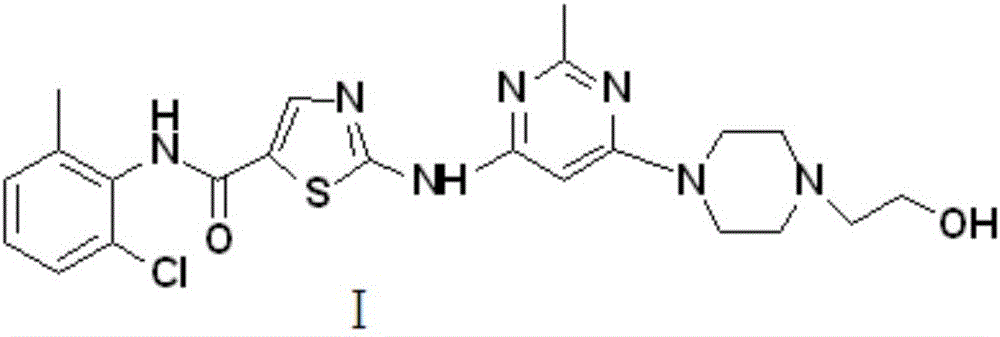
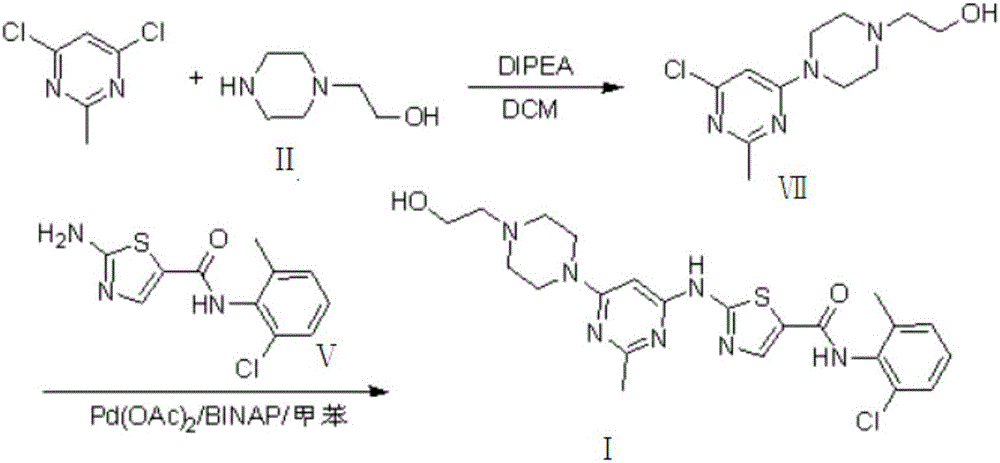
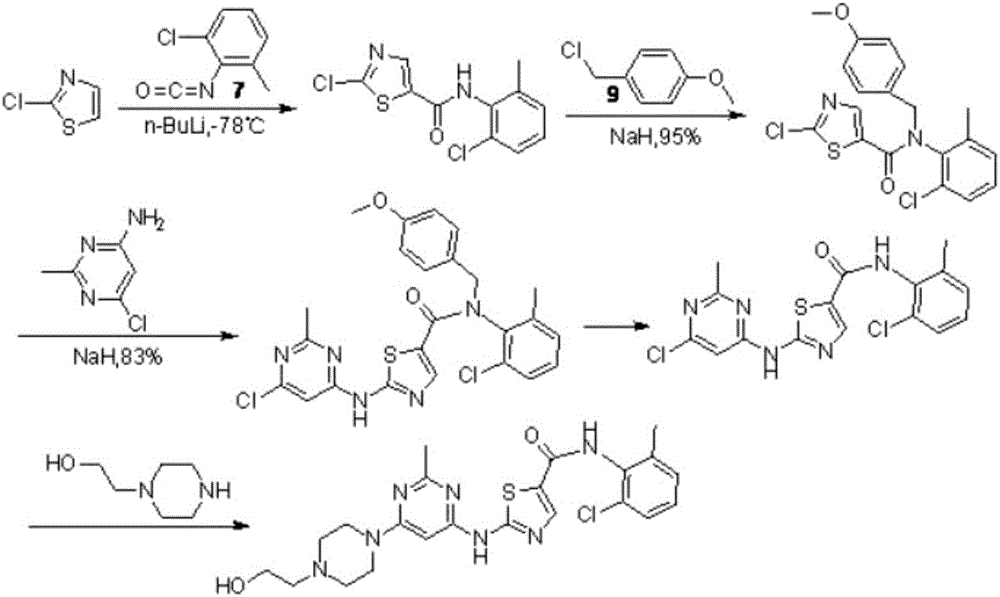
Preparation method for Dasatinib compound
A dasatinib and compound technology, applied in the field of drug synthesis, to achieve the effects of fewer by-products, improved product yield and purity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of compound IV
[0033] Take 13.0g of N-hydroxyethylpiperazine and place it in a 1000ml three-necked flask, add 500ml of dichloromethane, stir until dissolved, and react at room temperature; start to add 13.7g of methyl malonate chloride dropwise, and during the dropwise addition process 31.8g of sodium carbonate was added in batches, and after the low price was completed, stirred for 1.5h, the reaction was completed, filtered with suction, washed with distilled water, recrystallized with ethyl acetate, and dried in vacuo to obtain 20.6g of crystal powder with a yield of 88.9%. HPLC purity 99.3%.
Embodiment 2
[0034] Embodiment 2: the preparation of compound IV
[0035] Take 13.0g of N-hydroxyethylpiperazine and place it in a 1000ml three-neck flask, add 500ml of ethyl acetate, stir until dissolved, and react at room temperature; start to add 15.0g of methyl malonate chloride dropwise, Add 12.0 g of sodium hydroxide in batches, after the reduction is completed, stir for 1.5 h, after the reaction is complete, filter with suction, wash with distilled water, recrystallize with ethyl acetate, and dry in vacuo to obtain 21.1 g of crystal powder with a yield of 91.2%. , HPLC purity 99.5%.
Embodiment 3
[0036] Embodiment 3: the preparation of compound IV
[0037] Take 13.0g of N-hydroxyethylpiperazine and place it in a 1000ml three-necked flask, add 500ml of N,N-dimethylformamide, stir until dissolved, and react at room temperature; g, during the dropwise addition, add 10.0 g of potassium bicarbonate in batches, after the low price is completed, stir for 1.5 h, after the reaction is completed, filter with suction, wash with distilled water, recrystallize with ethyl acetate, and dry in vacuo to obtain crystal powder 20.1 g, yield 86.5%, HPLC purity 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com