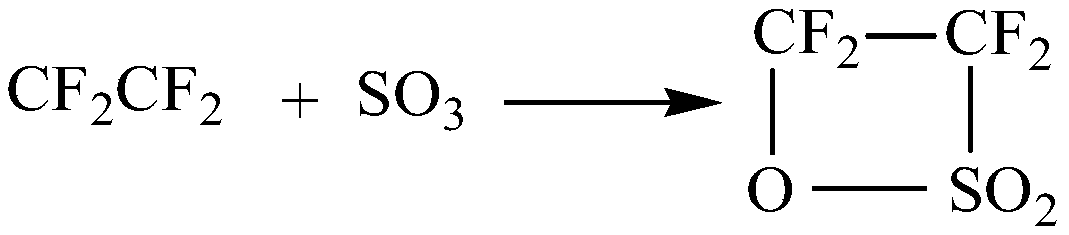Synthetic method of perfluoroalkyl sultone
A technology of perfluoroalkyl and synthetic methods, applied in chemical instruments and methods, chemical/physical/physical chemical processes, organic chemistry, etc., can solve the problems of long reaction time, etc. The effect of continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The raw materials sulfur trioxide and tetrafluoroethylene were respectively metered by metering pumps and then continuously entered into the micro-mixer (Labtrix@ R After mixing in S1), enter the microreactor (Labtrix@ R The continuous reaction is carried out in S1), the channel equivalent diameter of the micromixer is 30 μm, and the channel equivalent diameter of the microreactor is 200 μm. The molar ratio of sulfur trioxide and tetrafluoroethylene is 1:1.1, the reaction temperature is 45°C, the reaction pressure is 0.2MPa, and the residence time is 8min; after the reaction, the temperature is lowered, and the liquid product obtained is purified by distillation. , Tetrafluoroethane-β-sultone with a purity of more than 99.2% can be obtained.
Embodiment 2
[0030] The raw materials sulfur trioxide and tetrafluoroethylene are respectively metered by a metering pump and then continuously enter the micro-mixer (Kunshan Fuxi Engineering Technology Co., Ltd., T-type mixer) for mixing, then enter the micro-reactor (Corning, G1) for continuous reaction , the channel equivalent diameter of the micromixer is 70 μm, and the channel equivalent diameter of the microreactor is 200 μm. The molar ratio of sulfur trioxide and tetrafluoroethylene is 1:1.2, the reaction temperature is 50°C, the reaction pressure is 0.3MPa, and the residence time is 10min; after the reaction, the temperature is lowered, and the liquid product obtained is purified by distillation. , Tetrafluoroethane-β-sultone with a purity of more than 99.4% can be obtained.
Embodiment 3
[0032] The raw materials sulfur trioxide and hexafluoropropylene are respectively metered by a metering pump and then continuously entered into a micro-mixer (Kunshan Fuxi Engineering Technology Co., Ltd., Y-type mixer) for mixing, and then enter a micro-reactor (Corning, G1) for continuous reaction , the channel equivalent diameter of the micromixer is 20 μm, and the channel equivalent diameter of the microreactor is 200 μm. The molar ratio of sulfur trioxide and tetrafluoroethylene is 1:1.3, the reaction temperature is 55°C, the reaction pressure is 0.45MPa, and the residence time is 12min; after the reaction is completed, the temperature is lowered, and after gas-liquid separation, the obtained liquid product is distilled and purified , Hexafluoropropane-β-sultone with a purity of more than 99.3% can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com