A kind of production method of trigonelline synthetic perfume
A technology for synthesizing fenugreek lactone and fragrances, which is applied in the fields of essential oils/fragrances, fat production, organic chemistry, etc. It can solve the problems of harsh reaction conditions, complicated operations, and low total yield, and meet the requirements of fragrances and high purity. , easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
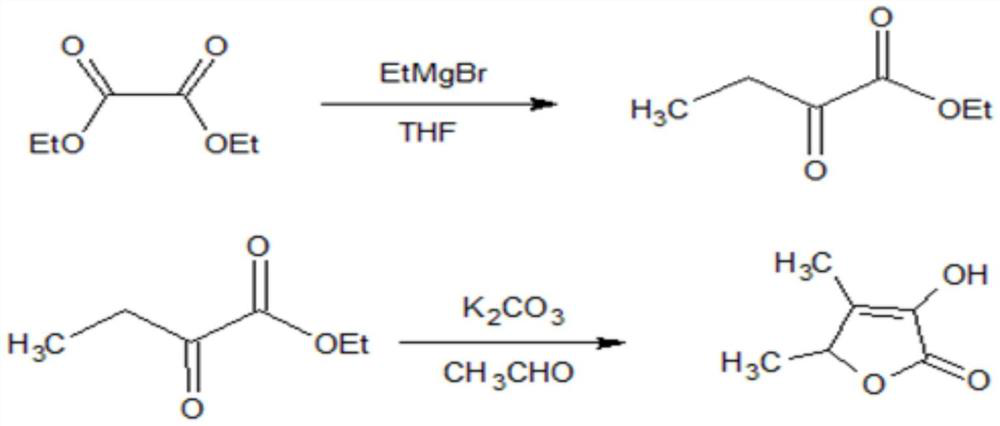
[0037] (1) First feed nitrogen into the 250L reaction kettle with a reflux device for replacement three times, then continue feeding nitrogen to keep the pressure in the kettle at 0.2Mpa.
[0038] (2) After the system pressure is stabilized, add 65kg of anhydrous tetrahydrofuran to the reactor through the anhydrous tetrahydrofuran head tank, after starting the stirrer, add 47kg of diethyl oxalate through the diethyl oxalate head tank, stir evenly, and Diethyl oxalate was well dissolved in THF.
[0039] (3) Open the frozen brine valve, cool the temperature of the reactor to -5°C, and slowly add 104kg of 39% ethylmagnesium bromide-tetrahydrofuran Grignard reagent dropwise through the flow meter in the reactor from the Grignard reagent head tank, Control the reaction temperature at 0~-5°C, and control the dropping time at 2h.
[0040](4) After the dropwise addition, continue to maintain the temperature, stir for 1h, and take a sample for gas chromatography detection. When the co...
Embodiment 2
[0058] (1) First pass nitrogen gas into the 2500L reactor with reflux device for replacement three times, then continue to feed nitrogen gas to maintain the pressure in the kettle at 0.2Mpa.
[0059] (2) After the system pressure is stabilized, add 265kg of anhydrous tetrahydrofuran to the reactor through the anhydrous tetrahydrofuran head tank. After opening the stirrer, add 185kg of diethyl oxalate through the diethyl oxalate head tank, stir evenly, and Diethyl oxalate was well dissolved in THF.
[0060] (3) Open the frozen brine valve, cool the temperature of the reactor to -5°C, slowly add 1240kg13% ethylmagnesium bromide-tetrahydrofuran Grignard reagent dropwise to the reactor through a flow meter from the Grignard reagent head tank, and control The reaction temperature is 0~-5°C, and the dropping time is controlled at 4h.
[0061] (4) After the dropwise addition, continue to maintain the temperature, stir for 2h, and take a sample for gas chromatography detection. When ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com