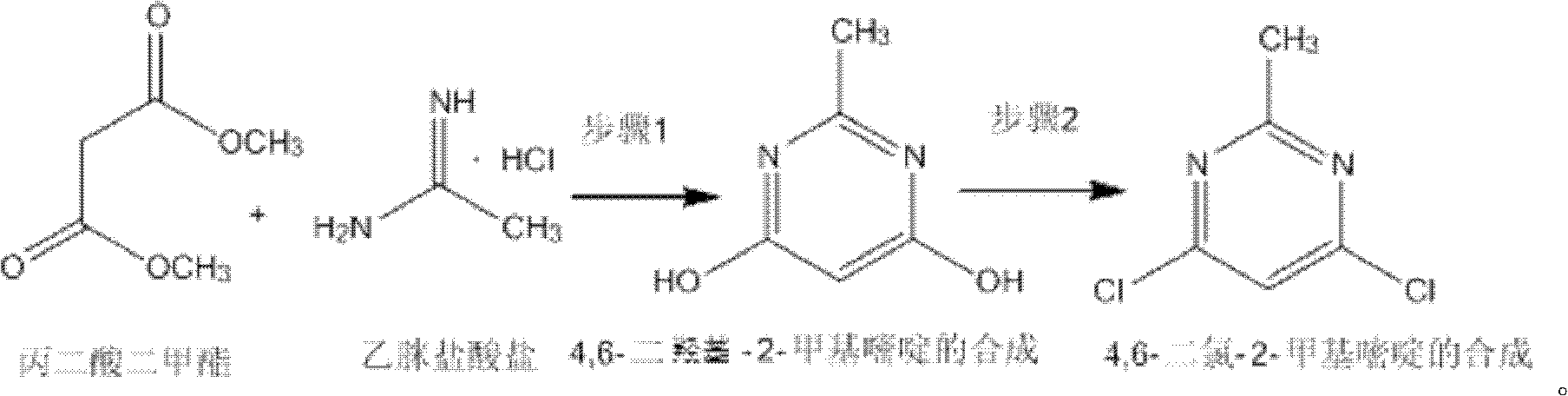
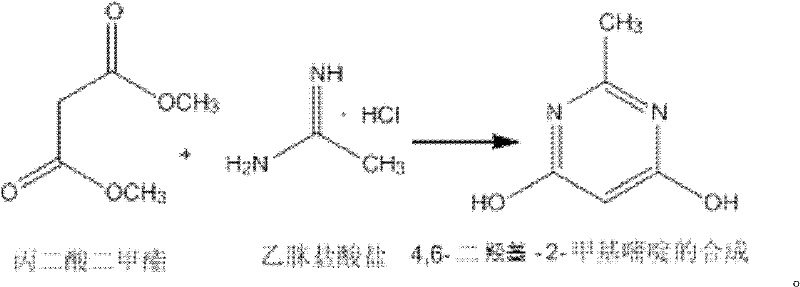
Method for synthetizing 4,6-dichloro-2-methyl pyridine
InactiveCN102432547AEasy to operateThe synthesis process is simpleOrganic chemistrySodium methoxideDistillation
The invention discloses a method for synthetizing 4,6-dichloro-2-methyl pyridine, which comprises the following steps of: under the ice-bath condition, adding sodium methoxide, dimethyl malonate and acetamidine hydrochloride into methanol; removing the ice bath, heating to a temperature of 18 to 25 DEG C and performing a reaction for 3 to 5 hours; carrying out reduced pressure distillation to remove the methanol; adding water for dissolving; regulating a pH value of the obtained solution to the range of 1 to 2; under the condition of a temperature of 0 DEG C, carrying out stirring and crystallization for 3 to 5 hours; carrying out extraction filtering, washing and drying to obtain white solid 4,6-dyhydroxyl-2-methyl pyridine; adding N,N-diethyl aniline and dichloroethane into the obtained 4,6-dyhydroxyl-2-methyl pyridine; heating to the reflux condition; then slowly adding a solution of triphosgene dichloroethane; performing a reflux reaction for 6 to 8 hours; washing reaction liquid; drying, filtering and concentrating an organic layer; and carrying out recrystallization and decolorization treatment on the obtain solid 4,6-dichloro-2-methyl pyridine. In the method, triphosgene is adopted to replace reagents with serious pollution to the environment and large toxicity, such as POC13, phosgene and the like. The method is safe, is easy to operate, has a simple synthetic process and is suitable for the industrial production.
Owner:太仓市运通化工厂
Synthesizing method for 4-amino-2-methyl-5-(brooethyl) pyrimidine hydrobromide
InactiveCN107382877ACheap to prepareRaw materials are easy to getOrganic chemistryHydrobromideSodium methoxide
The invention discloses a synthesizing method for 4-amino-2-methyl-5-(brooethyl) pyrimidine hydrobromide. The method comprises the following steps: acquiring formylated midbody sodium salt A by reacting 3-methoxypropionitrile with ethyl formate under the effect of sodium methylate; reacting the midbody sodium salt A with dimethyl sulfate, thereby acquiring a midbody B; condensing the midbody B with acetamidine hydrochloride, thereby acquiring a midbody C; and reacting the midbody C with hydrobromic acid, thereby acquiring a target product 4-amino-2-methyl-5-(brooethyl) pyrimidine hydrobromide I. According to the invention, the raw material source is wide and the cost is low; the synthesizing method is simple in production operation and has low requirement for processing equipment; the rigorous production conditions, such as, anhydrous condition, anaerobic condition and high-pressure hydrogenation, are not required; the reaction condition is mild; the method is easy for large-scale industrial production; the process is simple; the production period is short; the production efficiency is high; the production method is green and environmentally friendly and is suitable for large-scale industrial production.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Method for dissociating ethanamidine in acetamidine hydrochloride by liquid caustic soda and methyl alcohol mixed solution
InactiveCN107721879AIncrease profitReduce production safetyOrganic chemistryRotary evaporatorAlcohol
The invention provides a method for dissociating ethanamidine in acetamidine hydrochloride by a liquid caustic soda and methyl alcohol mixed solution. The method comprises the following steps: I, preparing the liquid caustic soda and methyl alcohol mixed solution, and preparing an acetamidine hydrochloride and methyl alcohol mixed solution; II, adding the liquid caustic soda and methyl alcohol mixed solution and the acetamidine hydrochloride and methyl alcohol mixed solution into a rotary evaporator, and evaporating the mixed solutions through the rotary evaporator; III, conveying the evaporated methyl alcohol solution into a distilling tower for recycling methyl alcohol; IV, filtering the solution in the rotary evaporator, washing an obtained filter cake with a little of methyl alcohol, and drying the filter cake to obtain a sodium chloride solid; V, generating 10 to 20 percent of liquid caustic soda and 10 to 20 percent of hydrochloric acid from the sodium chloride solid through a film treatment technology, concentrating the liquid caustic soda till the content is 32 percent, and putting the liquid caustic soda serving as a raw material into operation. According to the method, byadoption of liquid caustic soda and methyl alcohol for dissociation, the utilization rate of the liquid caustic soda is increased; after byproducts of the liquid caustic soda are washed and dried, the sodium chloride is obtained, and 100% recycle can be realized through the film treatment technology, so that the production cost is greatly reduced, and the environmental problems caused by free salt which are the byproducts are eliminated.
Owner:江苏兄弟维生素有限公司
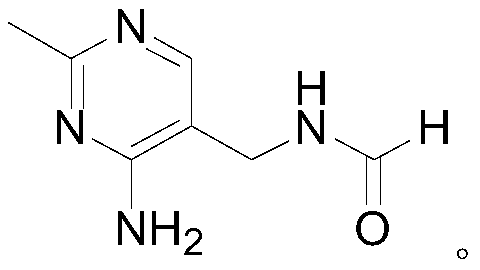
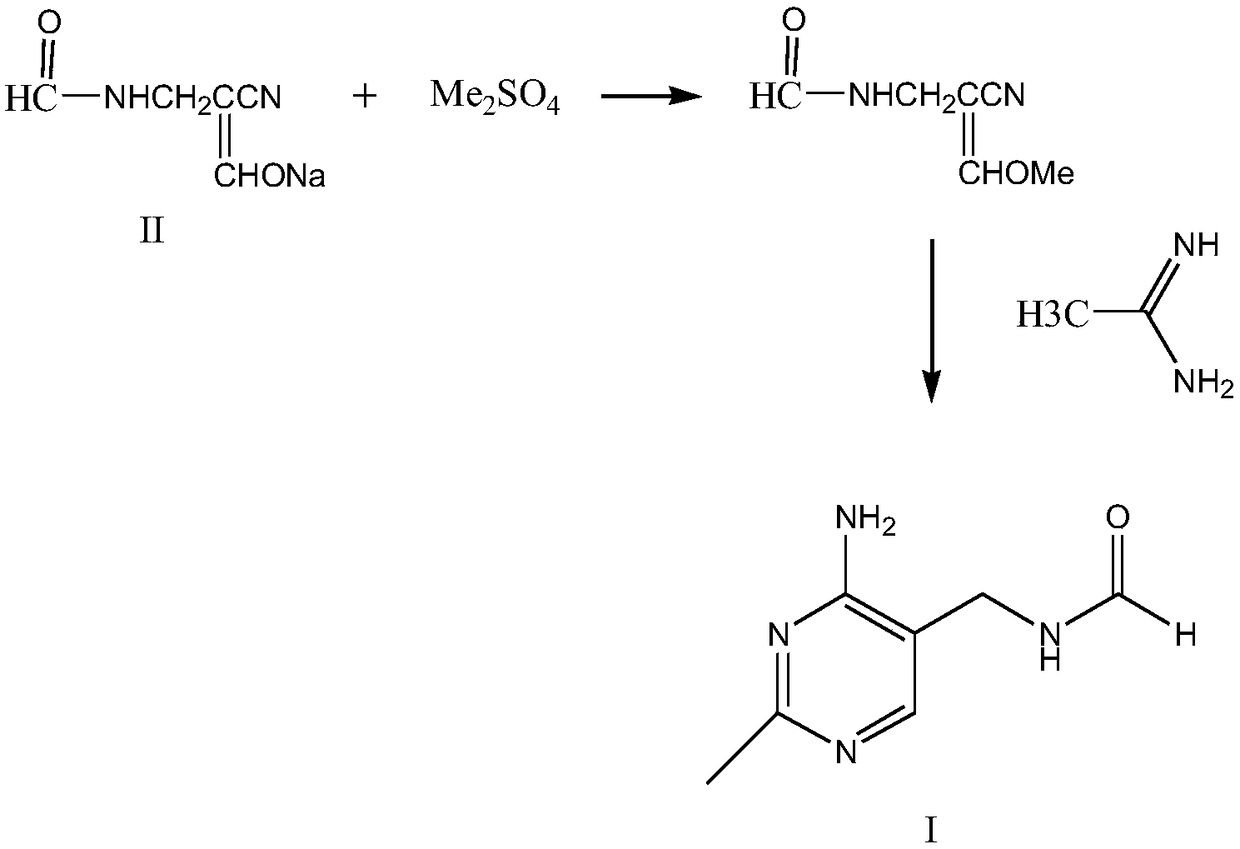
Method for synthesizing 2-methyl-4-amino-5-formamide methyl pyrimidine
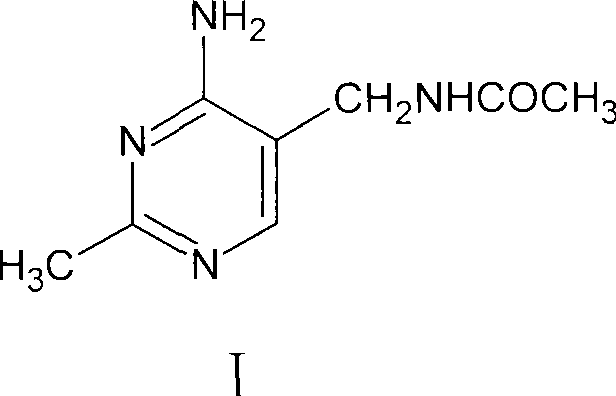
The invention discloses a method for synthesizing 2-methyl-4-amino-5-formamide methyl pyrimidine. The method comprises the following steps: conveying an organic ester solvent solution with beta-aminopropionitrile and an organic alcohol solution with sodium alcoholate into a pipeline reactor, performing a continuous reaction, discharging the materials, performing cooling, performing neutralizationto neutral, performing vacuum recycling on a solvent, continuously adding methylbenzene, and performing water washing so as to obtain a methylbenzene solution of 2-formyl-3-formyl amino-propionitrile;adding acetamidine hydrochloride into the organic alcohol solution with sodium alcoholate, performing filtration after addition, collecting filtrate, performing heating, putting the methylbenzene solution of 2-formyl-3-formyl amino-propionitrile; and adding acetamidine hydrochloride into the filtrate, under a vacuum condition, evaporating out the solvent and adding an alcohol of a corresponding volume at the same time, stopping the reaction when a solution of a volume equal to that of the organic alcohol solution with the sodium alcoholate is evaporated out, performing neutralization to neutral, performing vacuum crystallization, performing filtering, and performing drying, so as to obtain the compound. The method is simple, gentle in reaction condition, low in reaction equipment requirement, low in cost and high in yield.
Owner:XIAMEN KINGDOMWAY VI TAMIN INC +1
Preparation method for Dasatinib compound
ActiveCN106083839AMild reaction conditionsHigh yieldOrganic chemistryDasatinibCombinatorial chemistry
The invention discloses a preparation method for a Dasatinib compound. The synthetic method provided by the invention comprises the following steps: reacting initial raw material N-piperazine with methyl 3-chloro-3-oxopropanoate, thereby acquiring a compound IV; causing the compound IV react with the compound V, thereby acquiring a compound VI; cyclizing the compound VI and acetamidine hydrochloride, thereby acquiring the Dasatinib. According to the invention, the synthetic route is short, the operation is simple, the reaction condition is mild, the purity and yield are high and the method is fit for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
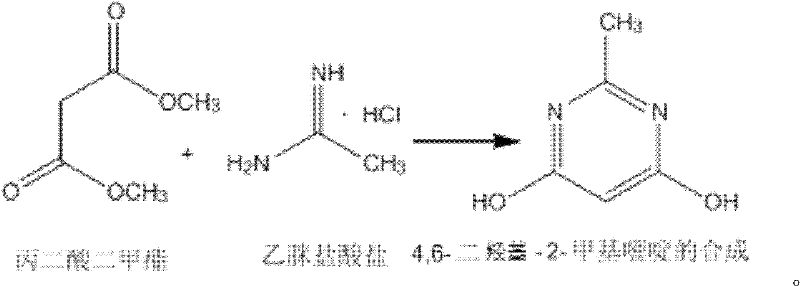
Synthesizing method of 4,6-dihydroxy-2-methylpyrimidine
InactiveCN102399196AEasy to operateThe synthesis process is simpleOrganic chemistrySodium methoxideDimethyl malonate
The invention discloses a synthesizing method of 4,6-dihydroxy-2-methylpyrimidine. The method comprises the following steps of adding sodium methoxide, dimethyl malonate and acetamidine hydrochloride in methanol under an ice-bath condition; removing the ice-bath; heating the mixture to 18-25 DEG C; reacting the mixture for 3-5 hours, distilling the reactant in reduced pressure to remove the methanol; adding water to dissolve the reactant; adjusting the PH value of the reactant to 1-2; and agitating the reactant at a temperature of 0 DEG C and devitrifying the reactant for 3-5 hours; filtering, washing and drying the product, obtaining white solid 4,6-dihydroxy-2-methylpyrimidine. In the invention, triphosgene is adopted to replace reagents such as POC13, phosgene and the like severely polluting the environment and with high toxicity, so that the synthesizing method provided by the invention is safe and easy to operate, the synthesizing process is simple and the synthesizing method is applicable to industrialization production.
Owner:太仓市运通化工厂
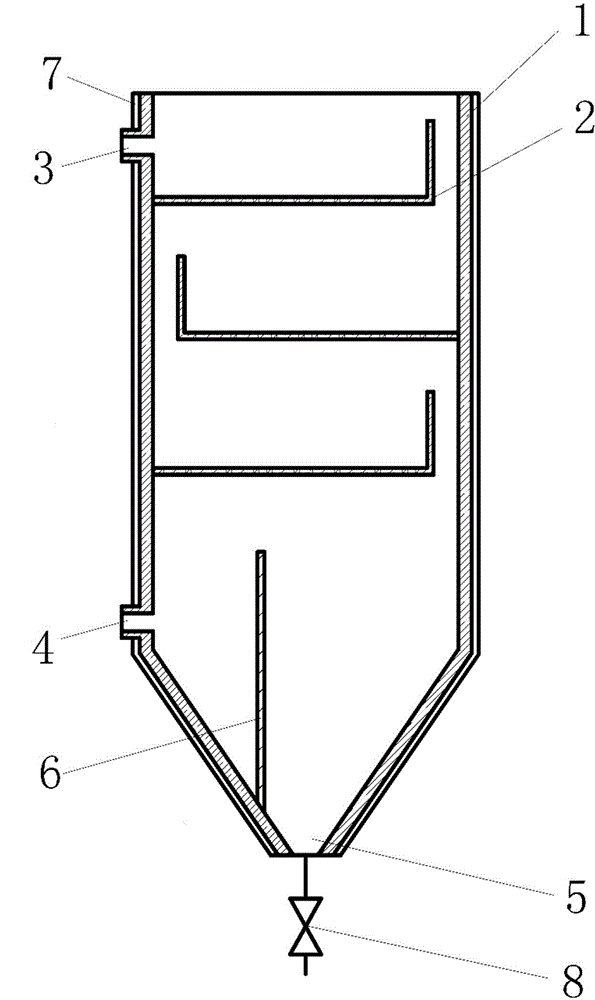
Device for rapidly and efficiently reducing content of ammonium chloride in acetamidine hydrochloride mother liquor
InactiveCN106268011AEfficient separationReduce contentOrganic chemistryFiltration circuitsSocial benefitsBiochemical engineering
The invention discloses a device for rapidly and efficiently reducing the content of ammonium chloride in acetamidine hydrochloride mother liquor. The device comprises a tank, inside which multiple overflow filter panels are arranged in a staggered manner from top to bottom. The upper part of the tank is provided with a water inlet, the lower part of the tank is equipped with a water outlet, and the bottom is provided with a drainage port. The water outlet is arranged on the sidewall of the tank and positioned at the opposite side of the opening of the lowest overflow filter panel. By potential difference overflow, multistage settlement and filtering, fine ammonium chloride particles precipitated out of the acetamidine hydrochloride mother liquor are separated rapidly and efficiently. Thus, the content of ammonium chloride in an acetamidine hydrochloride product is effectively reduced. No power source is required, and energy is saved. In addition, the separation is rapid and efficient. Production period of the product can be shortened, corresponding capital investment is reduced, and great social benefit and economic benefit can be generated.
Owner:YICHANG HENGYOU CHEM
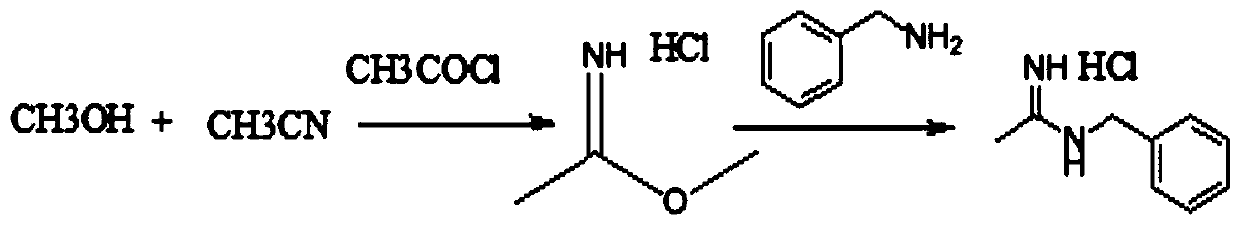
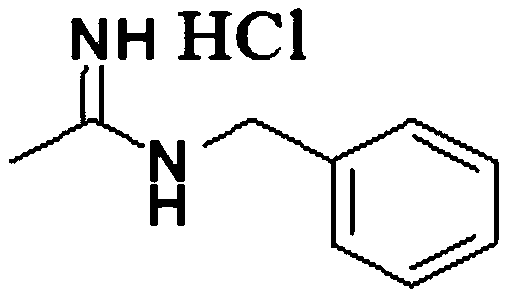
Synthesis method of N-benzyl acetamidine hydrochloride
ActiveCN110878032AMild reaction conditionsImprove product qualityOrganic chemistryHydroxylaminePtru catalyst
The invention discloses a synthesis method of N-benzyl acetamidine hydrochloride. The synthesis method mainly comprises the following three steps: 1, adding a benzyl hydroxylamine hydrochloride into toluene, adding a sodium hydroxide aqueous solution at a low temperature, and removing a water layer to obtain a benzyl hydroxylamine toluene solution; 2, adding acetonitrile into the benzyl hydroxylamine toluene solution, heating to react, and concentrating to remove the solvent after the reaction is finished, thereby obtaining an intermediate 1; and 3, adding the intermediate 1, ethyl acetate andraney nickel into an autoclave, introducing hydrogen for hydrogenation at a certain temperature, filtering to remove a catalyst, adding a hydrogen chloride methanol solution, cooling and crystallizing to obtain the target product with the purity higher than 95%. The synthetic route is mild in conditions, good in product quality, less in three wastes and simple and convenient in post-treatment, can meet the requirements of current green chemistry, has obvious economic and social benefits, and has a promising industrial application prospect.
Owner:苏州诚和医药化学有限公司
4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine preparation method
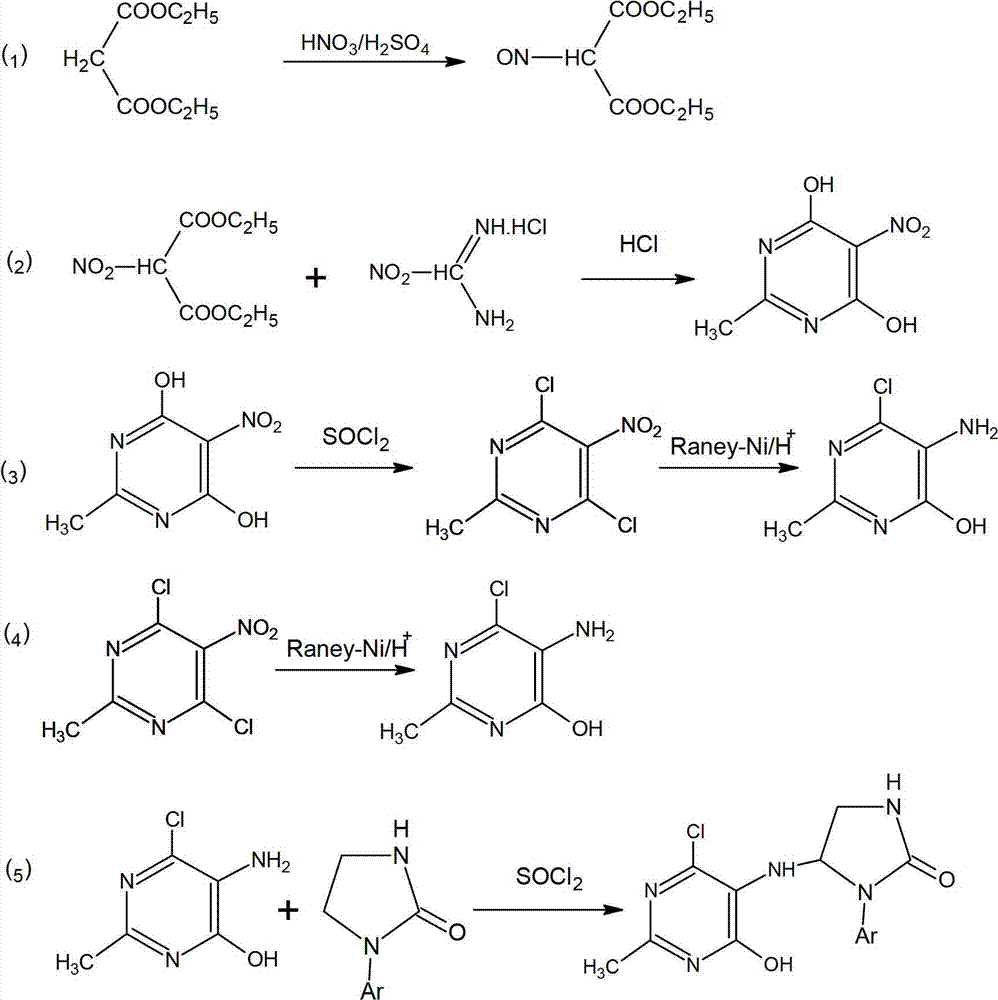
The invention discloses a 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine preparation method. The preparation method includes: firstly, feeding concentrated nitric acid and concentrated sulfuric acid into diethyl malonate for nitrifying diethyl malonate to obtain diethyl 2-nitromalonate; secondly, enabling the diethyl 2-nitromalonate and acetamidine hydrochloride to cyclize to obtain 2-methyl-5-nitro-4, 6-dihydroxypyrimidine under the action of 12N hydrochloric acid; thirdly, chloridizing the 2-methyl-5-nitro-4, 6-dihydroxypyrimidine with sulfoxide chloride serving as a chlorinating agent and hydrogenating and reducing the chloridized 2-methyl-5-nitro-4, 6-dihydroxypyrimidine with Raney nickel; and finally, condensing a product obtained in the third step and acetyl imidazo-2-one, so that 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine is obtained. The preparation method is short in reaction time, higher than 80% in yield and simple and convenient to operate as reaction processes are carried out under the normal pressure, the 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine is low in production cost, phosphorus wastewater is avoided, and emission of waste gases, wastewater and industrial residues is low.
Owner:上海旭东海普南通药业有限公司
Method for synthesizing acetamidine, acetamidine, application of acetamidine, and vitamin B1
ActiveCN111018744AHigh yieldReduce moisture contentOrganic chemistrySodium methoxideCombinatorial chemistry
The invention provides a method for synthesizing acetamidine, acetamidine and application thereof, and vitamin B1, belonging to the technical field of compound synthesis. The method for synthesizing the acetamidine comprises the following steps: reacting acetamidine hydrochloride with sodium methoxide to synthesize acetamidine, and adding methyl formate into a reaction system in the reaction process. The method is simple and convenient to operate, easy to implement and capable of effectively removing moisture in the acetamidine synthesis process and increasing the yield of acetamidine.
Owner:江苏兄弟维生素有限公司
Chemical pretreatment method of steel products and used pickling solution
The invention discloses a chemical pretreatment method of steel products and a used pickling solution. The chemical pretreatment method of steel products comprises four procedures of pickling, degreasing, washing, and phosphorizing, wherein coarse alkali is adopted in degreasing fluid; acrylonitrile-ethylene-styrene (AES) and anionic polyacrylamide (APAM) surfactants are respectively utilized to solve the demands of a normal temperature process on an active agent in the pickling and degreasing procedures; molybdate iron phosphating liquid is adopted in phosphorization; the used acid liquor comprises the following components in grams per liter: 101-135 g / L of sulfuric acid, 3 g / L of potassium rhodanide, 3 g / L of teiethylene tetramine-hexacetic acid, 3 g / L of acetamidine hydrochloride and 3 g / L of di-o-tolyl-thiourea; the mass concentration of the sulfuric acid is 15-19%. The method is simple in process, fewer in procedures, and free of an external heating device; about 80% of water can be saved; equipment investment can be reduced; about 70% of electricity can be saved in normal operation.
Owner:ZOOMLION HEAVY MASCH CO LTD
Preparation method of acetamidine hydrochloride
PendingCN113321598AOperational securityHigh yieldOrganic chemistryAcetamidine hydrochlorideMedicinal chemistry
The invention provides a preparation method of acetamidine hydrochloride. Specifically, an intermediate product acetamidine is dropwise added into aminomethanol to generate stable acetamidine hydrochloride by adopting a reverse dropwise adding mode, wherein the decomposition of acetamidine is effectively inhibited by adopting the reverse dropwise adding mode, and the yield of acetamidine hydrochloride can reach 95% or above. The method is safe to operate, high in yield, low in energy consumption and very suitable for large-scale application.
Owner:内蒙古益泽制药有限公司 +1
A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process
ActiveCN109400555BEliminate useLower synthesis costOrganic chemistryAcetamidine hydrochlorideSodium salt
The invention relates to the technical field of vitamin B synthesis intermediates, in particular to a process for dissociating acetamidine hydrochloride with alpha-acetyl-gamma-butyrolactone sodium salt. The process comprises the following steps: making alpha-acetyl-gamma-butyrolactone sodium salt react with the acetamidine hydrochloride and separating products to obtain acetamidine and alpha-acetyl-gamma-butyrolactone. According to the process, the intermediate product alpha-acetyl-gamma-butyrolactone sodium salt in the synthesis step of the alpha-acetyl-gamma-butyrolactone reacts with the acetamidine hydrochloride, the alpha-acetyl-gamma-butyrolactone sodium salt utilize hydrochloric acid coordinated in the acetamidine hydrochloride to achieve the effect that the alpha-acetyl-gamma-butyrolactone sodium salt produces the alpha-acetyl-gamma-butyrolactone, and the hydrochloric acid in the acetamidine hydrochloride is removed to form acetamidine. Synchronous production of two target products is achieved, the steps are saved, and the process is environmentally friendly and increases the revenue.
Owner:江苏兄弟维生素有限公司
A convenient synthetic method for the preparation of 2-methyl-4-amino-5-aminomethylpyrimidine by one-step cyclization
ActiveCN103724279BGood for condensationSimple and fast operationOrganic chemistryDecompositionSynthesis methods
The invention relates to a portable synthesis method for preparing 2-methyl-4-amino-5-aminoethylpyrimidine through one-step cyclization reaction. The method comprises the following steps: directly catalyzing acetamidine hydrochloride and 3-acyl aminopropionitrile to condense and to react with triester orthoformate for dealcoholization and cyclization by using lewis acid, and then hydrolyzing to obtain a vitamin B1 key intermediate, namely the 2-methyl-4-amino-5-aminoethylpyrimidine. According to the method disclosed by the invention, the raw materials are low in cost and easily obtained, and no sodium alcoholate needs to be used for dissociating acetamidine hydrochloride, thus the decomposition of acetamidine is reduced, and the high yield is achieved through reaction; the cyclization and hydrolysis reactions are sequentially carried out, products in all steps are not required to be separated and purified, and the synthesis method is simple and convenient to operate. Highly carcinogenic o-chloroaniline or other micromolecular aniline compounds are not used, and the portable synthesis method is environment-friendly in process, free of production of wastewater and beneficial to industrial production.
Owner:XINFA PHARMA
Process for preparing 2-methyl-4-amino-5-acetyl aminomethyl pyrimidine
The invention discloses a preparation method of 2-methyl-4-amino-5-acetylaminomethyl pyrimidine. The preparation method is characterized in that acetamidine hydrochloride reacts with sodium methoxide or sodium alcoholate to obtain alcoholic solution of ethanamidine after removing generated sodium chloride by filtration, and ethanamidine solution with a certain concentration is obtained after concentration; the ethanamidine solution react with acetal, methanol or ethanol is depressurized to dryness, then an obtained resultant is hydrolyzed to obtain crude acetylpyrimidine, and fixed amount of methanol or ethanol solution is added to separate refined acetylpyrimidine by crystallization. The methanol or the ethanol can be recovered from crystallization mother liquor in a distillation way, the recovered methanol or the ethanol can be applied to next batch, distillation residue is cooled and filtered after being added with a little water, and the acetylpyrimidine dissolved in a solvent is recovered. The preparation method has the advantages of high yield, good quality of the obtained product, no environmental pollution and the like.
Owner:HUAZHONG PHARMA
Preparation method and application of acetamidine hydrochloride
ActiveCN112679387ARealize continuous operationUniform and controllable residence timeOrganic compound preparationImino compound preparationPhysical chemistryAcetamidine hydrochloride
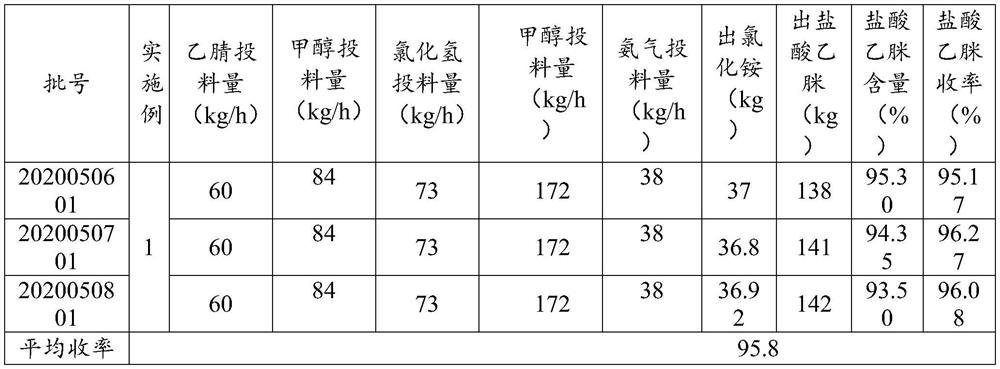
The invention provides a preparation method and application of acetamidine hydrochloride, and relates to the technical field of chemical engineering, and the preparation method comprises the following steps: a) mixing acetonitrile and methanol, and introducing the mixture and hydrogen chloride into a first micro-channel reactor for addition reaction to obtain acetamidine; b) introducing ammonia gas and methanol into a second micro-channel reactor for reaction to obtain aminomethanol; and c) simultaneously adding the acetamidine at the outlet of the first micro-channel reactor and the aminomethanol at the outlet of the second micro-channel reactor into the reaction kettle for ammoniation reaction to obtain the acetamidine hydrochloride. According to the method, the ethylidene amidine and the aminomethanol are obtained through the micro-channel reaction, then the acetamidine hydrochloride is obtained through ammonification, continuous operation is achieved, the cooling effect of the reaction is good, the cooling efficiency is high, the standing time is uniform and controllable, and side reactions caused by different reaction states can be avoided.
Owner:江苏兄弟维生素有限公司
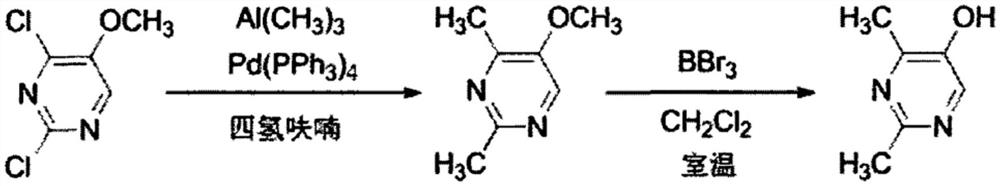
Method for synthesizing 2, 4-dimethyl pyrimidine-5-alcohol serving as intermediate of Leibolifera
The invention discloses a method for synthesizing 2, 4-dimethyl pyrimidine-5-alcohol serving as an intermediate of Lemborexant , and aims to solve the problems of safety risk and heavy environmental pollution of the 2, 4-dimethyl pyrimidine-5-alcohol in industrial production of the 2, 4-dimethyl pyrimidine-5-alcohol. The preparation method comprises the following steps: firstly, carrying out esterification reaction on benzoic acid serving as an initial raw material and chloroacetone; then, carrying out alkylation reaction and ring closing reaction with N, N-dimethylformamide dimethyl acetal and acetamidine hydrochloride; and finally, carrying out hydrolysis reaction to remove benzoic acid to obtain the 2, 4-dimethyl pyrimidine-5-alcohol. According to the method, benzoic acid widely existing in nature serves as a main raw material, the health problem caused in production is solved, the raw materials are non-toxic, and the method has the advantages that the EHS risk is low, the cost is low, the production efficiency is high, the yield is larger than or equal to 60%, and the purity is larger than or equal to 99.0%.
Owner:广西天铭药业有限公司
Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine*
ActiveCN105481863BLow reaction temperatureShort reaction timeOrganic chemistryMethyl benzeneReaction temperature
The invention provides a synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine. According to the invention, compound 4-bromo-1-[(4-methylphenyl) sulfonyl]-1,2,3,4-tetrahydro-5H-1-benzazepine-5-one represented by formula I is reacted with acetamidine hydrochloride in the presence of cesium carbonate in a reaction solvent. The synthetic method comprises following advantages compared with the prior art, reaction temperature is lower, reaction time is shorter, and finished product yield and purity are both increased to certain degrees.
Owner:科贝源(北京)生物医药科技有限公司
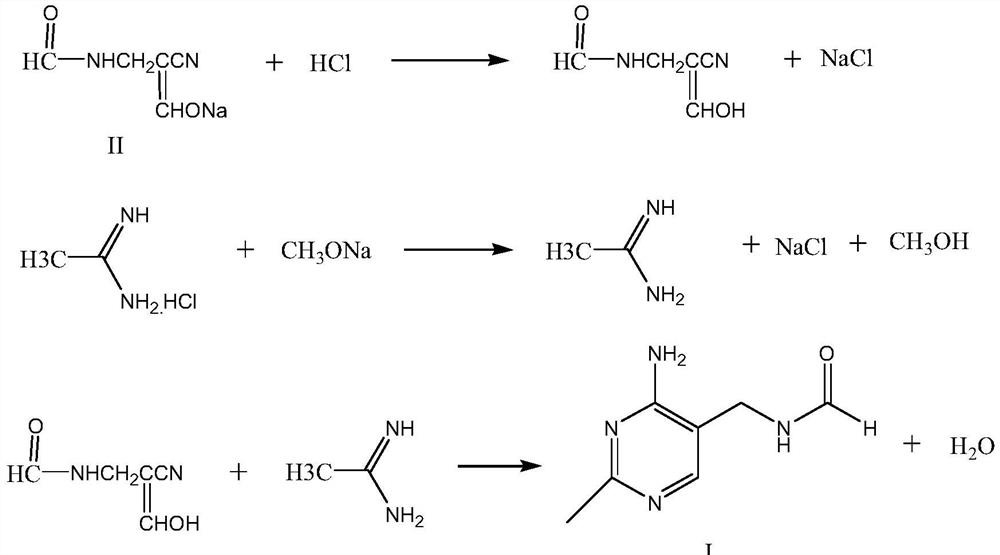
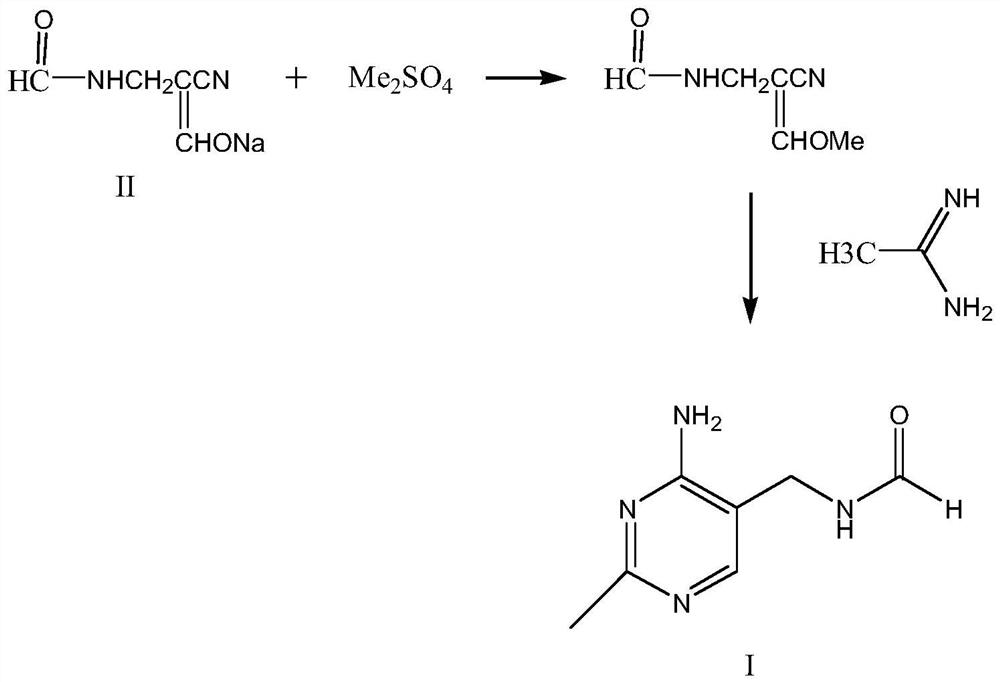
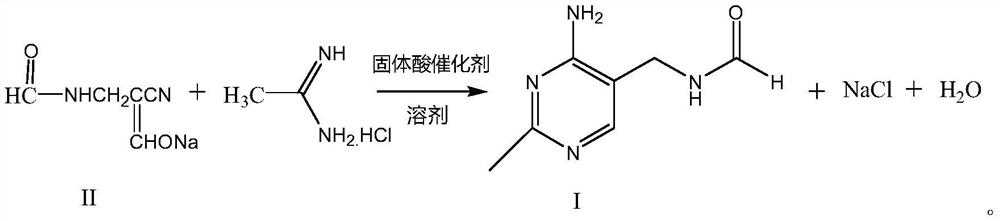
Synthetic method of 2-methyl-4-amino-5-formyl aminomethylpyrimidine
The invention relates to a synthetic method of 2-methyl-4-amino-5-formyl aminomethylpyrimidine. The method comprises the following steps: a benzene solvent and a solid acid catalyst are added to a reaction flask and stirred, alpha-sodio formyl-beta-formyl aminopropionitrile is added, heating is performed, an acetamidine hydrochloride solution dissolved in an alcohol solvent is added dropwise, a thermal reaction and filtering are performed, the solid acid catalyst is recovered and treated for recycling, and a filtrate is a 2-methyl-4-amino-5-formyl aminomethylpyrimidine solution. O-chloroaniline as a raw material used in the traditional synthetic process is not used, 2-methyl-4-amino-5-formyl aminomethylpyrimidine is directly synthesized from alpha-sodio formyl-beta-formyl aminopropionitrile and acetamidine hydrochloride under the action of the solid acid catalyst, intermediate processes are omitted, the method is an environment-friendly process because the solid acid catalyst is recyclable, and trace cancerogen residues in a vitamin B1 product are avoided; synthesis yield is high, and the requirement for current green development is met.
Owner:ZHEJIANG BENLI TECH CO LTD
Industrial preparation method for 4-chlorine-5-fluorine-2-methyl pyrimidine
InactiveCN104926735AAvoid safety hazardsSolve Post-Processing IssuesOrganic chemistryFluoroacetic acidPropanoic acid
The invention relates to an industrial preparation method for 4-chlorine-5-fluorine-2-methyl pyrimidine, and the problems that existing 4-chlorine-5-fluorine-2-methyl pyrimidine is not easy to amplify and low in yield coefficient are solved. According to the technical scheme, the industrial preparation method for the 4-chlorine-5-fluorine-2-methyl pyrimidine comprises the steps that 1 ethyl formate and ethyl fluoroacetate are reacted by potassium tert-butoxide in an alkali mode, and after a midbody of 2-fluorine-3-oxo, ethyl propionate sodium salt is obtained and reacted with acetamidine hydrochloride in a ring closing mode, 5-fluorine-2-methyl pyrimidine-4(3H)-ketone is obtained; 2 chlorination is conducted, phosphorus oxychloride serves as a chloride agent, organic alkali serves as an additive, and the 4-chlorine-5-fluorine-2-methyl pyrimidine is obtained in solvent through a reaction. The invention is used for the industrial preparation method for the 4-chlorine-5-fluorine-2-methyl pyrimidine.
Owner:SHANGHAI STA PHARMA R&D CO LTD +3
Micro-reaction system and method for continuously preparing 2-methyl-4-amino-5-cyanopyrimidine by using same
ActiveCN112851588AImprove heat transfer performanceExcellent material mixing performanceOrganic chemistryTransportation and packagingProcess engineeringMethylene radical
The invention belongs to the technical field of pharmaceutical engineering, and particularly relates to a micro-reaction system and a method for continuously preparing 2-methyl-4-amino-5-cyanopyrimidine by using the micro-reaction system. The preparation method comprises the following steps: respectively and simultaneously pumping an acetamidine hydrochloride solution and a (dimethyl aminomethylene) malononitrile solution into a micro-reaction system comprising a micro-mixer and an oscillating micro-channel reactor which are communicated in sequence, and carrying out a continuous condensation cyclization reaction to obtain the 2-methyl-4-amino-5-cyanopyrimidine. Compared with the prior art, the method has the advantages that the reaction time is shortened to be within 30 minutes, side reactions are inhibited to the maximum extent, the yield of the product 2-methyl-4-amino-5-cyanopyrimidine is increased to 90% or above, operation is easy and convenient, the technological process is continuous, the automation degree is high, the space time yield is high, energy consumption is greatly reduced, cost is low, and industrial application is easy.
Owner:FUDAN UNIV
A kind of steel chemical pretreatment method and used pickling solution
The invention discloses a chemical pretreatment method of steel products and a used pickling solution. The chemical pretreatment method of steel products comprises four procedures of pickling, degreasing, washing, and phosphorizing, wherein coarse alkali is adopted in degreasing fluid; acrylonitrile-ethylene-styrene (AES) and anionic polyacrylamide (APAM) surfactants are respectively utilized to solve the demands of a normal temperature process on an active agent in the pickling and degreasing procedures; molybdate iron phosphating liquid is adopted in phosphorization; the used acid liquor comprises the following components in grams per liter: 101-135 g / L of sulfuric acid, 3 g / L of potassium rhodanide, 3 g / L of teiethylene tetramine-hexacetic acid, 3 g / L of acetamidine hydrochloride and 3 g / L of di-o-tolyl-thiourea; the mass concentration of the sulfuric acid is 15-19%. The method is simple in process, fewer in procedures, and free of an external heating device; about 80% of water can be saved; equipment investment can be reduced; about 70% of electricity can be saved in normal operation.
Owner:ZOOMLION HEAVY MASCH CO LTD
Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine
ActiveCN105481863ALow reaction temperatureShort reaction timeOrganic chemistryMethyl benzeneReaction temperature
The invention provides a synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine. According to the invention, compound 4-bromo-1-[(4-methylphenyl) sulfonyl]-1,2,3,4-tetrahydro-5H-1-benzazepine-5-one represented by formula I is reacted with acetamidine hydrochloride in the presence of cesium carbonate in a reaction solvent. The synthetic method comprises following advantages compared with the prior art, reaction temperature is lower, reaction time is shorter, and finished product yield and purity are both increased to certain degrees.
Owner:科贝源(北京)生物医药科技有限公司
The synthetic method of 2-methyl-4-amino-5-formylaminomethylpyrimidine
ActiveCN109369540BReduce usageAvoid residueOrganic chemistryChemical recyclingAminopropionitrilePtru catalyst
The invention relates to a synthetic method of 2-methyl-4-amino-5-formyl aminomethylpyrimidine. The method comprises the following steps: a benzene solvent and a solid acid catalyst are added to a reaction flask and stirred, alpha-sodio formyl-beta-formyl aminopropionitrile is added, heating is performed, an acetamidine hydrochloride solution dissolved in an alcohol solvent is added dropwise, a thermal reaction and filtering are performed, the solid acid catalyst is recovered and treated for recycling, and a filtrate is a 2-methyl-4-amino-5-formyl aminomethylpyrimidine solution. O-chloroaniline as a raw material used in the traditional synthetic process is not used, 2-methyl-4-amino-5-formyl aminomethylpyrimidine is directly synthesized from alpha-sodio formyl-beta-formyl aminopropionitrile and acetamidine hydrochloride under the action of the solid acid catalyst, intermediate processes are omitted, the method is an environment-friendly process because the solid acid catalyst is recyclable, and trace cancerogen residues in a vitamin B1 product are avoided; synthesis yield is high, and the requirement for current green development is met.
Owner:ZHEJIANG BENLI TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

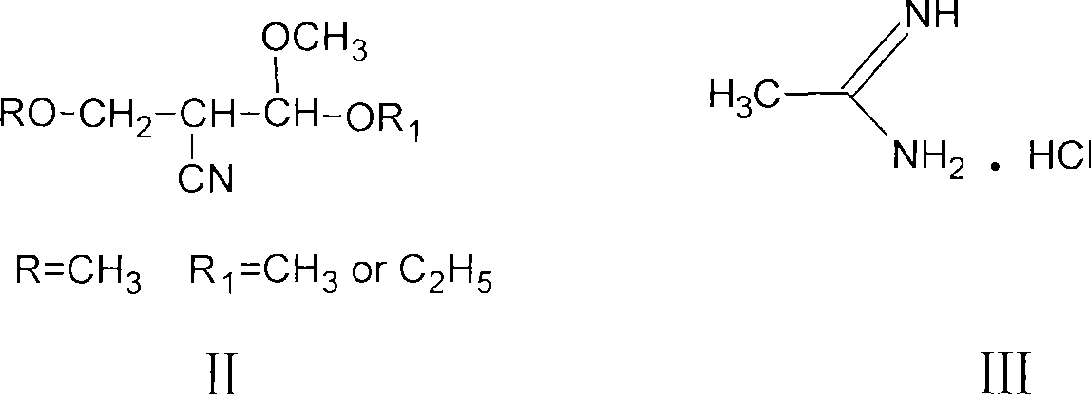











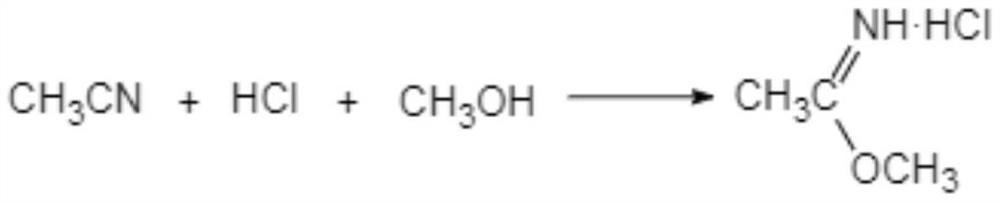


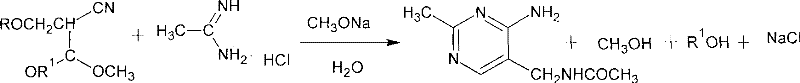





![Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine* Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine*](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5244286c-b6c2-401f-871c-e90b6ee5a18a/BDA0000871667950000012.png)
![Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine* Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine*](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5244286c-b6c2-401f-871c-e90b6ee5a18a/BDA0000871667950000013.png)
![Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine* Synthesis of 2-methyl-6-(4-methylbenzenesulfonyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine*](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5244286c-b6c2-401f-871c-e90b6ee5a18a/BDA0000871667950000021.png)








![Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac8214c9-10e3-4884-9238-01c214202f63/BDA0000871667950000012.PNG)
![Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac8214c9-10e3-4884-9238-01c214202f63/BDA0000871667950000013.PNG)
![Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine Synthetic method of 2-methyl-6-(4-methylphenyl)sulfonyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac8214c9-10e3-4884-9238-01c214202f63/BDA0000871667950000021.PNG)