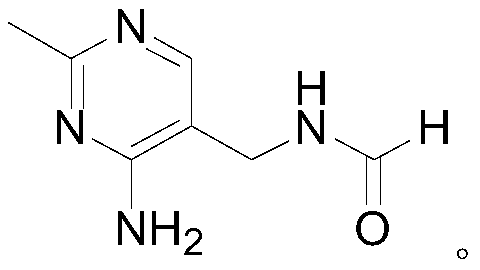
Method for synthesizing 2-methyl-4-amino-5-formamide methyl pyrimidine
A technology of formamide methyl pyrimidine and synthesis method, which is applied in the field of compound synthesis and can solve the problems of high construction cost and maintenance cost, residual end product and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 174g methyl formate (content 97.0%, 2.81mol) in the three-necked bottle, add 40g beta-aminopropionitrile (content 98.5%, 0.56mol) wherein, stir; Add 75.92g liquid methanol in another three-necked bottle Sodium (content 20%, 0.28mol); Setting pipeline reactor temperature is 55 ℃, opens feed pump, and above-mentioned two strands of materials are fed simultaneously, feed rate is 21.4g / min and 7.59g / min, reactor The residence time in medium is about 3min. After the reaction is stable, receive and discharge the material for 5min (i.e., take half of the product for the subsequent reaction, the following examples are the same), the feed liquid is cooled to room temperature, neutralized to neutral with acetic acid, and then decompressed The solvent was removed, 300 mL of toluene was added thereto, washed with water twice, and dried to obtain a toluene solution of 2-formyl-3-formylamino-propionitrile, which was set aside.
[0024] In the three-necked flask, 49.3g of sodium e...
Embodiment 2
[0032] Add 104.40g methyl formate (content 97.0%, 1.69mol) in the three-necked bottle, add 40g beta-aminopropionitrile (content 98.5%, 0.56mol) to it, stir; Add 45.55g liquid to another three-necked bottle Sodium methylate (content 20%, 0.16mol); Setting pipeline reactor temperature is 50 ℃, opens feed pump, and above-mentioned two strands of material are fed simultaneously, and feed rate is 14.44g / min and 4.55g / min, and reaction The residence time in the container is about 5 minutes. After the reaction is stable, receive and discharge the material for 5 minutes, cool the material liquid to room temperature, neutralize it with glacial acetic acid to neutrality, then remove the solvent under reduced pressure, add 300 mL of toluene to it, wash twice with water, and dry The toluene solution of 2-formyl-3-formylamino-propionitrile was obtained and set aside.
[0033]In the three-necked flask, 39.44g of sodium ethylate (content 97%, 0.56mol) was dissolved in 300mL of ethanol, after...
Embodiment 3
[0035] Add 278.40g methyl formate (content 97.0%, 4.50mol) in the three-necked bottle, add 40g beta-aminopropionitrile (content 98.5%, 0.56mol) to it, stir well; Add 151.83g liquid in another three-necked bottle Sodium methylate (content 20%, 0.56mol); Setting pipeline reactor temperature is 50 ℃, opens feed pump, and above-mentioned two strands of material are fed simultaneously, and feed rate is 31.84g / min and 15.18g / min, and reaction The residence time in the container is about 4 minutes. After the reaction is stable, receive and discharge the material for 5 minutes, cool the material liquid to room temperature, neutralize it with glacial acetic acid to neutrality, then remove the solvent under reduced pressure, add 300 mL of toluene to it, wash twice with water, and dry The toluene solution of 2-formyl-3-formylamino-propionitrile was obtained and set aside.
[0036] In the three-necked flask, 69.01g of sodium ethylate (content 97%, 0.98mol) was dissolved in 300mL of ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com