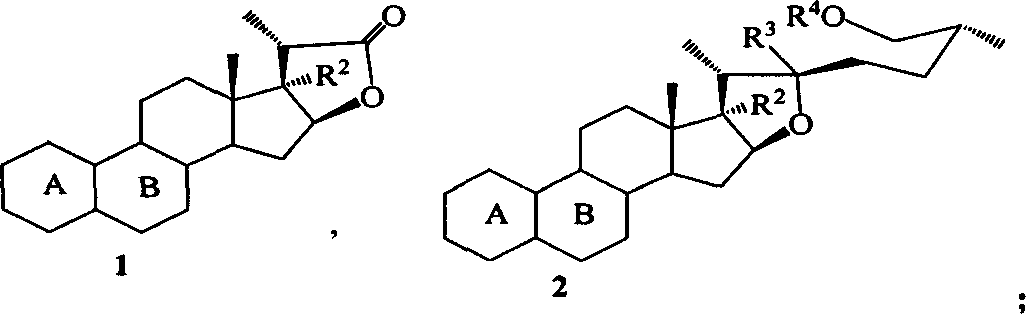
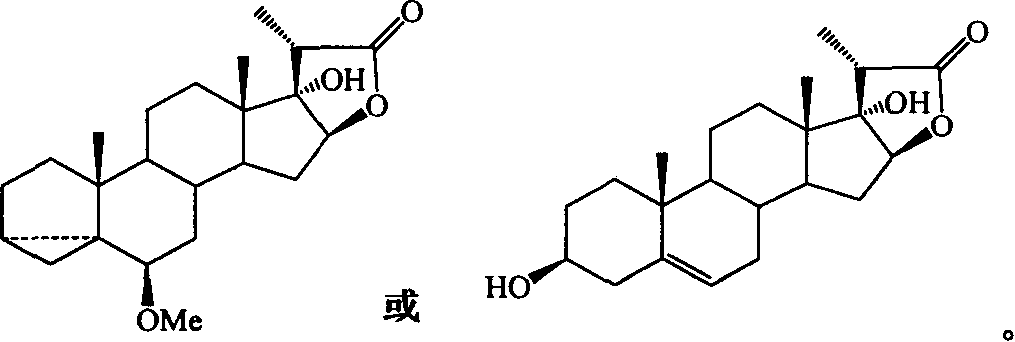
Process for synthesizing pennosapogenin by 17-hydroxy-steroid internal ester
A saponin and steroid technology, applied in the direction of steroids, chemical instruments and methods, steroid preparation, etc., can solve the problems of exacerbating the shortage of plant resources, affecting the ecological environment, and destroying vegetation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0034] Example 2 Synthesis of Compound 2h
[0035]
[0036] 5.0 equivalents of lithium reagent was dropped into the 5mL anhydrous THF solution of 150mg of internal steroid ester 1b, reacted at -20℃ until 1a no longer decreased, and saturated NH was added 4 The Cl solution terminates the reaction. Ethyl acetate extraction (40×2mL), the ester layer was saturated with NH 4 Cl solution wash, after anhydrous Na 2 SO 4 After drying, it was concentrated under reduced pressure. The obtained crude product was separated by flash column chromatography to obtain furostane compound 3,17,20R-furostatriol 2h 43mg (21% yield), and the recovered raw material 1b 108mg (72% yield).
[0037] Compound 2h:
[0038] 1 H-NMR(300MHz, CDCl 3 ): 5.57 (d, 1H, J = 8.7 Hz, H-6), 5.51 (s, 2H, MOM), 4.17 (dd, 1H, J = 4.5, 7.5 Hz, H-16), 3.54 (m, 1H , H-3), 3.27 (s, 3H, MOM), 2.21 (q, 1H, J = 7.5 Hz, H-20), 1.46 (t, J = 8.1 Hz, 2H, H-22), 1.04 (s , 3H, H-19), 0.94 (d, 3H, J=7.2 Hz, H-21), 0....
Embodiment 3
[0040] Example 3 Compound 2a removes silicon protecting group
[0041]
[0042] 31 mg (0.043 mmol) of furostane compound 2a was dissolved in 2 mL of anhydrous THF, and 0.43 mL (0.43 mmol) of 1M TBAF in THF was injected, the temperature was raised to -60°C, the reaction was stirred, and TLC tracked until the reaction was complete. Cool to room temperature, add 10 mL of water, extract with ethyl acetate (15×3 mL), and use saturated NaHCO for the ester layer 3 Wash with NaCl solution, after anhydrous Na 2 SO 4 After drying, it was concentrated under reduced pressure. The obtained crude product is separated by flash column chromatography to obtain furostanes desiliconized product: oily 3,5-cyclo-6-methoxy-26-tert-butyldiphenylsiloxy-17,20R- Furostediol (25R)-3,5-cyclo-6-methoxy-17,22R,26-furostetriol 2f 9mg (45.2% yield) and spirostane auto-ring closure product: oily (25R )-3,5-cyclo-6-methoxy-17-spirosterol 2g 10mg (52.4% yield).
[0043] Compound 2f:
[0044] 1 H...
Embodiment 4
[0047] Example 4 Synthesis of Pennogenin (Method 1: Synthesis from Compound 2g)
[0048]
[0049] 8 mg (0.018 mmol) of 2 g of spirostane compounds were dissolved in 0.9 mL of dioxane, and then 0.1 mL of water and 2 mg of p-toluenesulfonic acid were added, the temperature was raised to reflux, and TLC followed the reaction to completion. Cool to room temperature, add saturated NaHCO 3 The solution was 5mL, extracted with ethyl acetate (10×3mL), and the ester layer was saturated with NaHCO 3 And NH 4 Cl solution wash, after anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, the crude product obtained was separated by flash column chromatography to obtain 6 mg of powdered sapogenin (77.5% yield).
[0050] Pennogenin:
[0051] 1 H-NMR(300MHz, CDCl 3 ): 5.35 (d, 1H, J = 4.2 Hz, H-6), 3.98 (dd,'t'like, 1H, J = 7.6 Hz, H-16), 3.51 (dd, 1H, J = 3.6, 10.8 Hz, H-26), 3.38 (dd,'t'like, 1H, J = 10.8 Hz, H-26), 2.04 (q, 1H, J = 7.2 Hz, H-20), 1.03 (s, 3H, H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com