Method for preparing spirolactone by chemical-enzymatic method
A technology for enzymatic preparation of spironolactone, applied in chemical instruments and methods, organic chemistry, lactone steroids, etc., can solve the problems of expensive reagents, pollution, unfriendly environment, etc., achieve good economic benefits, high commercial competitiveness, and avoid Effects of Security Risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
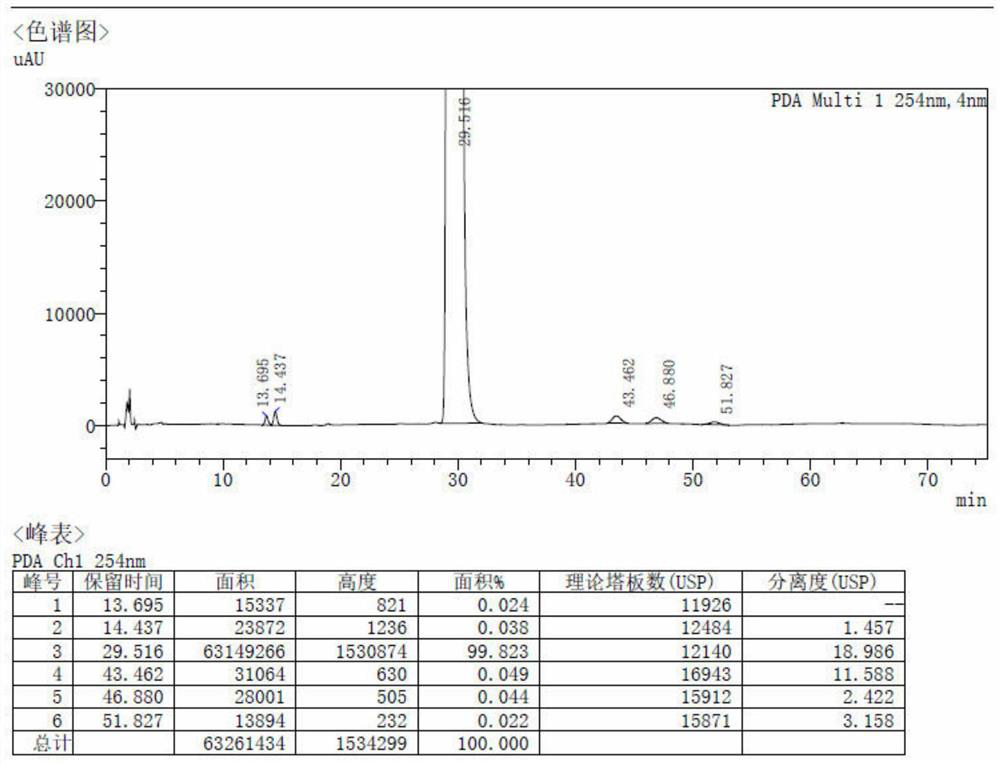
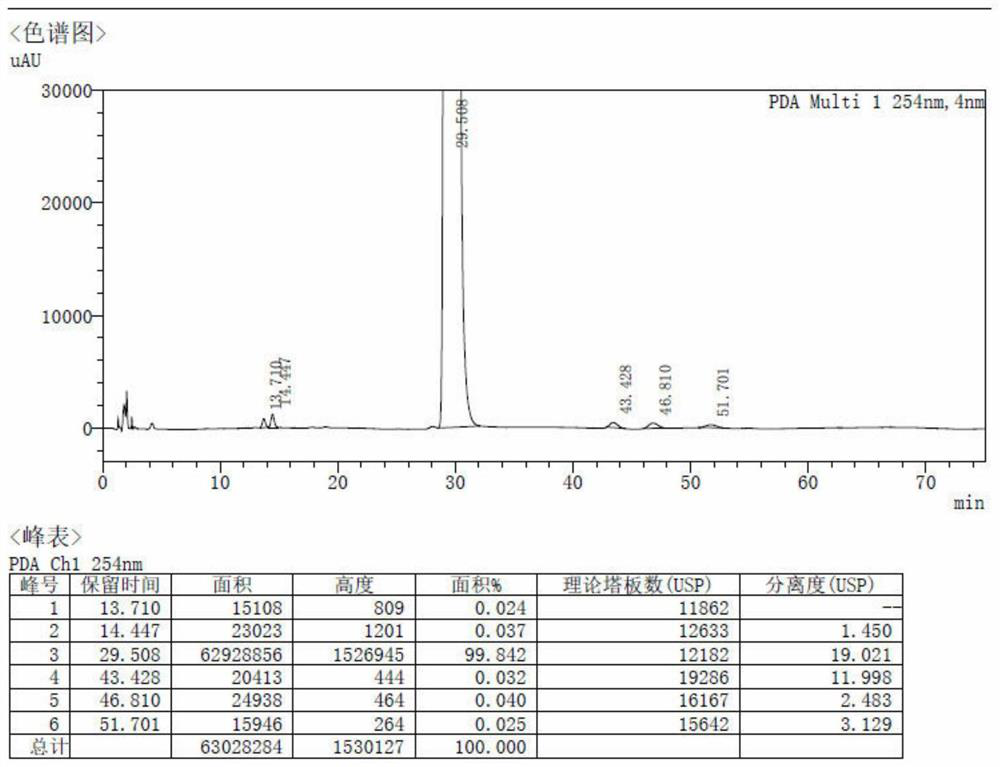
Image
Examples
example 1
[0041] Example 1 The preparation of 3-ethoxy-androst-3,5-dien-17-one
[0042] Put 30g of 4-androstene-3,17dione (4AD) into 15ml of absolute ethanol, add 18ml of triethyl orthoformate, 0.3g of p-toluenesulfonic acid, and keep warm at 40°C for reaction. After the reaction is completed, filter, Dry it to obtain 31.5 g of 3-ethoxy-androst-3,5-dien-17-one.
example 2
[0043]Example 2 Preparation 2 of 3-ethoxy-androst-3,5-dien-17-one
[0044] Put 30g of 4-androstene-3,17dione (4AD) into 150ml of absolute ethanol, add 60ml of triethyl orthoformate, 0.15g of p-toluenesulfonic acid, keep warm at 42°C, and filter after the reaction is completed. Dry it to obtain 31.3 g of 3-ethoxy-androst-3,5-dien-17-one.
example 3
[0045] Example 3 Preparation of 3-ethoxy-androst-3,5-dien-17-one
[0046] Put 30g of 4-androstene-3,17dione (4AD) into 300ml of absolute ethanol, add 90ml of triethyl orthoformate, 0.3g of p-toluenesulfonic acid, keep warm at 45°C for reaction, after the reaction is completed, filter, Dry it to obtain 31.2 g of 3-ethoxy-androst-3,5-dien-17-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com