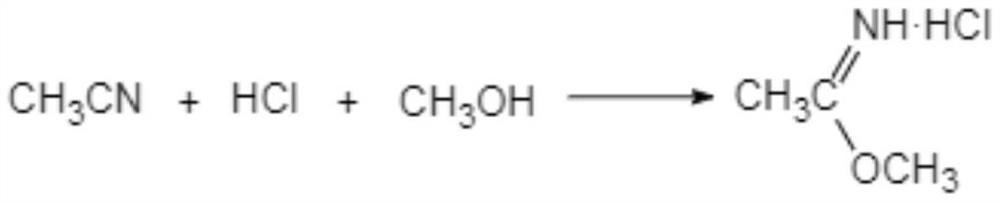Preparation method of acetamidine hydrochloride
A technology for acetamidine hydrochloride and acetamidine hydrochloride is applied in the field of preparation of acetamidine hydrochloride, and can solve the problems of unsafe ammoniation reaction, high consumption of acetonitrile and liquid ammonia, easy decomposition of reaction products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation of ethyleneamidine
[0037] The present invention has no special requirements on the preparation of ethyleneamidine, and methods commonly used in the art can be used.
[0038] Preferably, it is prepared by the following method:
[0039]
[0040] (i-1) Add acetonitrile dropwise to methanol hydrochloride, the initial dropping temperature is 0-5°C, the dropwise addition is completed within 10-15°C, and react at 10-15°C for 10-16 hours to obtain Acetamidine.
[0041] In another preferred example, in step (i-1), the mass percentage of HCl in methanol hydrochloride is 45-50%.
[0042] Preferably, the molar ratio of acetonitrile:HCl:methanol is 1:1.02-1.12:1.25-1.5.
[0043] Usually, the methanol hydrochloride is prepared by passing dry hydrogen chloride into methanol.
[0044] In the present invention, more preferably, the reaction solution obtained in step (i-1) is directly used in the synthesis of acetamidine hydrochloride.
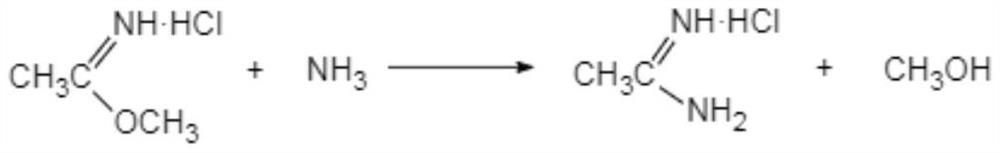
[0045] Preparation of acetam...
Embodiment 1
[0063] A preparation method of acetamidine hydrochloride, comprising the following steps:
[0064] (1) Synthesis of ethyleneamidine
[0065] Add 3.10 mol of methanol to a 500ml four-necked flask equipped with a stirrer and a thermometer, pass through 2.5 mol of dry HCl to make methanol hydrochloride, add 2.3 mol of acetonitrile to the methanol hydrochloride dropwise, and the initial dropping temperature is 5°C , The dropwise addition is completed within 10°C, and the temperature is kept at 10-15°C for 10 hours to synthesize ethyleneamidine.
[0066] (2) Synthesis of Acetamidine Hydrochloride
[0067] Add 4.5 mol of methanol dropwise to ethyleneamidine (reaction solution directly obtained in step (1)) to dilute, and add ethyleneamidine dropwise to 260.0 g mass concentration in 17% ammonia methanol (excessive amount of ammonia methanol), the initial drop The adding temperature is 0-5° C., the dropwise addition is completed within 15° C., and the temperature is kept at 25-28° C...
Embodiment 2
[0069] A preparation method of acetamidine hydrochloride, comprising the following steps:
[0070] (1) Synthesis of ethyleneamidine
[0071] Add 2.5mol of methanol to a 500ml four-necked flask equipped with a stirrer and a thermometer, and pass through 2.16mol of dry HCl to make methanol hydrochloride. Add 2.0mol of acetonitrile to the methanol hydrochloride dropwise, and the initial dropwise temperature is 7°C , The dropwise addition is completed within 10°C, and the temperature is kept at 10-15°C for 16 hours to synthesize ethyleneamidine.
[0072] (2) Synthesis of Acetamidine Hydrochloride
[0073] Add 4.0 mol of methanol dropwise to ethyleneamidine (reaction solution obtained directly in step (1)) to dilute, and add ethyleneamidine dropwise to 213.7 g of ammonia methanol with a mass concentration of 18.9%, and the initial dropwise temperature is 3 ℃, the dropwise addition is completed within 15 ℃, and the temperature is kept at 25-30 ℃ for 3.0 hours, and the pH=8-9 is de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com