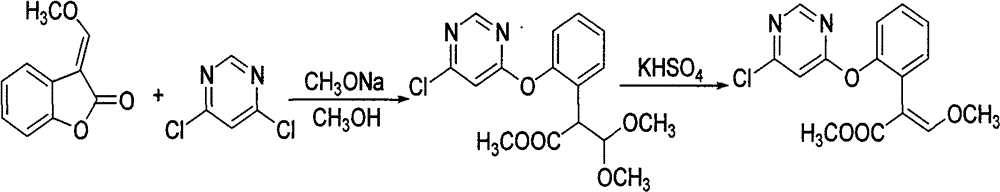
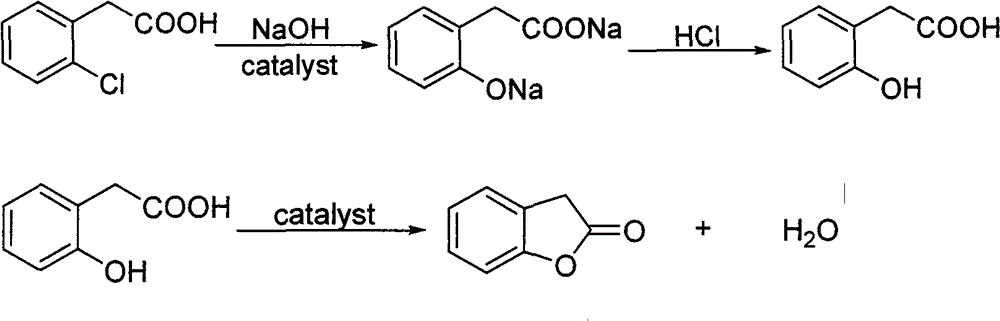
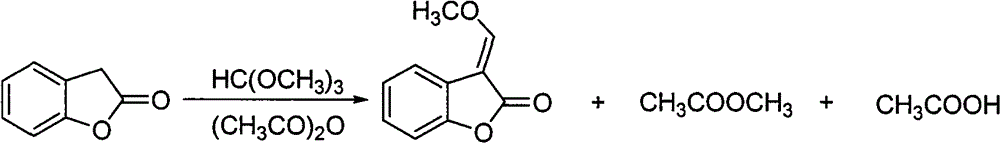
Synthesis of 3-(α-methoxy)methenylbenzofuran-2(3h)-one
A technology of methenylbenzene and methoxy, which is applied in the field of synthesis of chemical substances, can solve the problems of low atom economy, loss of products, and inability to increase yield, and achieve the effect of stable and reliable process and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Weigh 34.1g (0.2mol) of o-chlorophenylacetic acid, 7.04g (0.02mol) of catalyst 8-hydroxyquinoline copper and 253.6g of sodium hydroxide aqueous solution, put them into the autoclave at one time, and react at 170°C for 2h. After cooling to room temperature, the reaction solution was taken out and neutralized to pH 7 with concentrated hydrochloric acid. Filtrate, dry and recover the filter cake, continue to acidify the filtrate with concentrated hydrochloric acid until the pH value is 1, then transfer it to a three-necked round bottom flask with stirring, thermometer, and condenser tube, the oil bath temperature is 80 °C, and distill under reduced pressure at -0.095Mpa until the acid The water was basically evaporated to dryness, and 200 mL of toluene was added to the residue, and the temperature of the oil bath was adjusted to 115°C. Azeotropic distillation was carried out until no more water was evaporated from the system, and 15 mL of acetic acid was added to continue h...
Embodiment 2
[0057] Weigh 34.1g (0.2mol) of o-chlorophenylacetic acid, 7.04g (0.02mol) of catalyst 8-hydroxyquinoline copper and 253.6g of sodium hydroxide aqueous solution, put them into the autoclave at one time, and react at 170°C for 2h. After cooling to room temperature, the reaction solution was taken out and neutralized to pH 7 with concentrated hydrochloric acid. Filter, dry and recover the filter cake, continue to acidify the filtrate with concentrated hydrochloric acid until the pH value is 1, and then transfer it to a three-neck round bottom flask with stirring, thermometer, and condenser. The water was basically evaporated to dryness, and 250mL of toluene was added to the residue, and the temperature of the oil bath was adjusted to 120°C. Azeotropic distillation was carried out until no more water was evaporated from the system, and 20mL of acetic acid was added to continue heating until no more water was formed. The o-hydroxyl group was detected by HPLC. The content of phenyla...
Embodiment 3
[0059] Weigh 68.2g (0.4mol) of o-chlorophenylacetic acid, 14.08g (0.04mol) of catalyst 8-hydroxyquinoline copper and 507.3g of sodium hydroxide aqueous solution, put them into the autoclave at one time, and react at 170°C for 2h. After cooling to room temperature, the reaction solution was taken out and neutralized to pH 7 with concentrated hydrochloric acid. Filtrate, dry and recover the filter cake, continue to acidify the filtrate with concentrated hydrochloric acid until the pH value is 1, then transfer it to a three-neck round bottom flask with stirring, thermometer, and condenser tube, the oil bath temperature is 100 ° C, and distill under reduced pressure at -0.02Mpa until the acid The water was basically evaporated to dryness, and 500mL of toluene was added to the residue, and the temperature of the oil bath was adjusted to 125°C. Azeotropic distillation was carried out until no more water evaporated from the system, and 30mL of acetic acid was added to continue heating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com