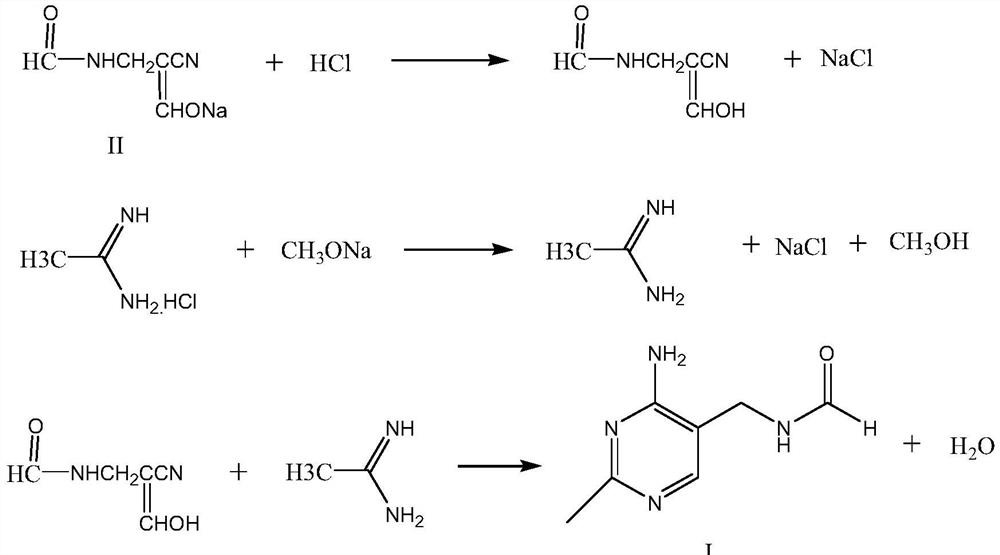
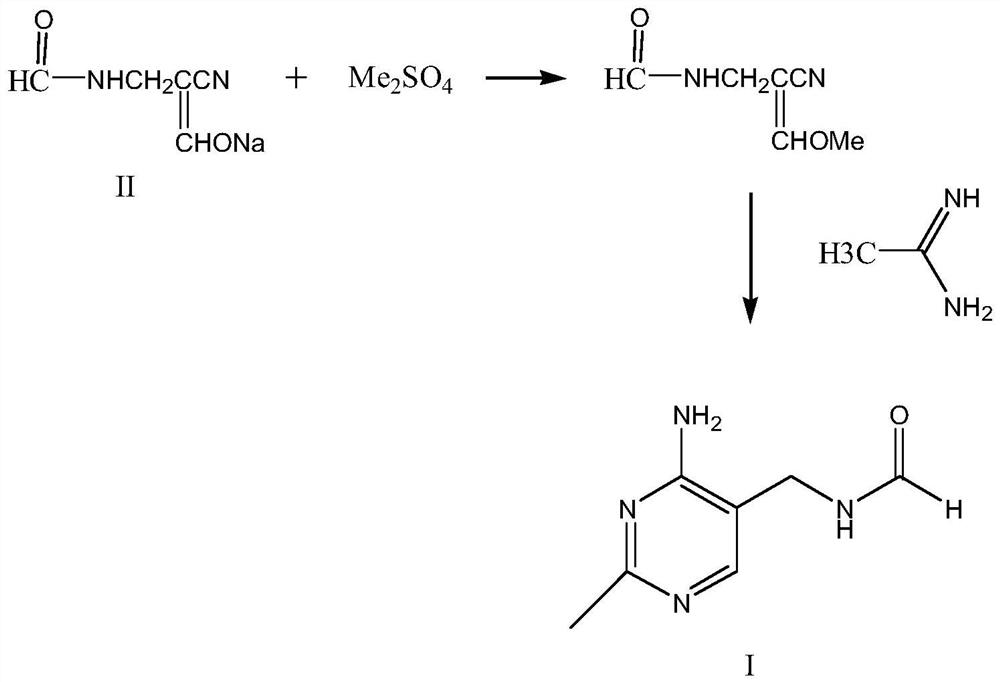
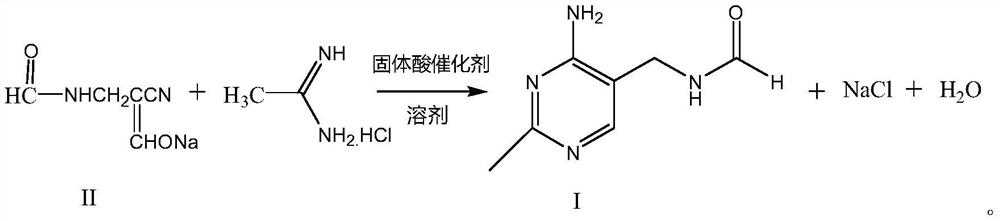
The synthetic method of 2-methyl-4-amino-5-formylaminomethylpyrimidine
A technology for the synthesis of formylaminomethylpyrimidine and its synthesis method, which is applied in the field of synthesis of vitamin B1 and its derivatives, which can solve the problems of long routes and residues, and achieve the effects of eliminating intermediate processes, simple operation, and avoiding trace carcinogen residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the reaction flask, add 500g toluene, 45g solid acid catalyst NbCl 5 / ZrO 2 / SiO 2 , start stirring, slowly add 148.1g of α-sodium formyl-β-formamidopropionitrile, heat up to 65-70°C, start to add dropwise a solution of 105g of acetamidine hydrochloride dissolved in 600g of methanol, after the dropwise addition, continue to keep warm After reacting for 12 hours, 2-methyl-4-amino-5-formylaminomethylpyrimidine reaction solution was obtained. Filtration, recovery of solid acid catalyst NbCl 5 / ZrO 2 / SiO 2 , can be reused after treatment; the filtrate is detected by HPLC, and the yield of 2-methyl-4-amino-5-formylaminomethylpyrimidine is 91.2%.
Embodiment 2
[0024] As described in Example 1, the difference is that the solid acid catalyst NbCl 5 / Al 2 o 3 / SiO 2 instead of solid acid catalyst NbCl 5 / ZrO 2 / SiO 2 Catalyzed reaction was carried out, the preparation steps and conditions were the same as in Example 1, and the obtained yields are shown in Table 1.
Embodiment 3
[0026] As described in Example 1, the difference is that the solid acid catalyst NbCl 5 / ZSM-5 zeolite instead of solid acid catalyst NbCl 5 / ZrO 2 / SiO 2 Catalyzed reaction was carried out, the preparation steps and conditions were the same as in Example 1, and the obtained yields are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com