Cyclic oxidation method and cyclic oxidation device for preparing epsilon-caprolactone
A technology of cyclic oxidation and caprolactone, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve problems such as high energy consumption and risk factor, increase system energy consumption, and difficult water removal, etc., to achieve Reduce energy consumption and danger, reduce difficulty, avoid the effect of peroxyacid and water residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A cyclic oxidation method for preparing ε-caprolactone, comprising the steps of:
[0032] (1) First place the solid acid in a continuous reactor; the hydrogen peroxide reaction solution flows through the solid acid to generate a solid peroxyacid; the solid acid is a weak acid ion exchange resin or an inorganic heteropolyacid;
[0033] (2) Secondly, pass the cleaning liquid through the solid peroxyacid to take away the residual moisture on the surface of the solid peroxyacid;
[0034] (3) Flow the cyclohexanone solution through the solid peroxyacid, react to generate caprolactone, and directly rectify to obtain the caprolactone product; the concentration of the cyclohexanone solution is 30%-100%, and the solvent is a cleaning solution
[0035] (4) The second cleaning solution flows through the continuous reactor to clean the residual cyclohexanone and caprolactone and repeat steps (1)-(4).
[0036] Wherein, the flow rate of the hydrogen peroxide reaction solution in ste...
Embodiment 2
[0045] Reactor preheating
[0046] Reactor R1 was preheated to reaction temperature 60°C using jacketed steam.
[0047] Peroxidation
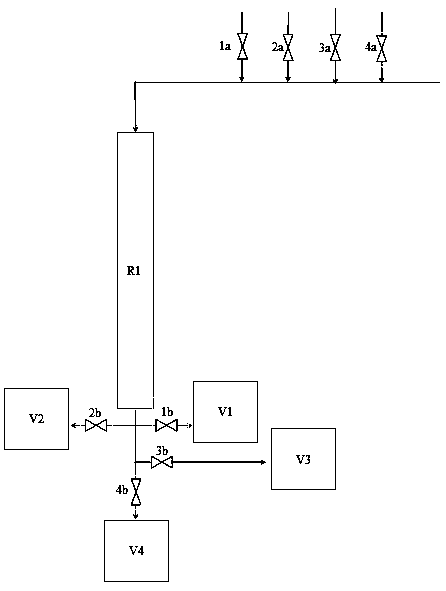
[0048] Open the valves 1a and 1b, and adjust the opening of the valve to control the flow rate to 2g·h -1 •g -1 固体酸 , the material flows out of R1 and enters into V1, and the peroxidation process is completed after 12 hours. Then, while closing 1a and 1b, open 2a and 2b, and control the flow rate to 2g·h -1 • g -1 固体酸, the material flows out of R1 and then flows into V2, and the removal of residual hydrogen peroxide and water is completed after 2 hours.
[0049] reaction
[0050] While closing 2a and 2b, open 3a and 3b, and adjust the opening of the valve to control the flow rate to 2 g·h -1 •g -1 固体酸 , the material flows out of R1 and then flows into V3, and the ε-caprolactone synthesis reaction is completed after 12 hours. The material concentration obtained at this time is: ε-caprolactone 50%, cyclohexanone 50%, water less than 5...
Embodiment 3
[0054] Reactor preheating
[0055] Reactor R1 was preheated to a reaction temperature of 90 °C using jacketed steam.
[0056] Peroxidation
[0057] Open the valves 1a and 1b, and adjust the opening of the valve to control the flow rate to 10g·h -1 •g -1 固体酸 , the material flows out of R1 and enters into V1, and the peroxidation process is completed after 5 hours. Then, while closing 1a and 1b, open 2a and 2b, and control the flow rate to 10g·h -1 •g -1 固体酸 For solid acid, the material flows out of R1 and then flows into V2, and the residual hydrogen peroxide and moisture removal are completed after 1 hour.
[0058] reaction
[0059] While closing 2a and 2b, open 3a and 3b, and adjust the opening of the valve to control the flow rate to 10 g·h -1 •g -1 固体酸 , the material flows out of R1 and then flows into V3, and the ε-caprolactone synthesis reaction is completed after 5 hours. The material concentration obtained at this time is: ε-caprolactone 30%, cyclohexanone 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com