Synthesis process of natural delta-decalactone
A synthesis process and decanolide technology, applied in the field of food additives, can solve the problems of complicated synthesis process, low yield, low yield and the like, and achieve the effects of easy availability of raw materials, abundant sources and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
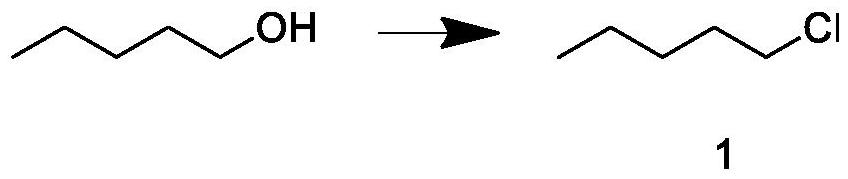
[0033] Build a 2000mL synthetic fine-separation integrated device with a constant temperature magnetic stirrer, rectification device, and tail gas absorption device, add 800g of n-pentanol and 1035g of hydrochloric acid solution with a mass fraction of 32% into the reaction kettle, start stirring, raise the temperature to 85°C, and keep warm React for 2 hours; continue to heat up to 94°C, keep warm for 20 hours, and the crude product is extracted at a reflux ratio of 3 / 5 during the reaction; after the reaction is completed, post-processing is carried out to obtain intermediate 1; the weight is 350g, and the GC content is 99.56%.
[0034] The aftertreatment process is as follows: the crude product at the bottom of the kettle is layered, wherein the oil phase is a mixture of amyl alcohol and pentyl ether, and the water phase is dilute hydrochloric acid. Add 5% sodium bicarbonate solution to the crude product to adjust the pH value to 7; Add concentrated sulfuric acid, the amount ...
Embodiment 2
[0036] Build a 2000mL synthetic fine-separation integrated device with a constant temperature magnetic stirrer, rectification device, and tail gas absorption device, add 800g of n-pentanol and 1035g of hydrochloric acid solution with a mass fraction of 32% into the reaction kettle, start stirring, raise the temperature to 90°C, and keep warm React for 2 hours; continue to heat up to 98°C, keep warm for 20 hours, and the crude product is extracted at a reflux ratio of 3 / 5 during the reaction; after the reaction is completed, post-processing is carried out to obtain intermediate 1; the weight is 352g, and the GC content is 99.55%.
[0037] The aftertreatment process is as follows: the crude product at the bottom of the kettle is layered, wherein the oil phase is a mixture of amyl alcohol and pentyl ether, and the water phase is dilute hydrochloric acid. Add 5% sodium bicarbonate solution to the crude product to adjust the pH value to 8; Add concentrated sulfuric acid, the amount ...
Embodiment 3
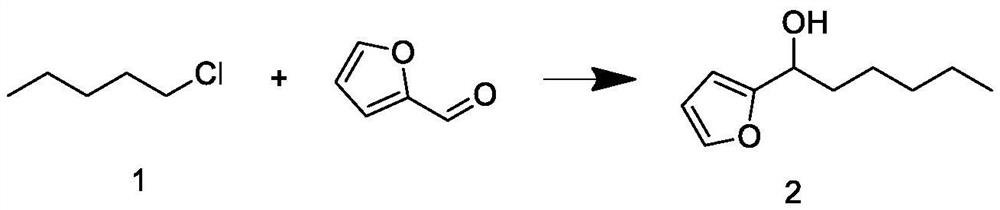
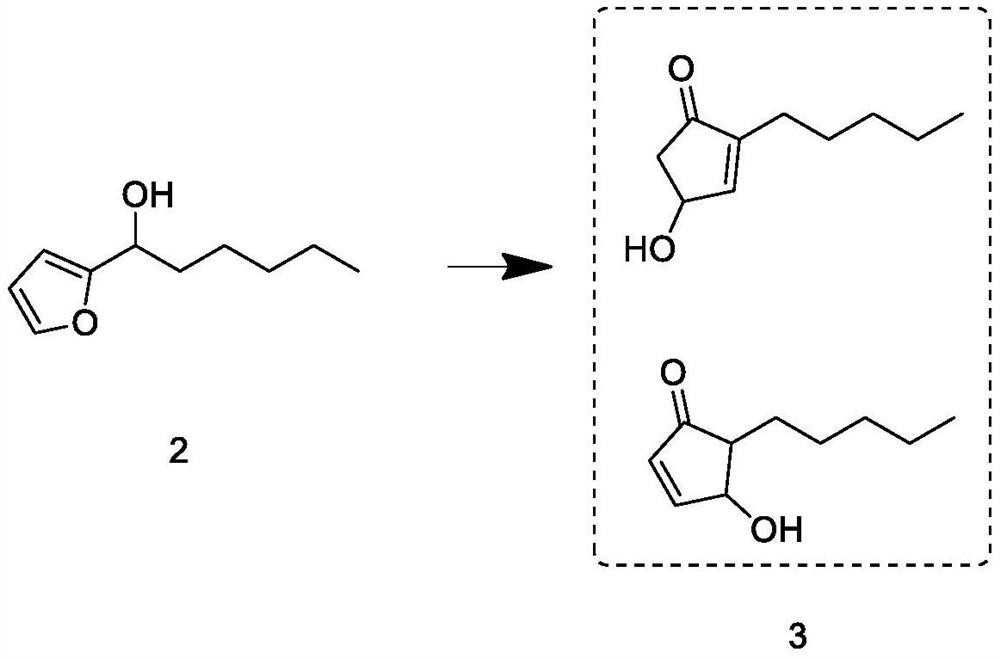
[0039]Build a 1000mL synthetic reaction kettle with a constant temperature magnetic stirrer, a spherical condenser, and a dropping device. Under nitrogen protection, add 57g of magnesium chips, 50g of tetrahydrofuran and 5g of bromopentane into the kettle, maintain the temperature at 20°C, and stir for 10min To obtain the Grignard reagent, keep the reaction kettle at 60°C, add 120g of intermediate 1 dropwise to the Grignard reagent, and the dropwise addition ends in 2h. After maintaining the temperature and continuing the reaction for 1h, cool down to 20°C, then add 100g of furfural dropwise, and the dropwise addition ends in 2h. The temperature was maintained and the reaction was continued for 1 h; after the reaction, after-treatment was carried out to obtain intermediate 2; the weight was 170 g, and the GC content was 88.33%.
[0040] The post-treatment process is as follows: raise the temperature of the reaction solution under normal pressure to recover 1 / 3 of tetrahydrofura...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com