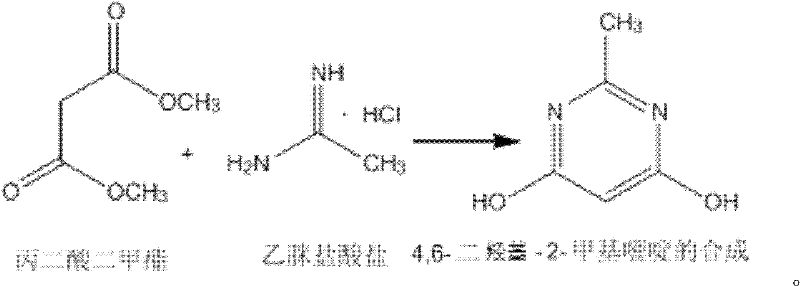
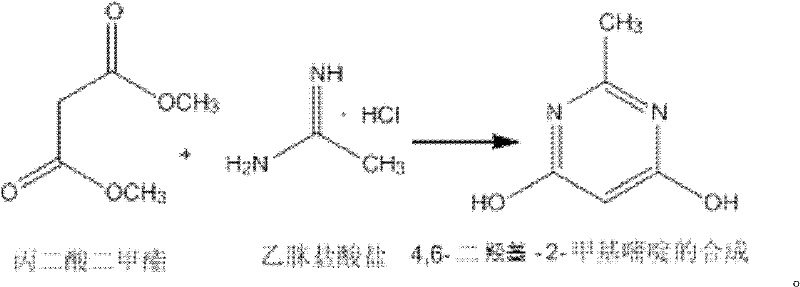
Synthesizing method of 4,6-dihydroxy-2-methylpyrimidine
A technology of methylpyrimidine and synthesis method, applied in 4 fields, can solve the problems of complex synthesis process route, serious environmental pollution, unsuitable for industrial production and the like, and achieves the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 150ml of methanol to a 500ml three-necked flask, and add 18.4g (0.34mol) of sodium methoxide while stirring under ice bath conditions, and add 13.2g (0.1mol) of dimethyl malonate and acetamidine salt after the dissolution is complete Acetate 9.45g (0.1mol), then remove the ice bath and heat up to 18-25°C for 4h reaction, the solution is milky white, after the reaction is completed, methanol is distilled off under reduced pressure (30-35°C), then add 50mL water to dissolve, and use 4mol / L dilute hydrochloric acid to adjust the pH to 1~2. At this time, a white solid precipitated. Stir and crystallize at 0°C for 4 hours, filter with suction, wash with ice water and ice methanol at 0~5°C, and dry to obtain a white solid. 10.8 g of 4,6-dihydroxy-2-methylpyrimidine, the yield is 86%.
Embodiment 2
[0017] Add 1060ml of methanol to a 3L three-necked flask, and add 108.2g (2mol) of sodium methylate while stirring under ice bath conditions, and add 105.6g (0.8mol) of dimethyl malonate and acetamidine hydrochloride after the dissolution is complete Salt 113.4g (1.2mol), then remove the ice bath and heat up to 18-25°C for 5h reaction, the solution is milky white, after the reaction is completed, methanol is distilled off under reduced pressure (30-35°C), then add 400mL water to dissolve, and use 4mol / L dilute hydrochloric acid to adjust the pH to 1-2. At this time, a white solid precipitated out. Stir and crystallize at 0°C for 4 hours, filter with suction, wash with ice water and ice methanol at 0-5°C, and dry to obtain a white solid 4 , 6-dihydroxy-2-methylpyrimidine 86g, the yield is 87%.
Embodiment 3
[0019] Add 3960ml of methanol into a 10L three-necked flask, and add 608.8g (11.25mol) of sodium methoxide while stirring under ice bath conditions, and add 330g (2.5mol) of dimethyl malonate and acetamidine hydrochloride after the dissolution is complete Salt 472.5g (5mol), then remove the ice bath and heat up to 18-25°C for 5h reaction, the solution is milky white, after the reaction is completed, methanol is distilled off under reduced pressure (30-35°C), then add 50mL water to dissolve, and use 4mol / L Dilute hydrochloric acid to adjust the pH to 1-2. At this time, a white solid precipitated out. Stir and crystallize at 0°C for 5 h, filter with suction, wash with ice water and ice methanol at 0-5°C, and dry to obtain white solid 4. 250 g of 6-dihydroxy-2-methylpyrimidine, the yield is 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com