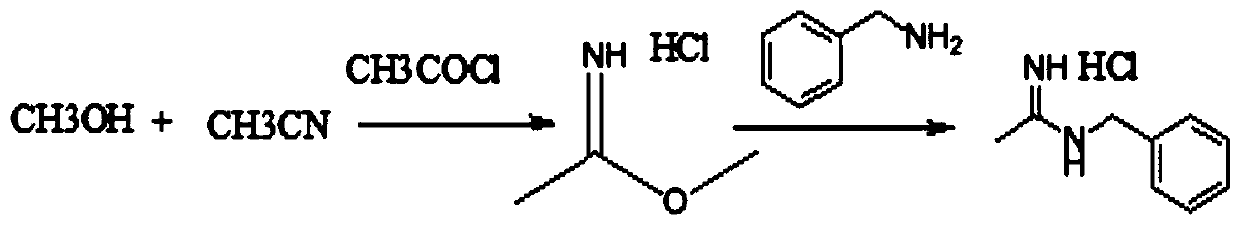
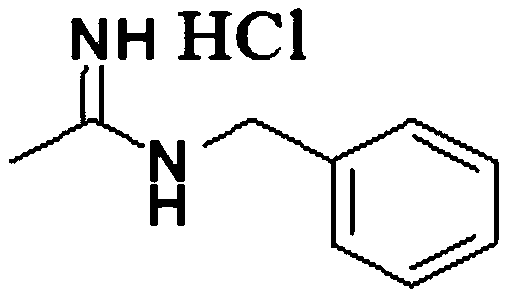
Synthesis method of N-benzyl acetamidine hydrochloride
A technology of benzylacetamidine hydrochloride and a synthesis method is applied in the field of synthesis technology of amidine compounds, can solve problems such as environmental pollution, and achieve the effects of simple post-processing, mild reaction conditions, and good prospects for industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of N-benzylacetamidine hydrochloride comprises the following steps:
[0024] I: Add 20g (0.125mol) of benzylhydroxylamine hydrochloride and 100ml of toluene into a 250ml reaction flask, stir and cool to 0°C under nitrogen protection for 2 hours, adjust the pH to 9-10 with 2M aqueous sodium hydroxide solution, and let it stand , the lower aqueous layer was removed, the organic layer was dried by adding 5 g of sodium sulfate, filtered, and the filtrate was added to the reaction flask;
[0025] II: Add 10.25 g (0.25 mol) of acetonitrile into the above reaction flask, raise the temperature to 50°C under the protection of nitrogen, keep it warm for 3 hours, and concentrate under negative pressure until no liquid comes out to obtain 17.2 g of intermediate 1 with a yield of 84%. ;
[0026] III: Put 17.2g of intermediate 1, 100ml of ethyl acetate, and 2g of Raney nickel into the autoclave, replace with nitrogen twice, and then pass in hydrogen to 0.6MPa, raise th...
Embodiment 2
[0028] The synthesis of N-benzylacetamidine hydrochloride comprises the following steps:
[0029] I: Add 20g (0.125mol) of benzylhydroxylamine hydrochloride and 100ml of toluene to a 250ml reaction bottle, stir and cool to 5°C under nitrogen protection for 3 hours, adjust the pH to 9-10 with 2M aqueous sodium hydroxide solution, and let it stand , the lower aqueous layer was removed, the organic layer was dried by adding 5 g of sodium sulfate, filtered, and the filtrate was added to the reaction flask;
[0030] II: Add 10.25 g (0.25 mol) of acetonitrile to the above reaction flask, raise the temperature to 70°C under the protection of nitrogen, keep it warm for 5 hours, concentrate under negative pressure until no liquid comes out, and obtain 18.9 g of intermediate 1 with a yield of 92%;
[0031] III: Put 18.9g of intermediate 1, 100ml of ethyl acetate, and 2g of Raney nickel into the autoclave, replace with nitrogen for 3 times, then pass in hydrogen to 0.8MPa, raise the temp...
Embodiment 3
[0033] The synthesis of N-benzylacetamidine hydrochloride comprises the following steps:
[0034] I: Add 20g (0.125mol) of benzylhydroxylamine hydrochloride and 100ml of toluene into a 250ml reaction flask, stir and cool to 5°C for 4 hours under nitrogen protection, adjust the pH to 9-10 with 2M aqueous sodium hydroxide solution, and let it stand , the lower aqueous layer was removed, the organic layer was dried by adding 5 g of sodium sulfate, filtered, and the filtrate was added to the reaction flask;
[0035] II: Add 10.25 g (0.25 mol) of acetonitrile into the above reaction flask, raise the temperature to 80°C under the protection of nitrogen, keep it warm for 8 hours, concentrate under negative pressure until no liquid comes out, and obtain 18.2 g of intermediate 1 with a yield of 88%;
[0036] III: Put 18.2g of intermediate 1, 100ml of ethyl acetate, and 2g of Raney nickel into the autoclave, replace with nitrogen for 3 times, and then pass in hydrogen to 1.0MPa, raise t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com