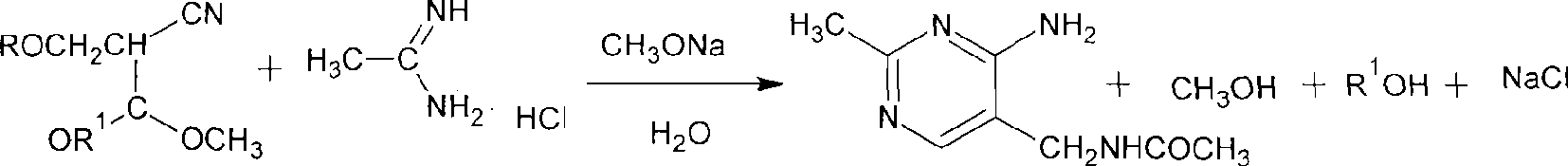
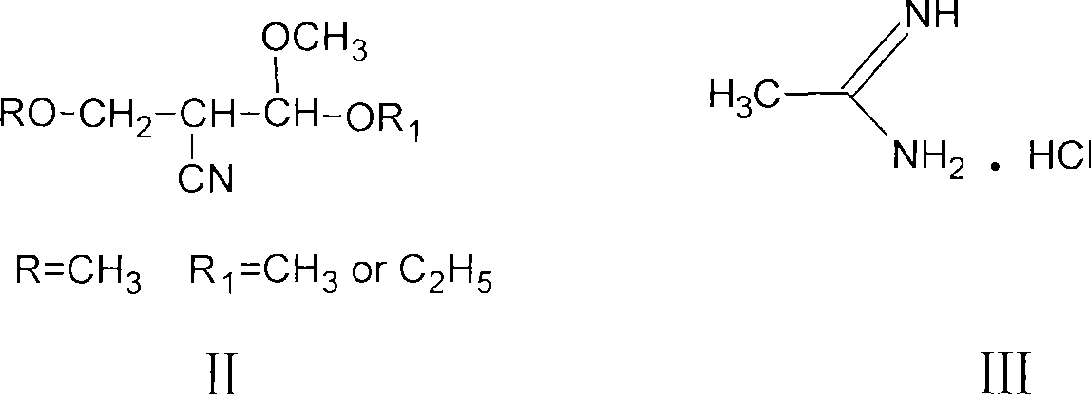Process for preparing 2-methyl-4-amino-5-acetyl aminomethyl pyrimidine
An acetamidomethyl pyrimidine and preparation technology technology, which is applied in the field of preparation technology of 2-methyl-4-amino-5-acetamidomethyl pyrimidine, can solve the problem that the process conditions are not easy to control, the content of acetopyrimidine is low, and the technology Long time and other problems, to achieve the effect of easy control of process conditions, excellent quality, and avoidance of salty wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1. With the methanol solution of 100 grams of 28% sodium methylate, 300g acetamidine hydrochloride (93%, 2.951mol), drop in the dry three-necked flask of 500ml band stirring, stir reaction 3 hours, filter desalting, the free The liquid was warmed up, and the filtrate was concentrated under reduced pressure to make the concentration of acetamidine reach 30%. Put the concentrated solution and 350g acetal (95%, 2.089mol) into a 1000ml dry three-necked flask with stirring, react at 20~25°C for 2 hours, dry formazan and ethanol under reduced pressure, and then add 200g pure water into the three-necked flask , heat up to 70°C, recover the low boiling point under vacuum, then heat up to 90°C for 30 minutes and then hydrolyze it for 30 minutes, add 600g of absolute ethanol, cool down to 0°C, filter to obtain 98.9% purity Product 247.4 g. The ethanol in the filtered mother liquor was recovered, and 28.3 grams of acetamidomethylpyrimidine with a content of 96.0% was obta...
example 2
[0018] Example 2. The operation is the same as in Example 1. 100 grams of 28% sodium methylate, 300g acetamidine hydrochloride (93%, 2.951mol), stirred and reacted for 3 hours, filtered to desalinate, and concentrated the filtrate under reduced pressure to obtain the concentration of 80% acetamidine concentration solution and 350g acetal (95%, 2.089mol) reacted at 25-28°C for 2 hours, dried methyl alcohol under reduced pressure, then added 200g pure water into the three-necked flask, raised the temperature to 70°C, recovered the low boiling point in vacuum, and then After heating up to 90°C for 30 minutes for hydrolysis, methanol was added, the temperature was lowered to 0°C, and 266.2 g of a finished product with a purity of 98.0% was obtained by filtration. The ethanol in the filtered mother liquor was recovered, and 30.0 g of acetamidomethylpyrimidine with a content of 95.0% was obtained simultaneously.
[0019] Example 3. According to the method for example 1, the acetamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com