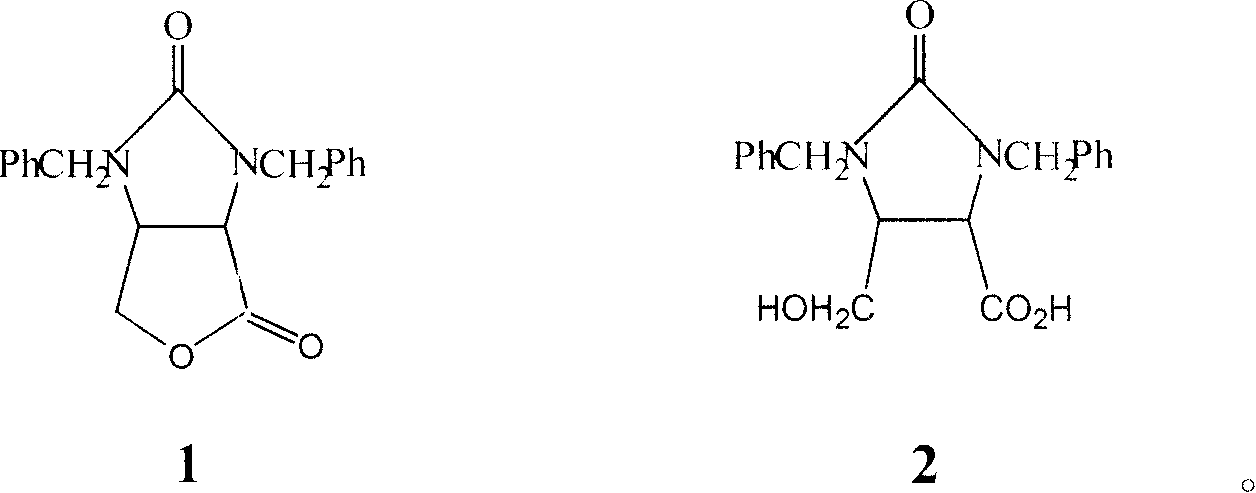Method for preparing dextral biotin intermediate lactone
An intermediate, biotin technology, applied in the field of preparation of dextro-biotin intermediate lactone, can solve the problems of high condition control requirements, low yield, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
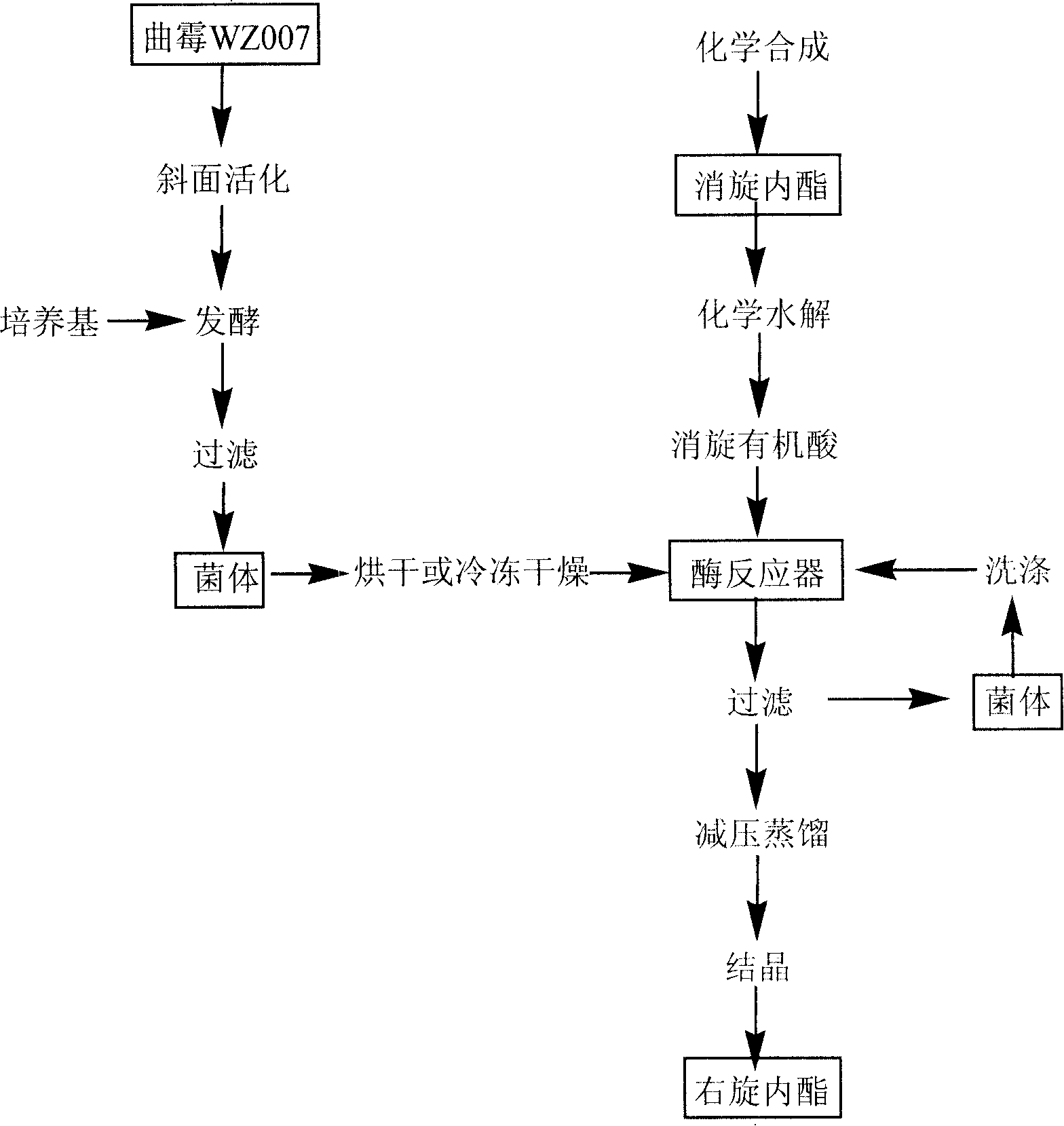
[0050] Slant medium: NaNO 3 0.2%, K 2 HPO 4 0.1%, KCl 0.5%, FeSO 4 0.001%, MgSO 4 0.05%, 3% sucrose, 1.5% agar, natural pH, sterilized at 121°C for 20 minutes, inoculated with Aspergillus oryzae CCTCC No.M 206105 (Aspergillusoryzae WZ007) after sterilization, and cultivated at 30°C for 3 days as slant activated seeds.
[0051] The liquid fermentation medium is glucose 20g, peptone 20g, sucrose 10g, urea 3g, NaCl 5g, k 2 HPO 4 2g, MgSO 4 1g,, (NH 4 ) 2 SO 4 5g, constant volume to 1L, natural pH, 500ml triangular bottle, 80ml liquid, sterilized at 121°C for 20 minutes, cooled and inoculated with slant seeds after sterilization, cultivated at 30°C for 48 hours, filtered to obtain mycelium, and dried to obtain 4 grams as enzyme powder 1.
[0052] Enzyme activity determination method: Take 0.1g of dry mycelium, add 10ml of toluene solution with 1% mixed organic acid 2 concentration to the bacteria, at 40°C, stir in a water bath at a speed of 200rpm, react for 60min,...
Embodiment 2
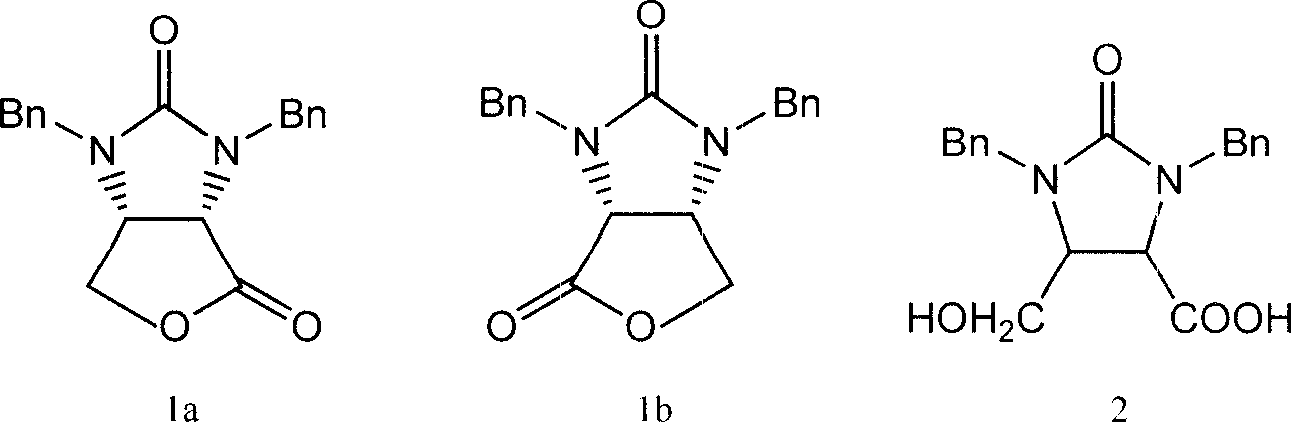
[0055] 0.1 g of Aspergillus oryzaeWZ007 enzyme powder 1 was added to 20 mL of toluene containing 2% racemic organic acid 2, and reacted for 12 hours at 40° C., the reaction conversion rate was 32.6%. After the reaction was completed and filtered, the organic solvent was distilled under reduced pressure at a pressure of 0.1 MPa to obtain the product lactone. The optical purity of the product lactone was determined by an automatic polarimeter, and the enantiomeric excess value was about 96.2%.
Embodiment 3
[0057] Add 0.1g of Aspergillus oryzaeWZ007 enzyme powder 1 to 20mL of toluene containing 2% racemic organic acid 2, and react for 12 hours at 40°C to convert the converted reaction solution, remove the bacteria by filtration, and add a new reaction substrate Continue to react, repeat like this, and reuse 5 times. The enzymatic hydrolysis results are shown in Table 1. After lactonization, the product d-biotin intermediate lactone was concentrated to 5mL under reduced pressure at 0.1Mp, and crystallized at room temperature to obtain D-biotin intermediate lactone, with an enantiomeric excess of over 96% and a yield of 93%. , with a purity of over 98%. After using the enzyme powder 1 for 5 times, the enzyme conversion rate still did not drop significantly.
[0058] Table 1: Results of repeated batch enzyme conversion
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| esterification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com