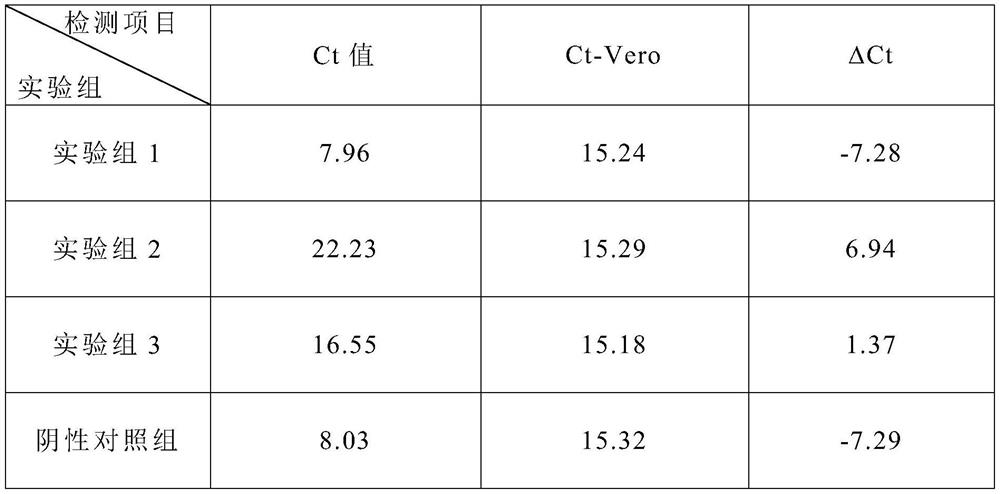
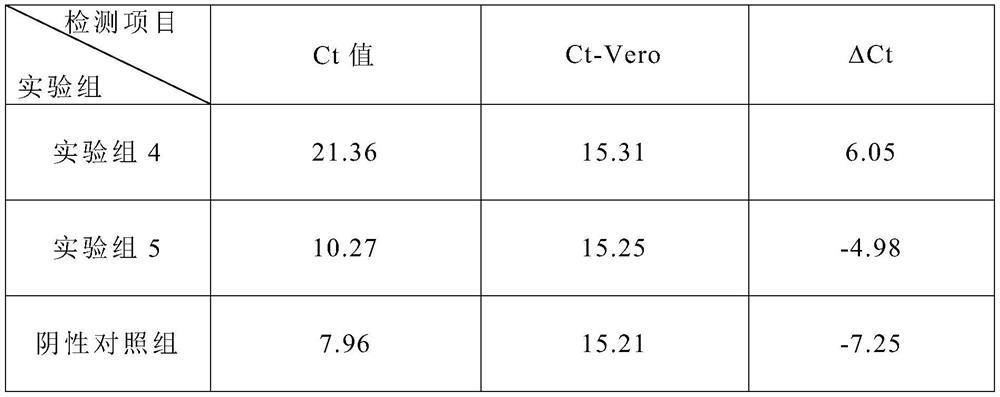
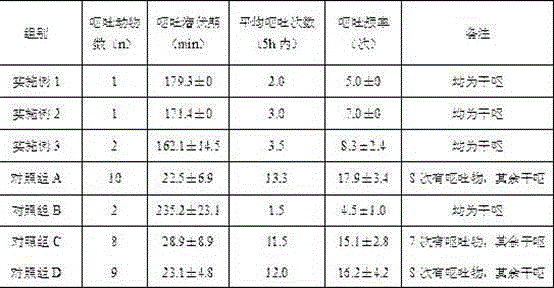
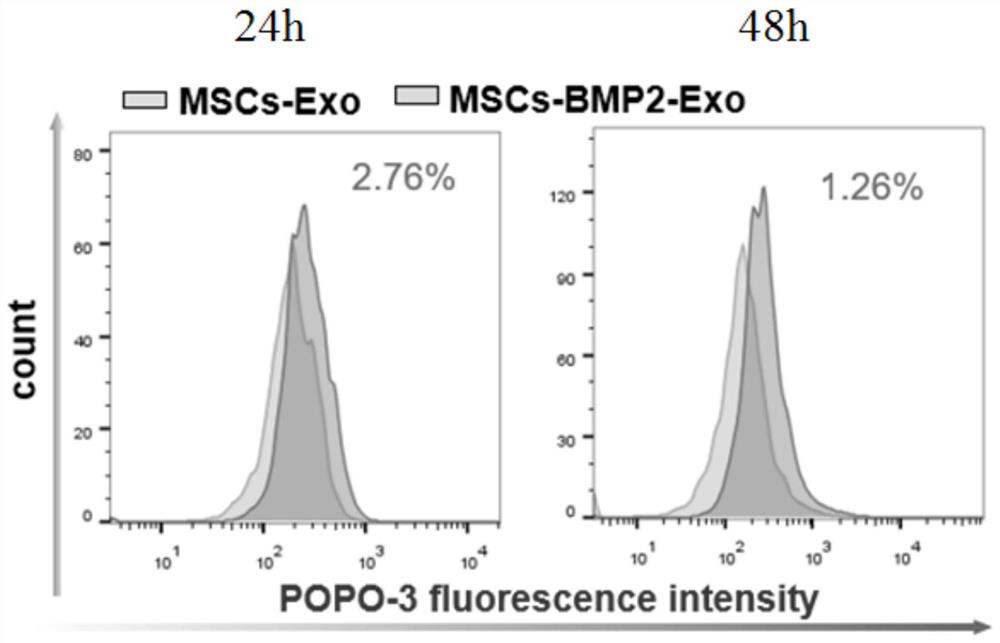
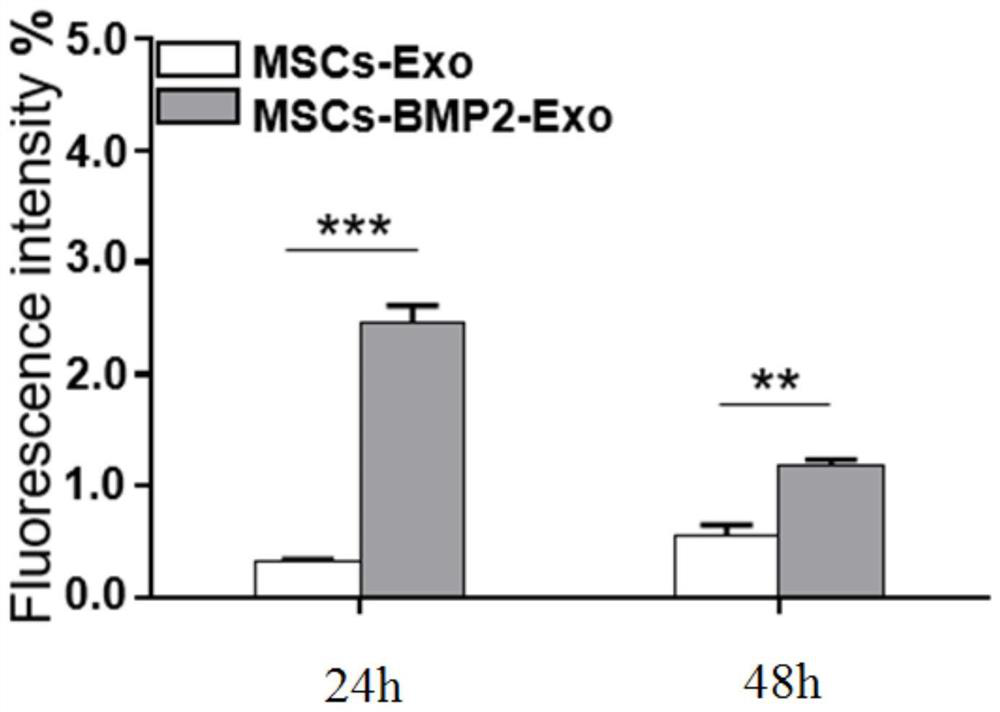
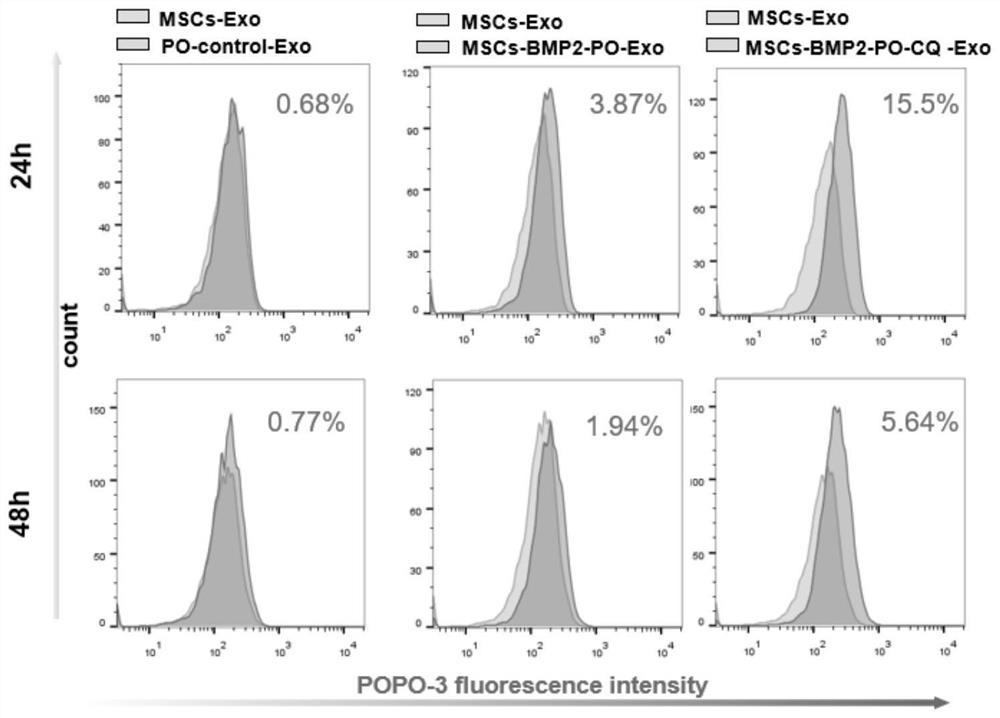
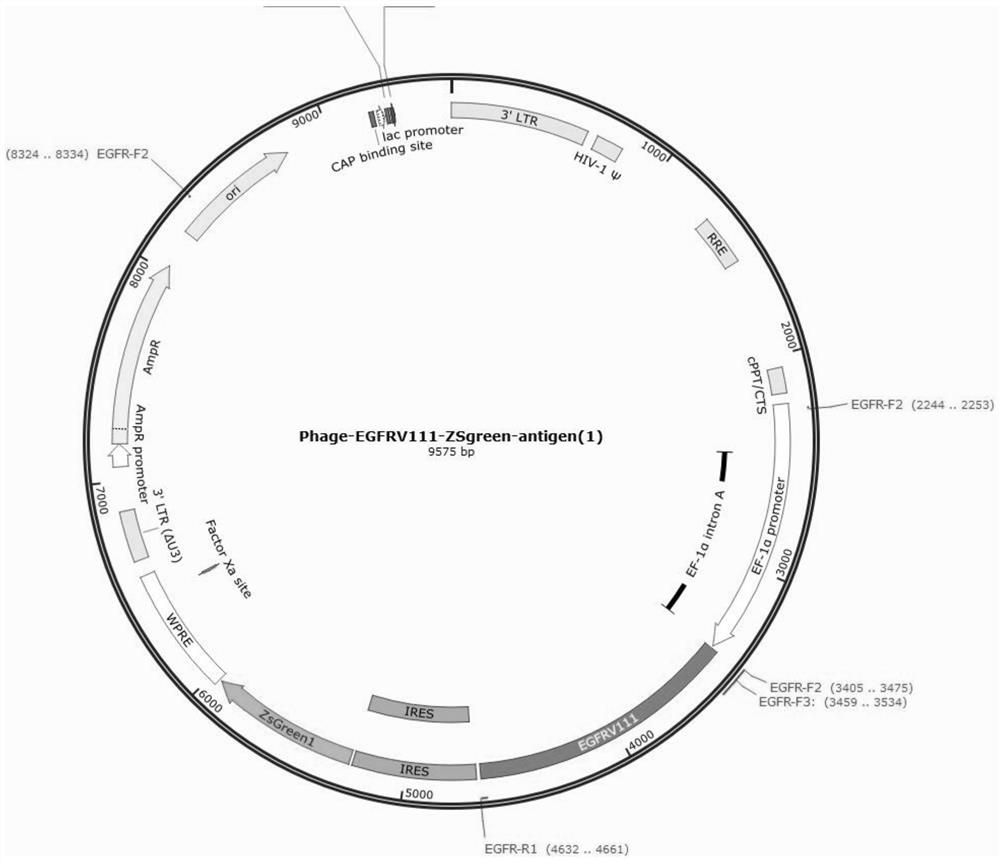
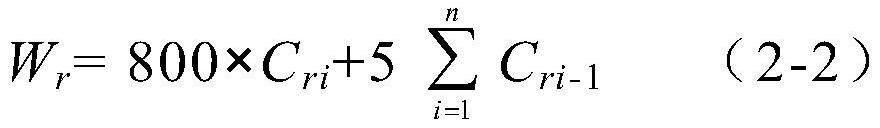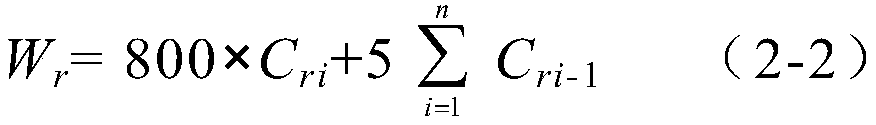Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Chloroquine Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
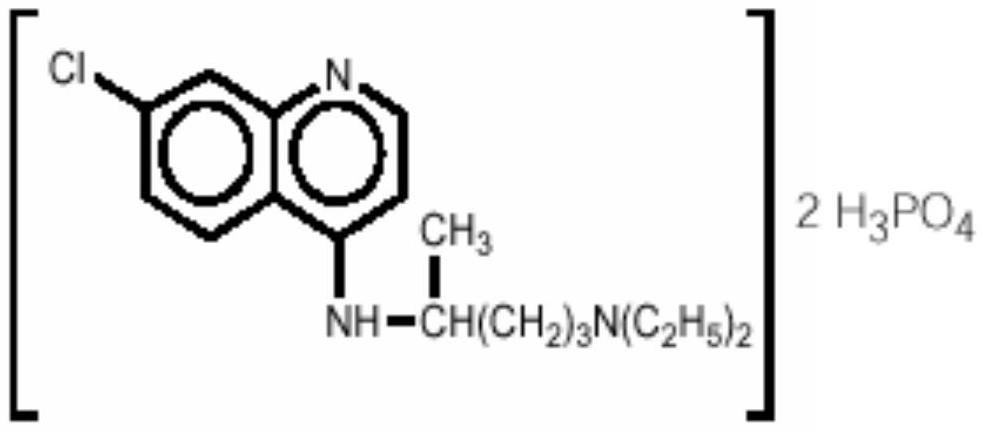
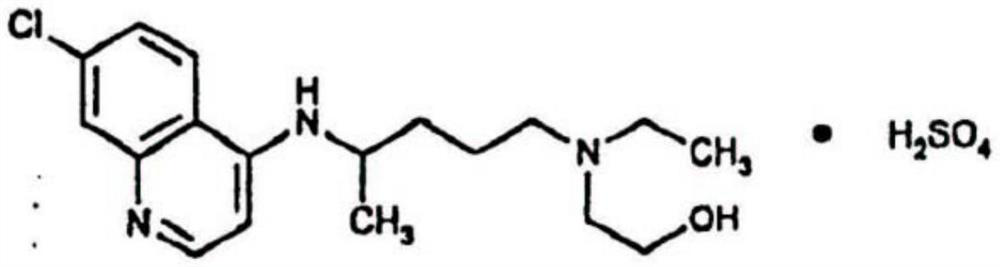
The phosphate salt of chloroquine, a quinoline compound with antimalarial and anti-inflammatory properties. Chloroquine is the most widely used drug against malaria, except for those cases caused by chloroquine resistant Plasmodium falciparum. Although the mechanism of action is not fully understood, chloroquine is shown to inhibit the parasitic enzyme heme polymerase that converts the toxic heme into non-toxic hemazoin, thereby resulting in the accumulation of toxic heme within the parasite. Chloroquine may also interfere with the biosynthesis of nucleic acids.
Process for dip-coating fiber surface with emulsion polymerization chloroquine diphosphate microcapsules
InactiveCN111636212ALong effective timeReduce releaseBiochemical fibre treatmentConjugated cellulose/protein artificial filamentsCelluloseFiber
The invention belongs to the technical field of spinning, and particularly relates to a process for dip-coating fiber surface with emulsion polymerization chloroquine diphosphate microcapsules. The process comprises the steps that a fiber main material and hydroxypropyl methyl cellulose are added into a solvent, a stock solution is prepared by heating and dissolving, and then the stock solution isadded into a spinning box; the stock solution in the spinning box is ejected by a spinning jet, and is solidified in a DMAC aqueous solution to obtain a composite fiber; and the surface of the composite fiber is coated with the microcapsules with an antiviral agent containing chloroquine diphosphate as capsule cores and the fiber main material as capsule walls to obtain a finished fiber. According to the process, the antiviral agent containing chloroquine diphosphate is taken as the capsule cores of the microcapsules, then the surface of the composite fiber is coated with the microcapsules toobtain the finished fiber, so that in the use process, the antiviral agent on the surface of the finished fiber has a slow-release property, and the antiviral effective time of the finished fiber isprolonged.
Owner:SHAOXING BIAODIAN TEXTILE TECH
Compound preparation for treating cecal granuloma and preparation method thereof
InactiveCN105920081AEffective expansionLittle side effectsPeptide/protein ingredientsDigestive systemIntestinal wallsChlorogenic acid
The invention discloses a compound preparation for treating cecal granuloma and a preparation method thereof. The compound preparation comprises chloroquine phosphate, diiodohydroxyquinoline, chlorogenic acid, liquiritin, cistanoside, ursolic acid, polysavone, levamisole, albendazole, okra powder, lactein, vitamin B1, lactalbumin and arginine. The compound preparation can effectively expand intestinal tubes, prevent intestinal obstruction, prevent mesentery and intestinal wall inflammatory infiltration and edema, prevent intestinal wall fibrosis, adjust body metabolism, improve immunity, reduce drug side effects and treat cecal granuloma and has a wide application prospect.
Owner:于爱华
Compound reserpine orally disintegrating tablet for treating hypertension and preparation method of compound reserpine orally disintegrating tablet
InactiveCN105213425AEasy to takeAvoid side effectsOrganic active ingredientsPill deliveryVitamin b6Orally disintegrating tablet
The invention belongs to the field of pharmacy, and particularly relates to a compound reserpine orally disintegrating tablet for treating hypertension and a preparation method of the compound reserpine orally disintegrating tablet. The compound reserpine orally disintegrating tablet is prepared from the following raw and auxiliary materials in parts by weight: 0.03 part of reserpine, 1 part of hydralazine hydrochloride, 0.025 part of cyclopenthiazide, 1.5 parts of hydrochlorothiazide, 2 parts of promethazine hydrochloride, 30 parts of potassium chloride, 5 parts of rutin, 2.5 parts of phosphate chloroquine, 1 part of vitamin B1, 1 part of vitamin B6, 2-10 parts of a stabilizer fumaric acid and other pharmaceutical adjuvants. An experiment result shows that the orally disintegrating tablet is short in onset time, long in medicine duration time, and convenient for treatment of a patient for a long period of time; the medication safety is improved; meanwhile, the prescription is high in process stability, and is free of effects of environmental temperature and humidity; and the difference between batches of the preparations is significantly reduced; and the stability of a sample is improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Compound antihypertensive drug and preparation method thereof
ActiveCN101548985ADecreased blood flowPharmaceutical non-active ingredientsAluminium/calcium/magnesium active ingredientsSide effectPotassium
The invention discloses a compound drug, particularly relating to a compound antihypertensive drug and a preparation method thereof and belonging to the field of medicine technology. The compound antihypertensive drug is prepared by the following bulk drugs according to parts by weight: 2 parts of hydrochlorothiazide, 1.5 parts of dihydralazine sulfate, 2 parts of promethazine hydrochloride, 5 parts of rutin, 2.5 parts of chloroquine phosphate, 30 parts of potassium chloride, 1 part of thiamine hydrochloride, 1 part of pyridoxin hydrochloride, 0.03 part of reserpine, 30 parts of magnesium trisilicate, 2 to 5 parts of starch, 0.1 to 0.8 part of magnesium stearate and 10 to 30 parts of starch slurry. The compound antihypertensive drug generates low fluctuation of blood pressure in the process of reducing blood pressure for hypertension and the compound antihypertensive drug is also characterized by excellent treatment effect, low side effect, high treatment efficiency and long-lasting drug effect.
Owner:SHENZHEN OASIS PHARMA
Compound reserpine medicine composition for treating hypertension and preparation method of compound reserpine medicine composition
InactiveCN105193841AImprove stabilityImprove standardsPill deliveryPharmaceutical non-active ingredientsVitamin b6Efficacy
The invention belongs to the field of pharmacy, and particularly relates to a compound reserpine medicine composition for treating hypertension and a preparation method of the compound reserpine medicine composition. The compound reserpine medicine composition comprises raw materials in parts by weight as follows: 0.03 parts of reserpine, 1 part of hydralazine hydrochloride, 0.025 parts of cyclopenthiazide, 1.5 parts of hydrochlorothiazide, 2 parts of promethazine hydrochloride, 30 parts of potassium chloride, 5 parts of rutin, 2.5 parts of chloroquine phosphate, 1 part of vitamin B1, 1 part of vitamin B6, 2-10 parts of a stabilizer fumaric acid and other pharmaceutical adjuvants. Experiment results show that the medicine composition takes effect quickly, has long-lasting medicine efficacy, facilitates long-term treatment of a patient and is safe to take; besides, the formula has a high technological stability and is not influenced by environmental temperature and humidity, differences between different bathes of preparations are remarkably reduced, and the stability of samples is improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Combination kit used in the treatment of malaria
A combination kit for the treatment of malaria caused by Plasmodium vivax (P. vivax) having individual doses of an anti-malarial agent, 3-[1-[[4-[(6-methoxy-8-quinolinyl)amino]pentyl]amino]ethylidene]-dihydro-2(3H)-furanone (I) in the form of capsules; individual doses of the anti-malarial agent, chloroquine in the form of tablets; and instruction material for the administration of the two anti-malarial drugs. The combination kit is particularly suited for a 6 days treatment regimen where the treatment is rendered by five tablets containing 500 mg of chloroquine phosphate (equivalent to 300 mg base), three to be taken on day one and one each on days two and three; and five capsules containing 25 mg of 3-[1-[[4-[(6-methoxy-8-quinolinyl)amino]pentyl]amino]ethylidene]-dihydro-2(3H)-furanone (I), one each to be taken on days two to six.
Owner:NICHOLAS PIRAMAL INDIA LTD +1
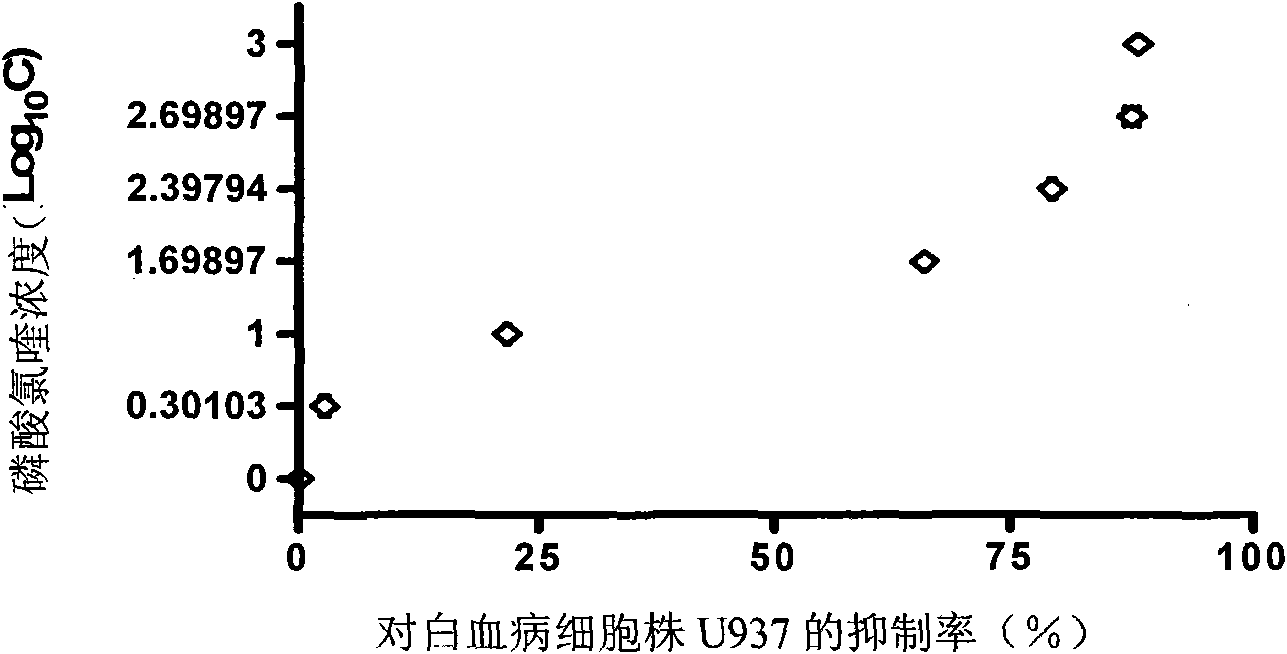
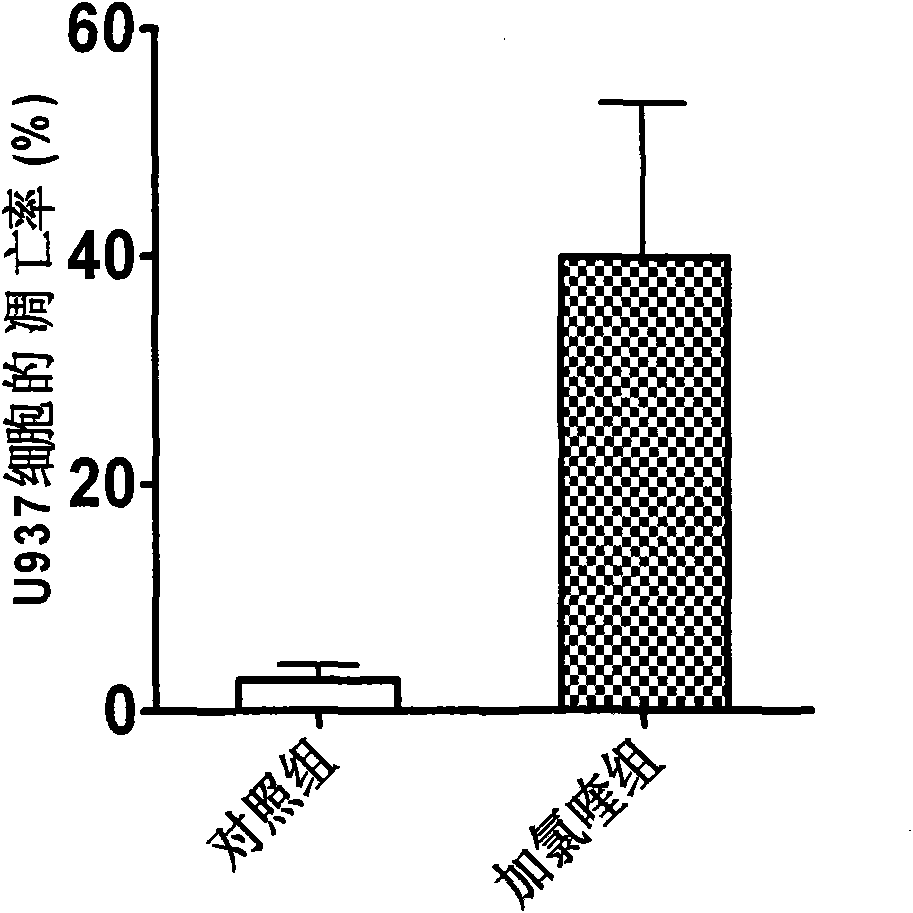
Application of chloroquine compound or salt thereof in preparation of medicament for treating acute leukemia
InactiveCN101829115APrevent seepagePro-apoptoticOrganic active ingredientsAntineoplastic agentsHydroxychloroquineApoptosis
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Novel application of minor radix buplenri granules combined chloroquine phosphate tablets
ActiveCN111329982AImprove the level ofImprove the ability to scavenge free radicalsAntibacterial agentsOrganic active ingredientsAzithromycinSuperoxide dismutases
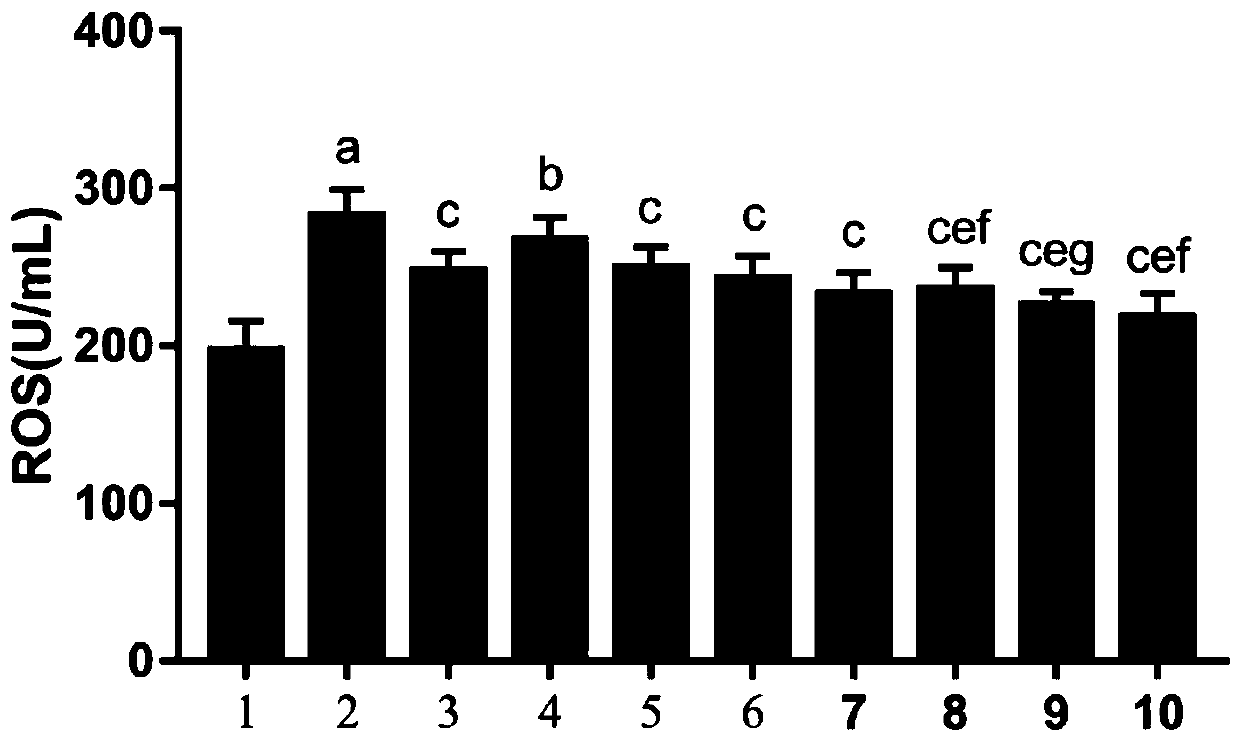
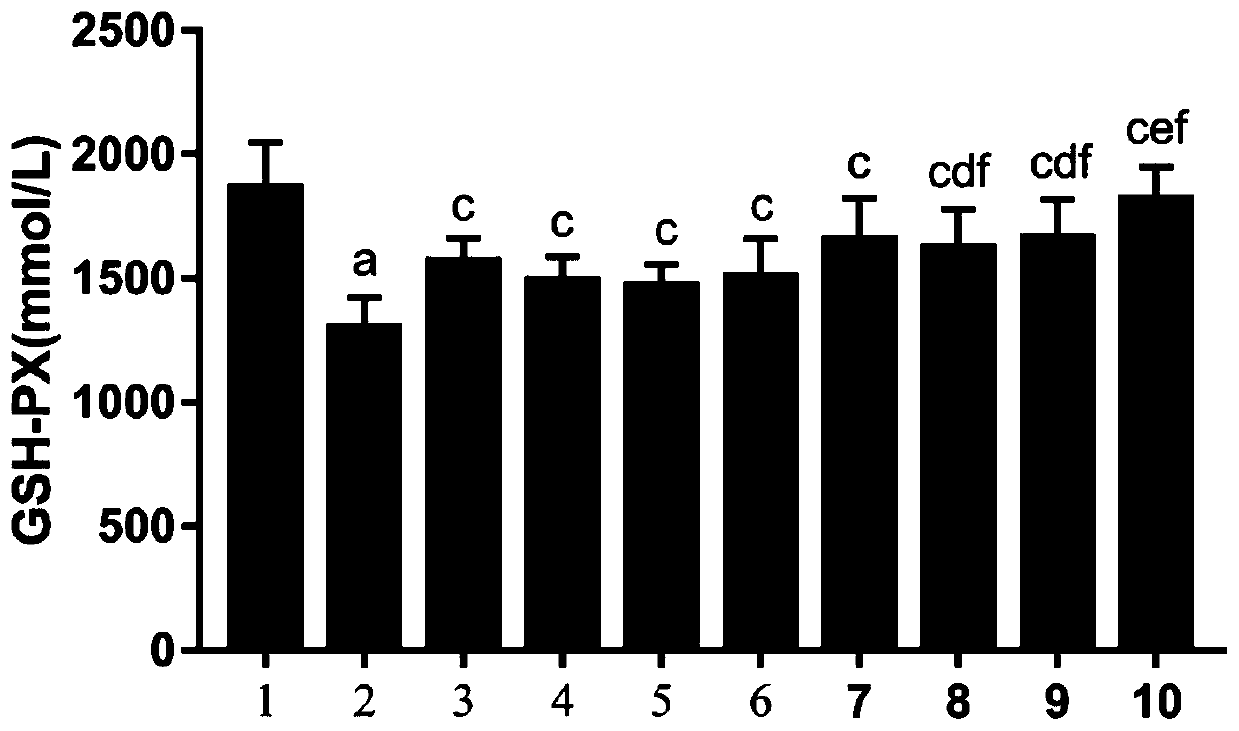
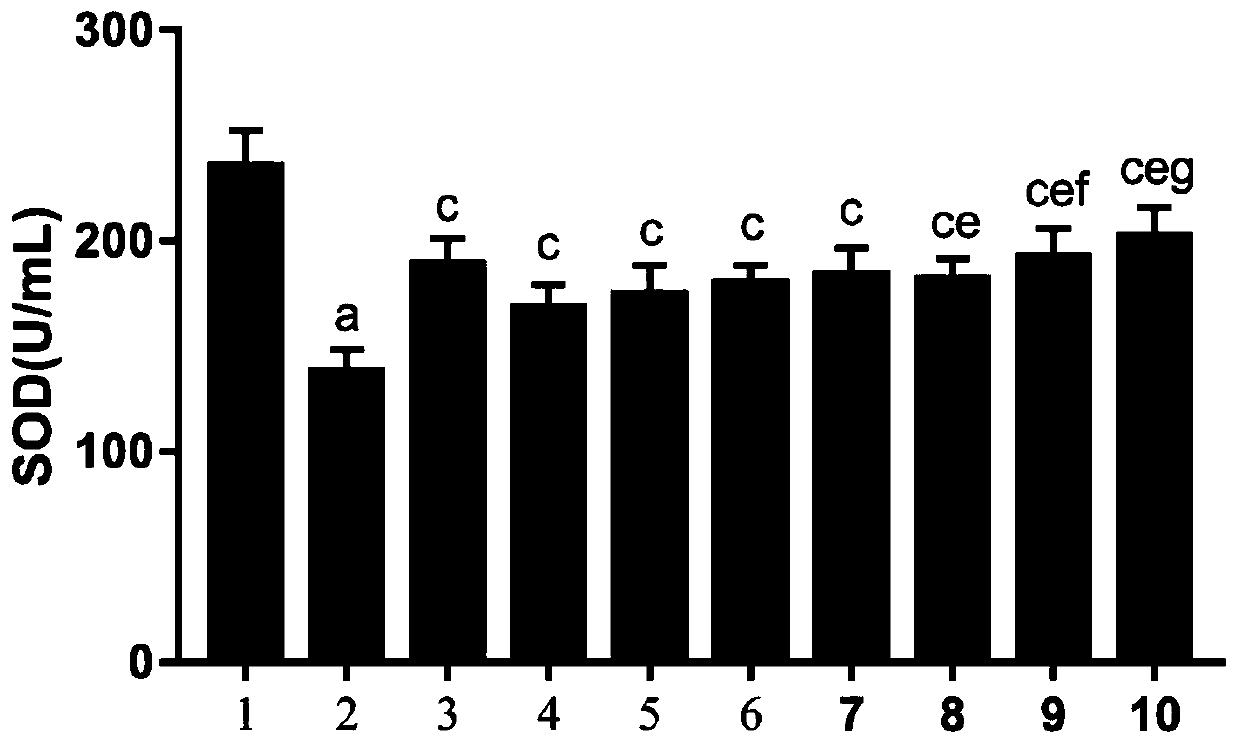
According to the invention, an SD rat model infected by mycoplasma pneumoniae is adopted for experiments, and it is found that minor radix buplenri granules can remove excessive free radicals caused by mycoplasma pneumoniae infection. In particular, through combination of the minor radix buplenri granules and chloroquine phosphate, the levels of glutathione peroxidase, superoxide dismutase and catalase in serum can be obviously increased, and the content of malondialdehyde can be reduced, so that combined administration can improve the free radical removing capability of an organism and reducecytotoxicity generated by free radical accumulation. The minor radix buplenri granules combined chloroquine phosphate tablets have a purpose of removing excessive free radicals caused by mycoplasma pneumoniae infection, solve the problem of excessive free radicals in mycoplasma pneumoniae infection, have a better sensitization effect on azithromycin, and thus can be used for preventing and treating mycoplasma pneumoniae.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Application of polycyclic polyketone compound in preparation of medicine for resisting novel coronavirus
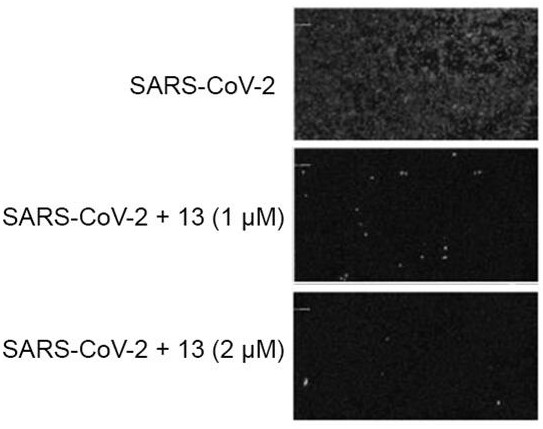
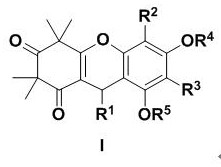
The invention discloses an application of a polycyclic polyketone compound in preparation of a medicine for resisting a novel coronavirus SARS-CoV-2. The polyketone compound disclosed by the invention has an inhibition effect on a novel coronavirus SARS-CoV-2 at a cellular level, can be used for remarkably reducing the virus titer of the virus in cells and inhibiting cytopathy induced by the virus, and has concentration dependence. In addition, the polyketone compound has chemical structure types different from those of the ridecevir and the chloroquine phosphate, and is expected to be developed into a novel medicine for resisting the novel coronavirus SARS-CoV-2. Therefore, the compound has a good application prospect in treatment of related diseases caused by infection of novel coronavirus SARS-CoV-2.
Owner:JINAN UNIVERSITY
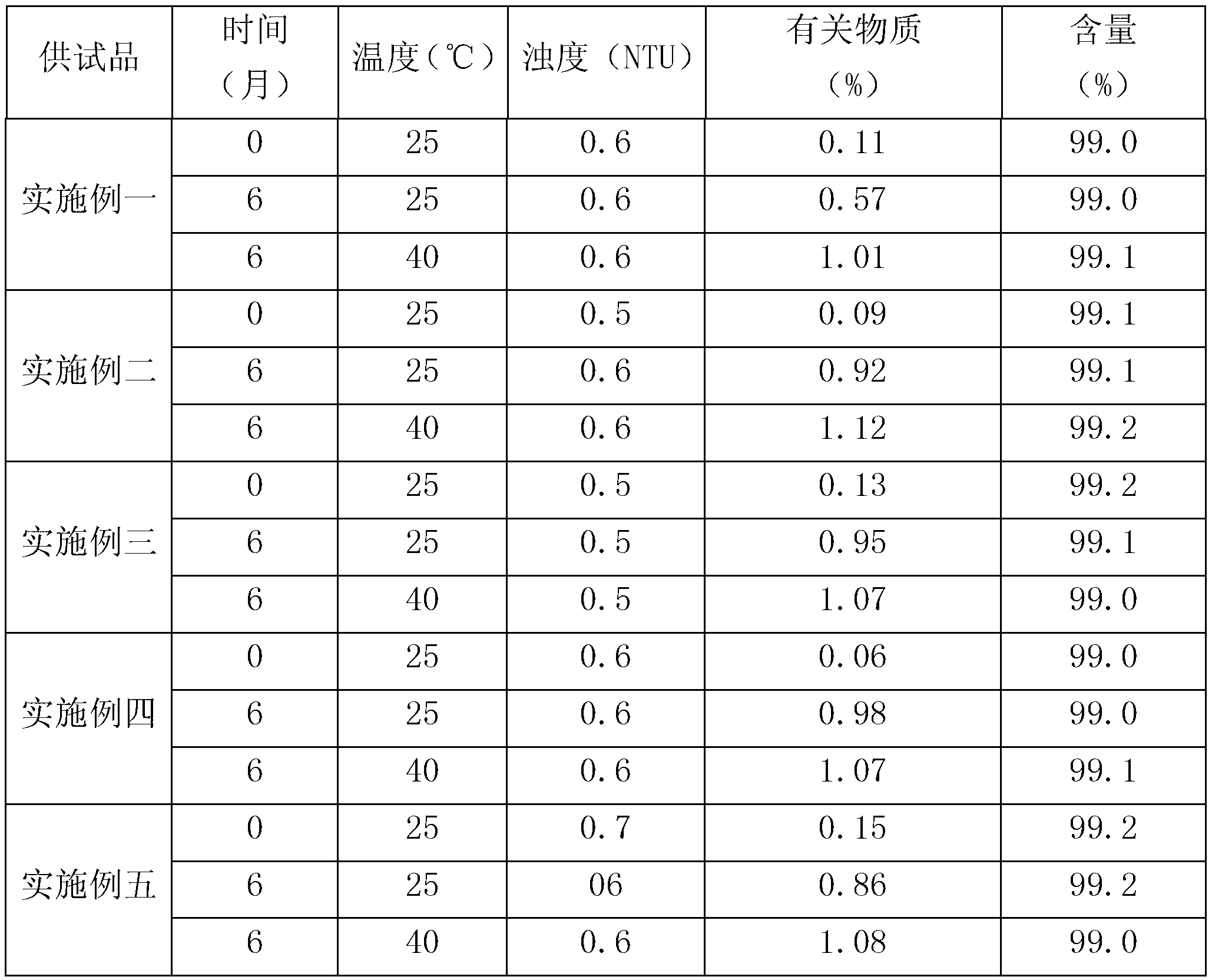
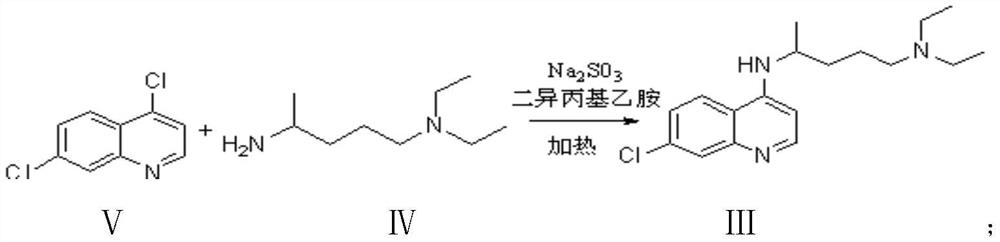
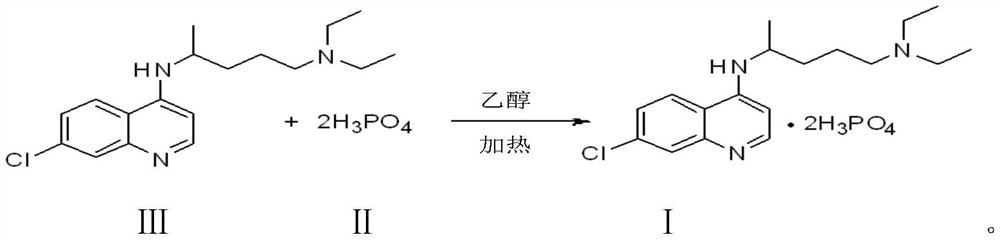
Preparation process of chloroquine phosphate
ActiveCN111662229AReduce usageHigh purityOrganic chemistryAgainst vector-borne diseasesO-Phosphoric AcidPtru catalyst
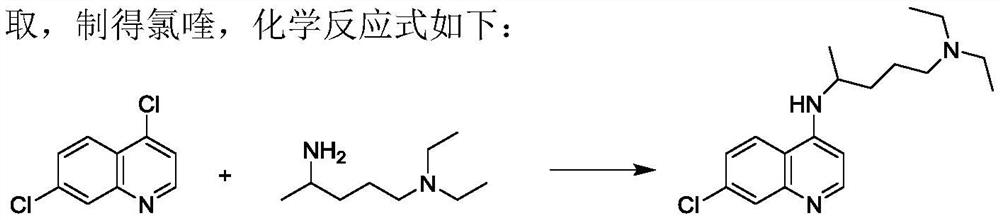
The invention discloses a preparation process of chloroquine phosphate. The preparation process of the chloroquine phosphate comprises the following steps of: (1) taking 4, 7-dichloroquinoline as an initial raw material, carrying out condensation reaction with 2-amino-5-diethylaminopentane, and carrying out alkalization extraction to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) with phosphoric acid to obtain chloroquine phosphate. The invention provides a preparation process of chloroquine phosphate. In the preparation process, the use of organic solvents suchas benzene and other catalysts such as phenol is avoided, and a condensation crystallization impurity removal step is introduced, so that the product purity is high, the individual impurity is less than 0.1%, the green chemical requirements and pharmacopeia standards are met, the operation is simple and convenient, the process is simplified, the production efficiency is improved, and the method is suitable for industrial production.
Owner:JINGHUA PHARMA GRP NANTONG
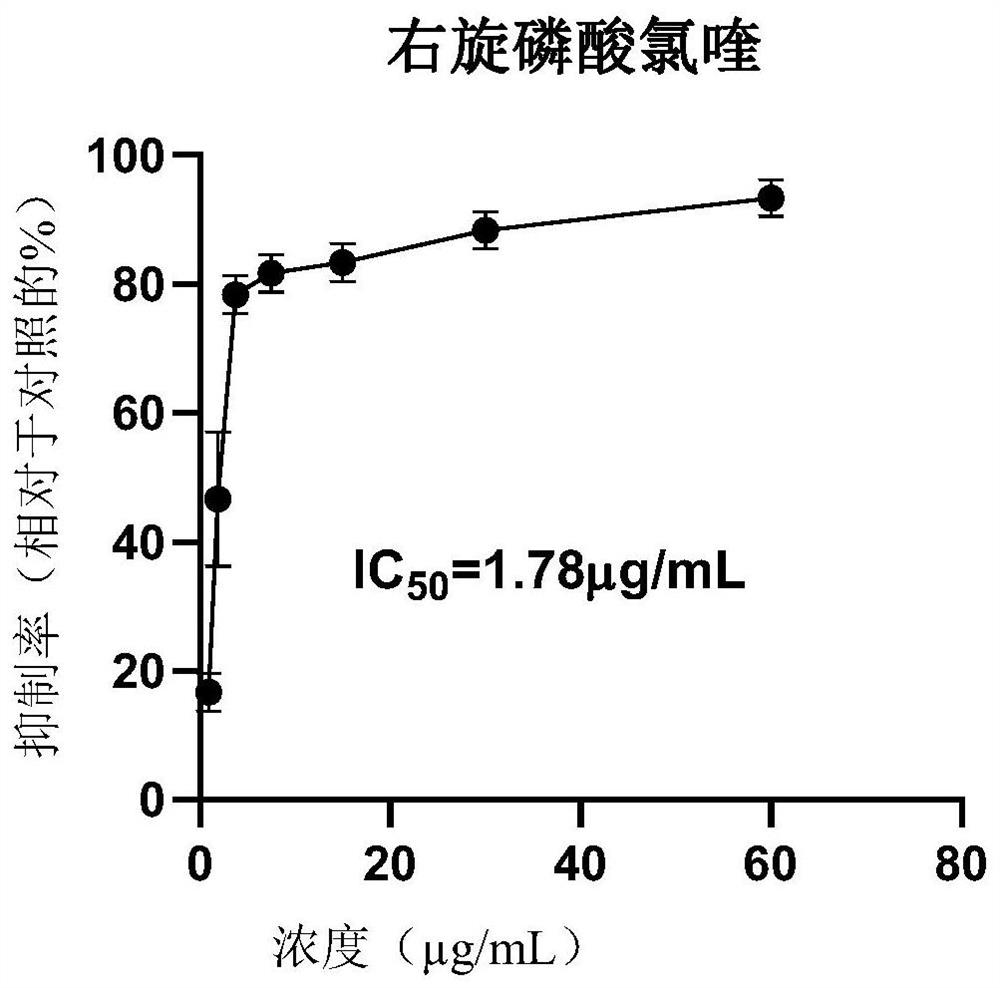
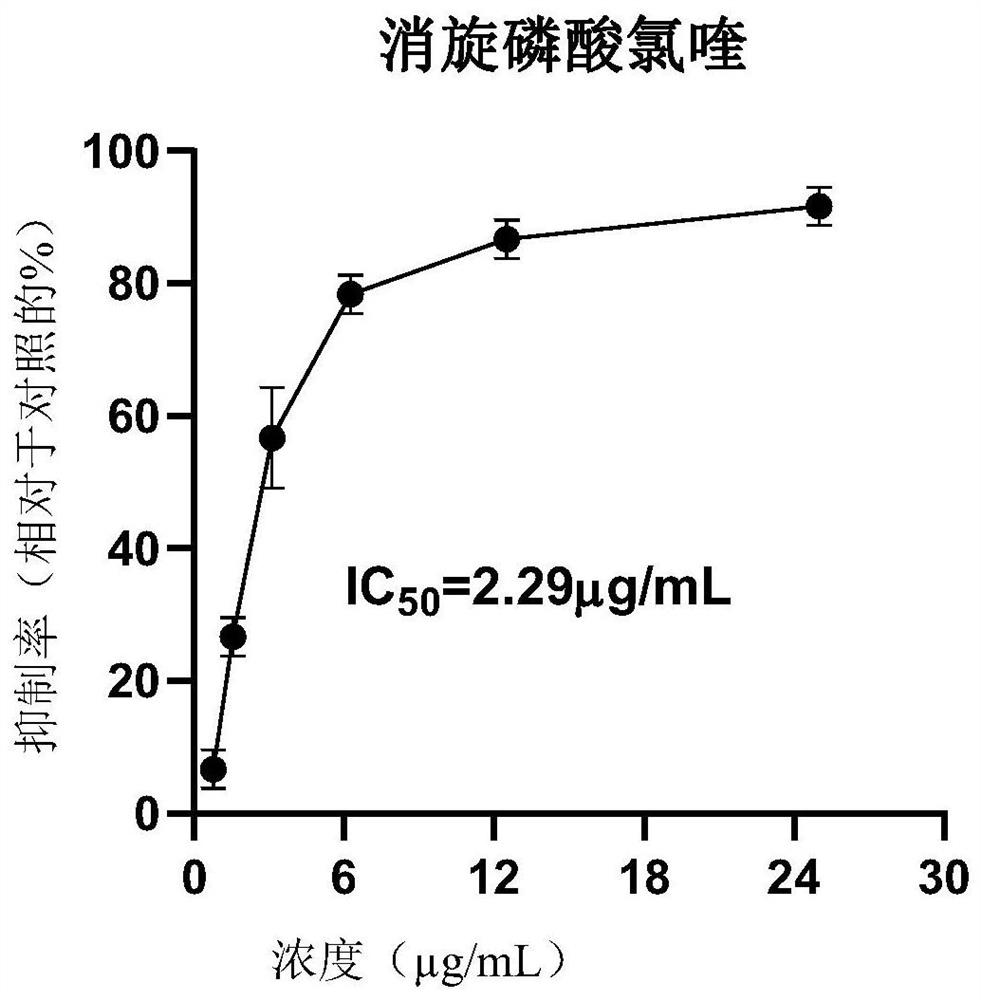
Application of dextro-chiral chloroquine phosphate in preparation of medicine for treating coronavirus
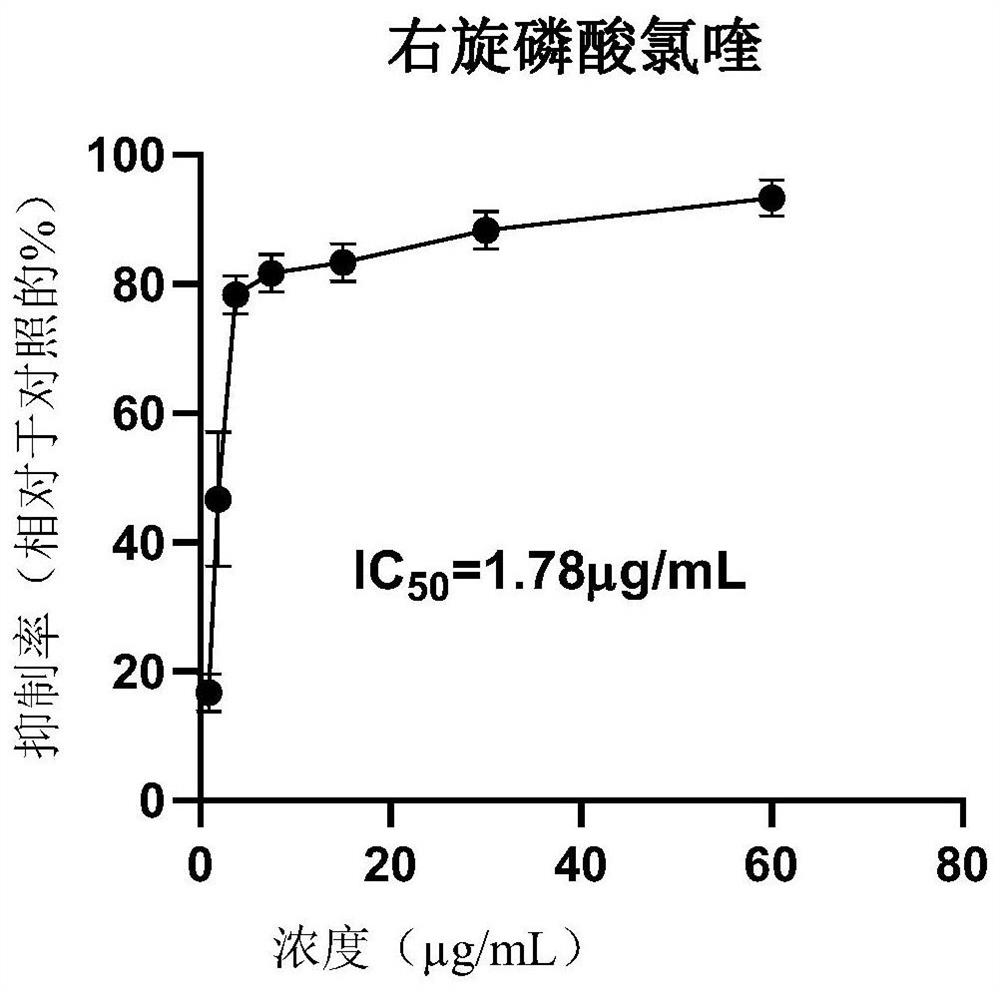
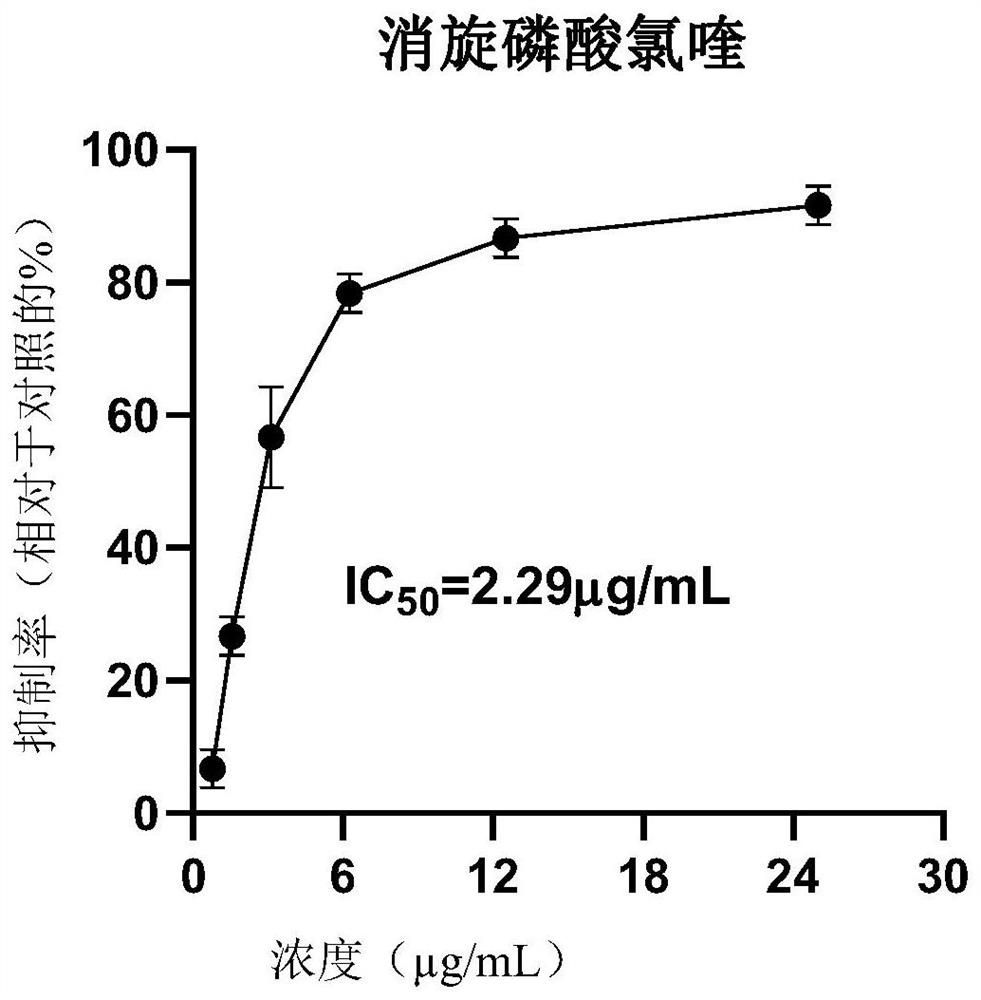
The invention discloses application of dextro-chiral chloroquine phosphate in preparation of a medicine for treating coronavirus. The inventor of the invention finds that in an in-vitro cell experiment, the dextro-chiral chloroquine phosphate has a better anti-novel coronavirus effect than the currently applied chloroquine phosphate, and under the condition that the inhibition rate (78.33%) is the same, the drug concentration of the dextro-chiral chloroquine phosphate is 3.75 mu g / mL, and the drug concentration of racemic chloroquine is 6.25 mu g / mL. The invention provides a new way for treating coronavirus.
Owner:SOUTH CHINA UNIV OF TECH
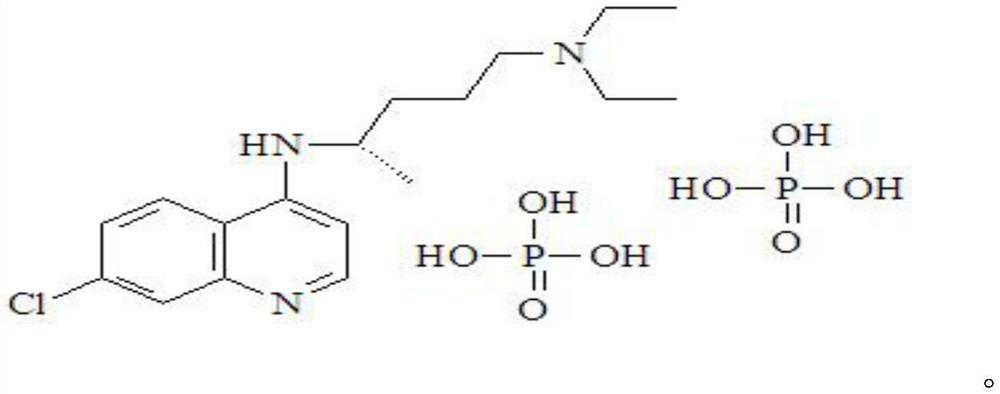
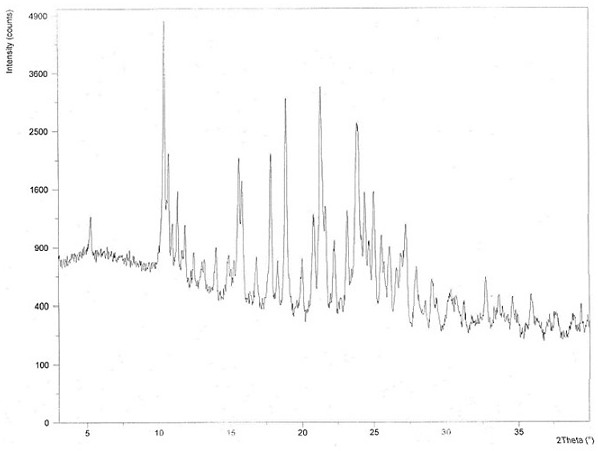
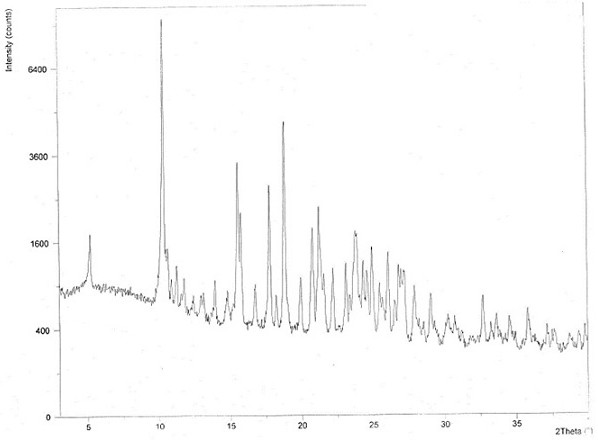
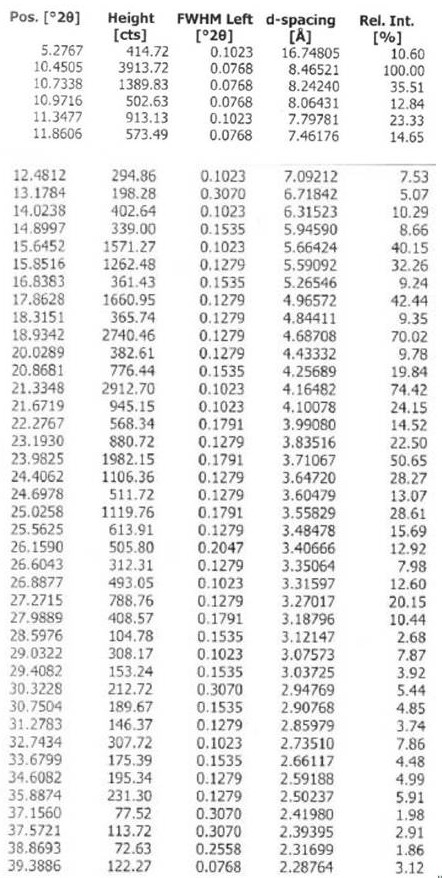
Chloroquine phosphate enantiomer crystal form and preparation method thereof
PendingCN111732539AEase of industrial productionQuality improvementAmino preparation from aminesOrganic compound preparationEnantiomerPhosphoric acid
The invention discloses a chloroquine phosphate enantiomer crystal form and a preparation method thereof. The invention provides (R) / (S)-chloroquine phosphate and a preparation method thereof. According to the (S)-chloroquine phosphate provided by the invention, an X-ray powder diffraction pattern expressed by a 2theta angle has characteristic peaks at 10.4 degrees, 10.7 degrees, 15.6 degrees, 15.8 degrees, 17.8 degrees, 18.9 degrees, 21.3 degrees and 23.9 degrees + / -0.2 degrees. According to the (R)-chloroquine phosphate provided by the invention, an X-ray powder diffraction pattern expressedby a 2theta angle has characteristic peaks at 10.3 degrees, 15.5 degrees, 15.7 degrees, 17.8 degrees and 18.8 degrees + / -0.2 degrees.
Owner:珠海润都制药股份有限公司
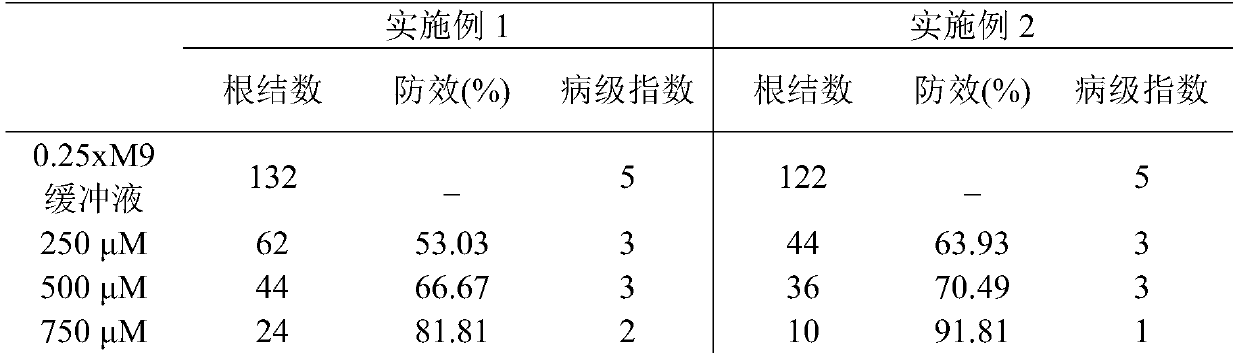
New use of chloroquine in prevention and control of root-knot nematodes
The invention discloses a new use of chloroquine in prevention and control of root-knot nematodes, and belongs to the technical field of the prevention and control of root-knot nematodes. The invention relates to a use of chloroquine in prevention and control of root-knot nematodes and a use of chloroquine in inhibition of infection, development and / or oviposition of root-knot nematodes, wherein the chloroquine is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. The invention also relates to a use of chloroquine in preparation of a root-knot nematode pesticide or a root-knot nematode infection, development and / or oviposition inhibitor, wherein the active component of the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate; and the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is an aqueous solution of chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. According to the present invention, the prevention and control of root-knot nematodes with the aqueous solution of chloroquine are safe, non-toxic and effective.
Owner:YUNNAN UNIV
Composition containing chloroquine phosphate
InactiveCN103191113AImprove stabilityStrong specificityOrganic active ingredientsPharmaceutical non-active ingredientsHistidineXylitol
The invention discloses a composition containing chloroquine phosphate. The composition also contains the following components: 5-20wt% of histidine sodium, 10-20wt% of xylitol, 30-50wt% of alkyl trimethyl ammonium bromide and 10-20wt% of sodium caprylate. The composition has the beneficial effect of good stability.
Owner:天津市嘉凡生物科技有限公司
Preparation method of chloroquine phosphate
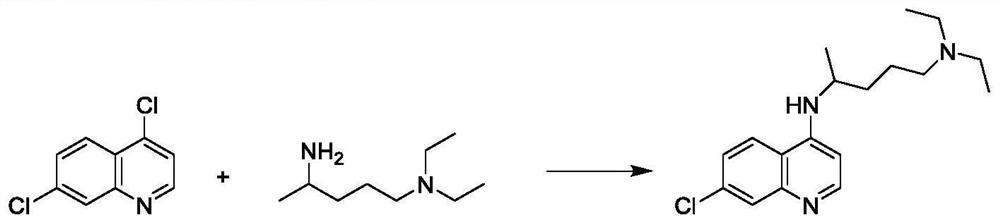
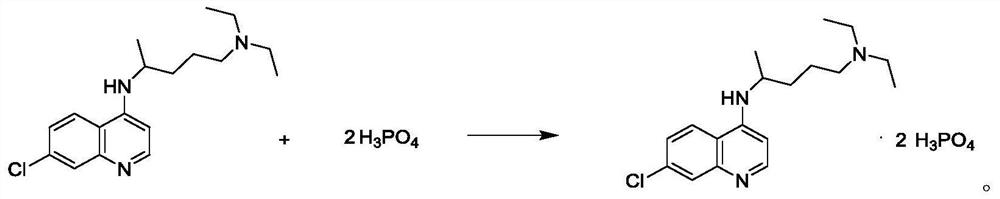
The invention discloses a preparation method of chloroquine phosphate, namely 7-chloro-4-(4-diethylamino-1-methylbutylamino)quinoline diphosphate. The preparation method comprises the following steps: (1) carrying out a condensation reaction on 4,7-dichloroquinoline and 2-amino-5-diethylaminopentane, and carrying out alkalization extraction, concentration and crystallization to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) and phosphoric acid to obtain chloroquine phosphate. The method avoids the use of phenol, is simple in reaction, and is beneficial to industrial production; and the chloroquine is crystallized to improve the purity and then salified with the phosphoric acid, so product appearance is good, the purity of the produced chloroquine phosphate is greater than or equal to 99.5%, and a single impurity is less than 0.1% (according to an HPLC area normalization method).
Owner:CHONGQING KANGLE PHARMA
Production method of exosome carrying plasmid
ActiveCN113801894AUpregulation efficiencyNo significant immune rejectionGenetically modified cellsSkeletal/connective tissue cellsDiseaseLiposome
The invention discloses a production method of an exosome carrying plasmid. The method comprises the following steps of: 1) fusing human mesenchymal stem cells cultured in vitro to 85-95%; 2) discarding the culture supernatant, adding a plasmid liposome compound, and performing transfection culture in a cell incubator; 3) after the transfection culture is completed, removing the supernatant carrying the plasmid liposome compound, adding an alpha-MEM culture medium containing exosome-free fetal calf serum and chloroquine or chloroquine phosphate solution, and culturing in the cell incubator; and 4) collecting the culture supernatant, and separating and purifying by using ultracentrifugation or an exosome extraction kit to obtain the exosome carrying plasmid. The exosome obtained by the production method disclosed by the invention can be used as a novel drug, and can be widely used for treating various diseases by carrying different plasmids.
Owner:THE UNIV OF HONG KONG SHENZHEN HOSPITAL
Prescription of compound bendazol and hydrochlorothiazide capsule and its preparation process
ActiveCN1729982ASolve the problem of chemical instabilitySolve the problem that the content is very small and it is not easy to mix evenlyOrganic active ingredientsCapsule deliveryVitamin b6CALCIUM LACTOBIONATE
The invention relates to a compound dibazole and hydrochlorothiazidum capsule and process for preparation, wherein the raw materials include dibazole, promethazine, chloroquine phosphate, guanoxan sulfate, vitamin B6, potassium chloride, crystoserpine, hydrochlorothiazidum, calcium lactate, vitamin B1, magnesium trisilicate and medicinal starch. The end product can effectively lower blood pressure.
Owner:FUZHOU CHENXING PHARMA
Chloroquine phosphate inhalation aerosol and preparation method thereof
InactiveCN111110634AImprove stabilityAccurate doseOrganic active ingredientsDispersion deliveryInhalationPhosphoric acid
The invention belongs to the technical field of medicine, and discloses a chloroquine phosphate inhalation aerosol and a preparation method thereof. The chloroquine phosphate inhalation aerosol is composed of active ingredient chloroquine phosphate and a certain proportion of a propellant, a flavoring agent, a pH regulator and water for injection. The aerosol inhaler is administered through mouthsand directly acts on lungs to realize targeted drug delivery. The inhalation preparation provided by the invention can target lesion, has an accurate dosage and a fast effect, can quickly improve theinfection conditions of the lungs, facilitates improving the adaptability of infected people, and avoids absorption through gastrointestinal tracts to reduce gastrointestinal side effects.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
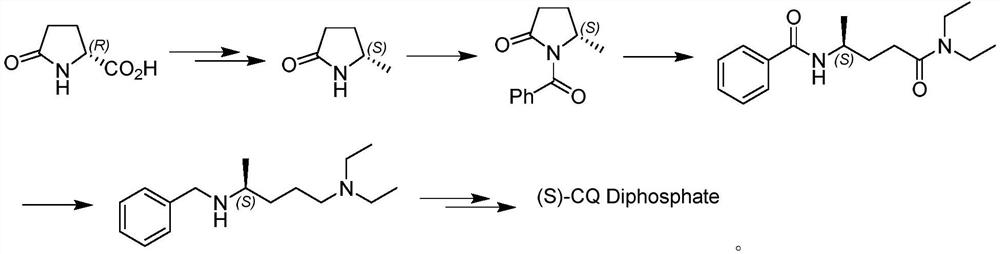
Asymmetric synthesis method of (S)-chloroquine phosphate
PendingCN112266356AFew reaction stepsHigh selectivityOrganic chemistry methodsO-Phosphoric AcidSide chain
The invention provides a novel asymmetric synthesis method of (S) chloroquine. According to the invention, 5-(N-diethylamino)-2-pentanone and (S)-alpha-methyl benzylamine are adopted as raw materials,and in the presence of Lewis acid, an asymmetric reductive amination reaction is carried out to obtain (S,S)-5-(N'-diethylamino)-N-((1-phenyl)ethyl)-2-pentylamine, catalytic hydrogenation is performed to remove benzyl to obtain a side chain (S)-5-(N'-diethylamino)-2-amyl amine for synthesizing chloroquine, the (S)-5-(N'-diethylamino)-2-amyl amine is condensed with 4,7-dichloroquinoline to obtain(S)-chloroquine, salifying is performed with phosphoric acid, and recrystallizing is performed to obtain (S)-chloroquine phosphate with ee value of more than 99%, wherein the the total yield of the four-step reaction exceeds 70%; and the preparation method is stable, reliable, economical, efficient and easy to industrialize.
Owner:江苏百奥信康医药科技有限公司
Systematic novel anti-virus treatment method for coronavirus pneumonia
PendingCN113786478ASystematize the treatment processImprove treatment conditionsRespiratorsOrganic active ingredientsInitial treatmentDisease
The invention discloses a systematic novel anti-virus treatment method for coronavirus pneumonia. According to the systematic novel anti-virus treatment method, [alpha]-interferon, ibovitamide, ribavirin, chloroquine phosphate and arbidol are included. The whole novel anti-virus treatment method for coronavirus pneumonia is optimized, so that the treatment process is more systematized, a patient can be examined before suffering from a disease, and measures such as standard initial examination, initial treatment, anti-virus treatment and critical treatment are implemented when the patient suffers from the disease; the curative effect of antiviral treatment is improved to the greatest extent, a high-level medical service system is built, and high-level diagnosis and treatment services are provided for patients; and when the illness state of the patient deteriorates, the patient can be actively rescued and treated by selecting treatment schemes and measures for the critically ill patient, so that the life of the patient is actively saved, the rescue and treatment survival rate of the critically ill patient is improved, and the case fatality rate is reduced.
Owner:成都市公共卫生临床医疗中心
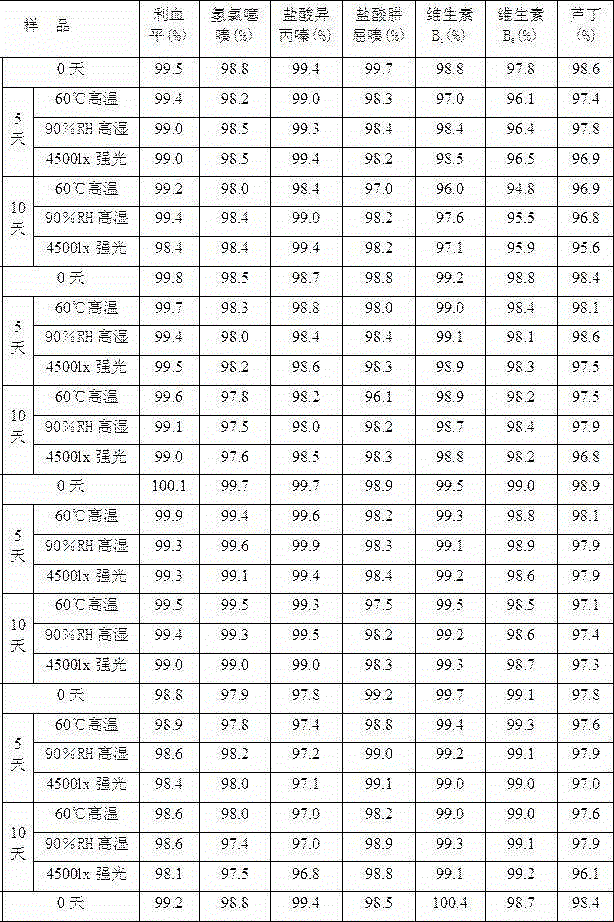
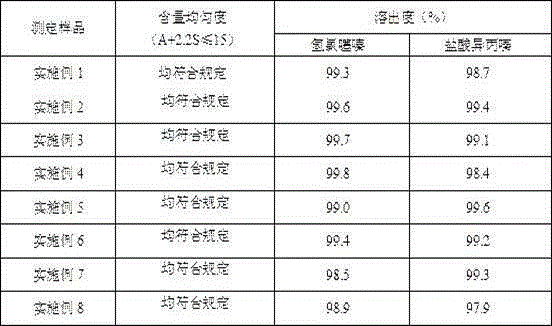
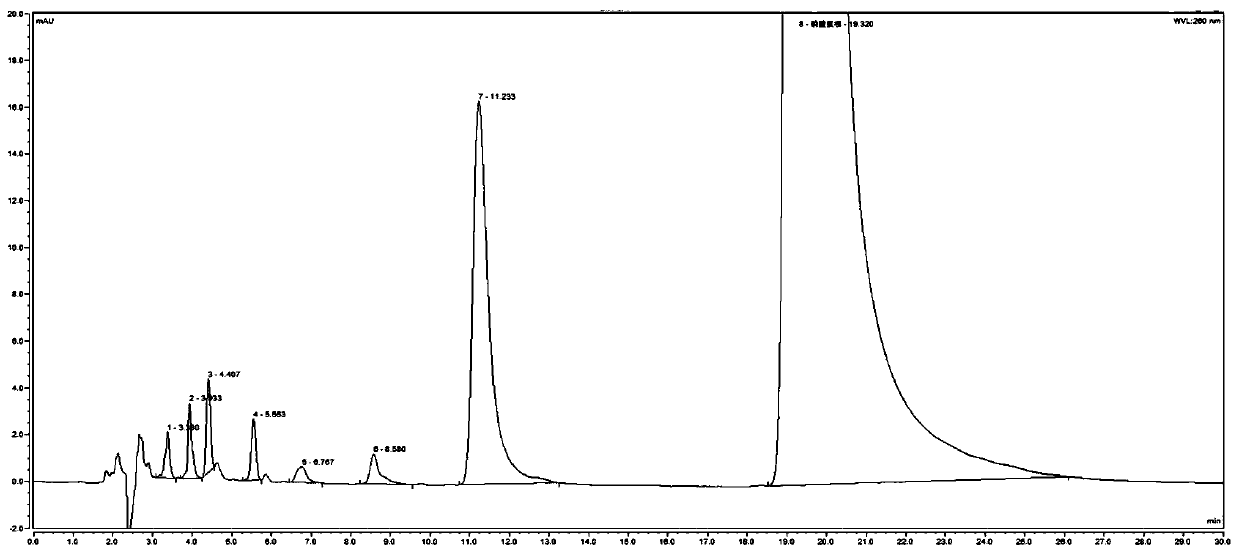
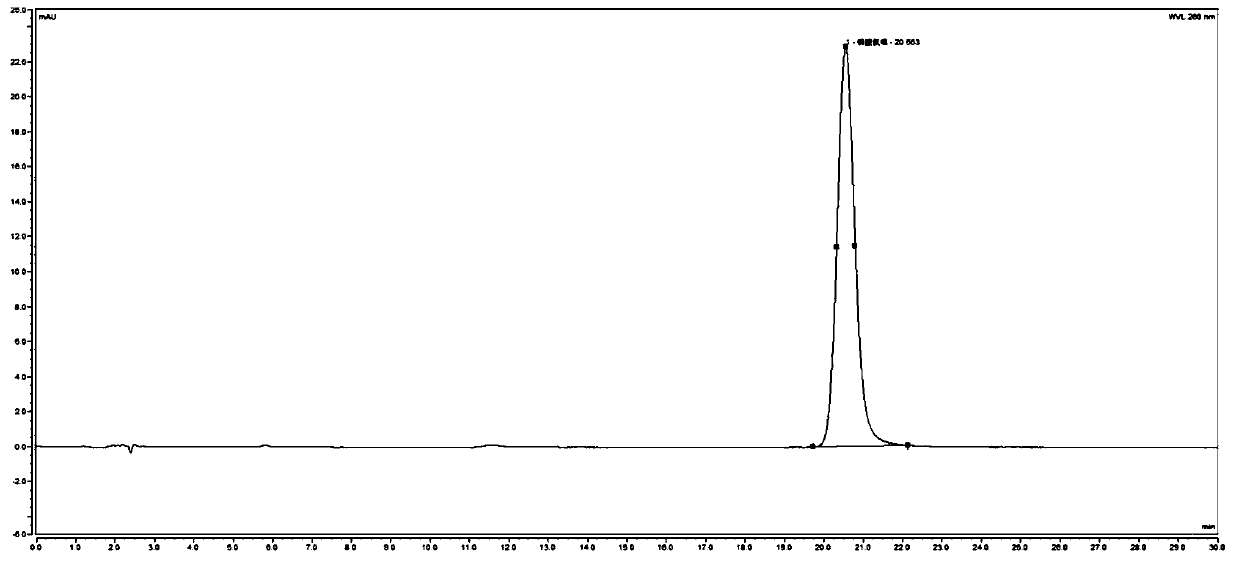
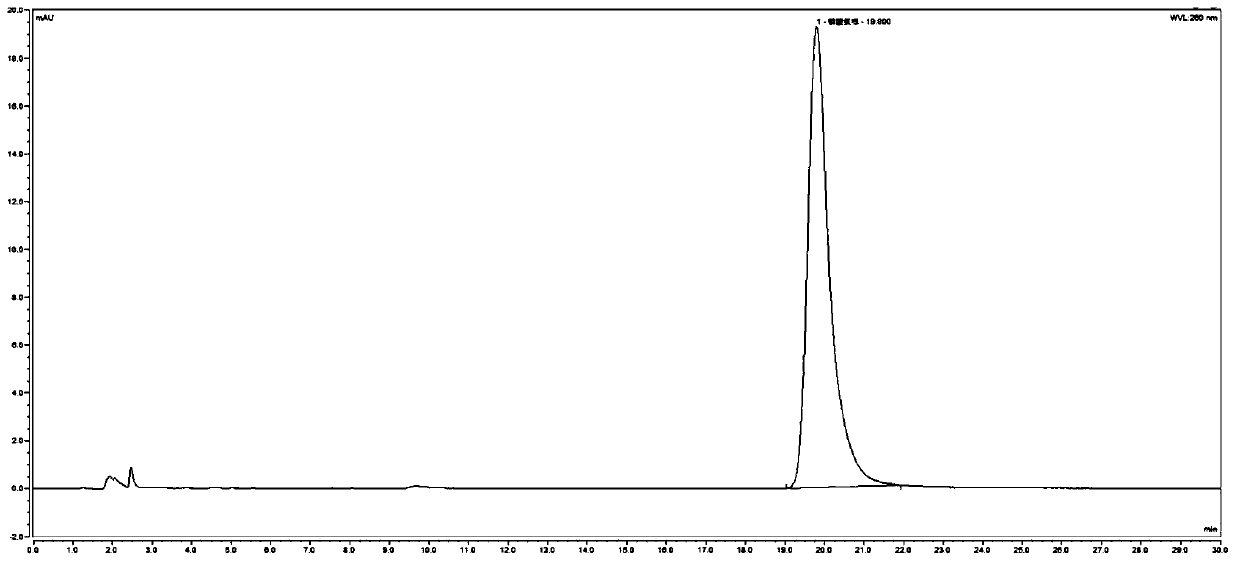
Method for detecting related substances in chloroquine phosphate tablet
ActiveCN111272937AEasy to separateEfficient separationComponent separationAgainst vector-borne diseasesUltraviolet detectorsPhysical chemistry
The invention relates to a method for detecting related substances in a chloroquine phosphate tablet. According to the method, the ultraviolet detector is connected with the electrospray detector (CAD) in series, so that the ultraviolet relative response factor of each impurity can be quickly, conveniently and accurately calculated, the content of known and unknown impurities in the chloroquine phosphate tablet can be accurately determined, the sensitivity and specificity of the method are high, and the effective control on related substances of the chloroquine phosphate tablet is realized. The method comprises the following steps: preparing a test solution, taking chloroquine phosphate tablets, dissolving the chloroquine phosphate tablets with a mobile phase, performing filtering, and taking subsequent filtrate as the test solution; preparing a system adaptive solution, namely taking the test solution, and irradiating the test solution by using an ultraviolet lamp to obtain the systemadaptive solution; preparing a contrast solution, namely diluting the test solution with a mobile phase to obtain the contrast solution; preparing a reference substance solution, namely dissolving achloroquine phosphate reference substance by using a mobile phase to obtain the reference substance solution; and injecting the system adaptive solution, the test solution, the reference solution andthe reference solution into a liquid chromatograph for determination.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Method for manufacturing anti-coronavirus acrylic fibers
PendingCN111472066AChloroquine PhosphateReduce the temperatureArtificial filament washing/dryingConjugated cellulose/protein artificial filamentsPolymer scienceSpinning
The invention belongs to the technical field of textile, and particularly relates to a method for manufacturing anti-coronavirus acrylic fibers. The method comprises the steps that a stock solution where a polyacrylonitrile composition and phosphate chloroquine are dissolved is added into a spinning box; and then the stock solution in the spinning box is sprayed out through a spinning head and issolidified in an aqueous solution of DMAC, and the anti-coronavirus acrylic fibers are obtained. According to the method, the phosphate chloroquine capable of effectively inhibiting coronavirus on thecellular level is added into the spinning box in the spinning process, so that the obtained acrylic fibers and a fabric made from the acrylic fibers have certain anti-coronavirus effect, and therefore the aim of assisting people in resisting the coronavirus is achieved; and polyacrylonitrile resin is mixed with EVA resin, montmorillonoid and a plasticizer to obtain a polyacrylonitrile composition, the heating and dissolving temperature of the polyacrylonitrile composition is relatively low, and thus the probability that the phosphate chloroquine is decomposed can be reduced.
Owner:SHAOXING BIAODIAN TEXTILE TECH
Disinfectant
InactiveCN112167273AChange permeabilityGood sterilization and antibacterial effectBiocideDisinfectantsBiotechnologyAntimicrobial action
The invention provides a disinfectant. The disinfectant comprises the following components: citric acid, lactic acid, malic acid, baicalin alcohol extract, chinaroot greenbrier alcohol extract, bruceajavanica alcohol extract, echinacea purpurea alcohol extract, chloroquine phosphate and water. Through the synergistic effect of citric acid, lactic acid and malic acid, the sterilization and antibacterial effects on bacteria are well achieved, and after baicalin alcohol extract, brucea javanica alcohol extract and echinacea purpurea alcohol extract are compounded, the obvious killing effect on respiratory syncytial viruses and influenza viruses can be well achieved; through the compounding effect of chloroquine phosphate, the inhibition effect on herpes viruses and parotitis viruses is improved, the chinaroot greenbrier alcohol extract plays a good role in inhibiting bacteria, new bacteria and viruses are prevented from growing in the sterilized environment again, and the sterilized environment is kept from being polluted.
Owner:重庆赛力格柯网络科技有限公司
Liquid-changing culture medium and application thereof
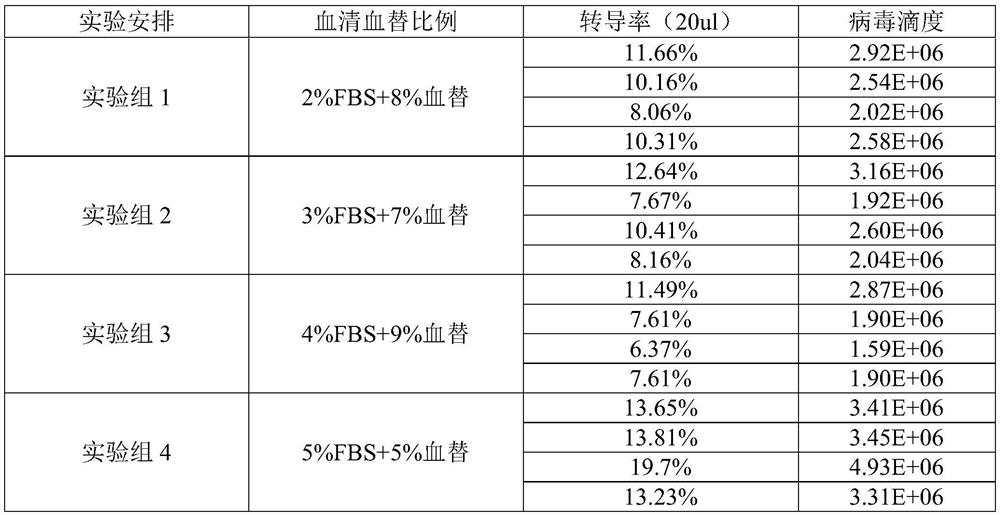
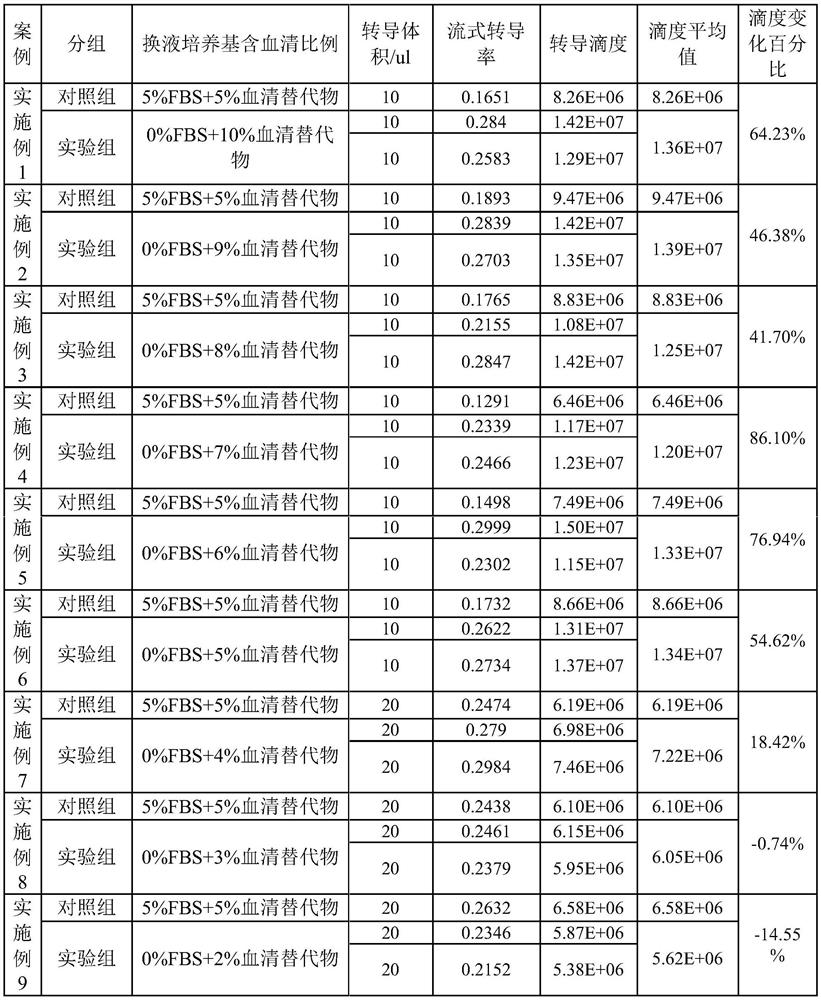
PendingCN114214287AHigh titerSupplements to maintain cells in good conditionGenetically modified cellsCulture processViral vectorNutrients substances
The invention relates to a liquid-changing culture medium and application thereof, and belongs to the technical field of virus vector packaging. The liquid-changing culture medium comprises a basic culture medium and a form maintaining agent, wherein the form maintaining agent is prepared from the following components in percentage by volume: 3 to 10 percent of serum substitute, 1 + / -0.1 g / L of insulin, 0.55 + / -0.1 g / L of transferrin, 0.00067 + / -0.0001 percent of sodium selenite, 0.20 + / -0.05 g / L of ethanolamine, 1 + / -0.02 mmol / L of sodium pyruvate and 25 + / -5 mu mol / L of chloroquine phosphate. The form maintenance agent is added into the basic culture medium, so that the good cell state is maintained through the supplement of nutrient substances, and the lentivirus with higher titer than the lentivirus packaged by 10% fetal calf serum can be obtained by matching with a small amount of serum substitute (serum substitute).
Owner:GUANGZHOU FINELMMUNE BIOTECHNOLOGY CO LTD
Inhalant containing chloroquine therapeutic agent and preparation method of inhalant
InactiveCN113559086AImprove stabilityImprove the protective effectOrganic active ingredientsPowder deliveryOral medicationInhalation
The invention provides a chloroquine inhalant and a preparation method thereof, and belongs to the field of medicine preparations. The inhalant comprises chloroquine phosphate or hydroxychloroquine sulfate, a stabilizer and a diluent, can improve use efficiency, has a preventive effect, such as a new epidemic situation-COVID-19, and can take effect quickly. The powder inhalation has better stability, has the effects of masking taste, enhancing stability and protecting organism biological membranes on chloroquine phosphate or hydroxychloroquine sulfate after being wrapped by the stabilizer, is absorbed through the lungs, has a high targeting effect, also can effectively avoid the first-pass effect caused by the liver during oral administration, and improves bioavailability.
Owner:DISCOVERY SHENZHEN NEW MEDICINES DEV CO LTD
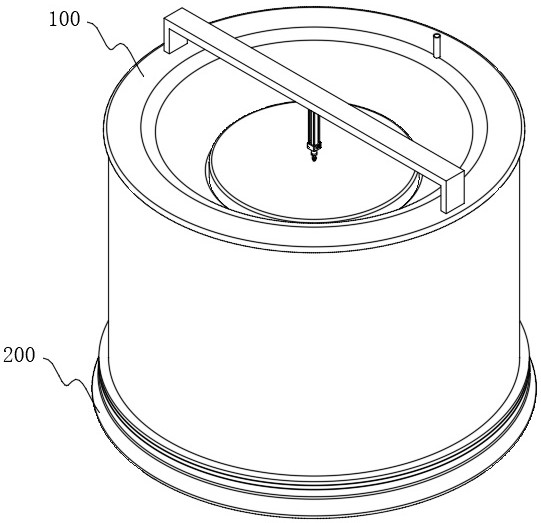
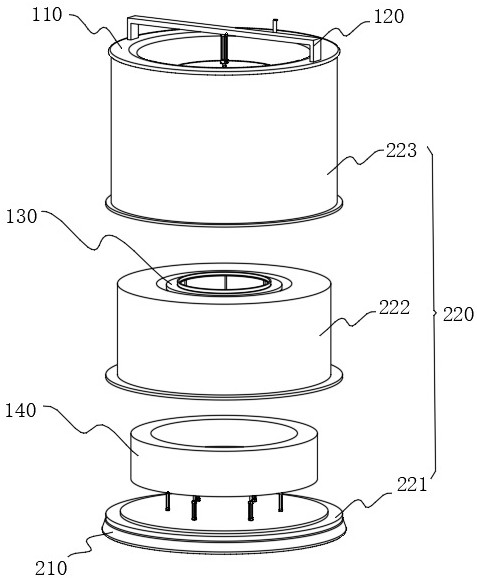
Device for producing chloroquine phosphate and preparation process
ActiveCN114832493AGuaranteed purityImprove product qualityOrganic chemistrySolid sorbent liquid separationFiltrationPhosphoric acid
The invention relates to the technical field of chloroquine phosphate production, in particular to a device for producing chloroquine phosphate and a preparation process. The device comprises a production device and a base arranged at the bottom of the production device, the base comprises a chassis, the production device comprises a decoloring pot and a concentrating pot, the decoloring pot is arranged at the top of the concentrating pot, a pressing assembly is arranged at the top of the decoloring pot, and a cooling shell is arranged at the bottom of the concentrating pot. According to the invention, mixing of purified water and the chloroquine phosphate crude product is realized through cooperation of the decolorizing pot and the pressure plate, a mixed solution flows into the top of the decolorizing pot from the top of the pressure plate through lifting of the pressure plate, and at the moment, pressure filtration of the mixed solution by a filter screen is realized through downward pressing of the pressure plate. Through cooperation of the bottom plate, the inner supporting plate and the outer supporting plate, the whole process is completed in a sealed environment, so that the purity of prepared chloroquine phosphate is guaranteed, and the preparation quality is improved.
Owner:JINGHUA PHARMA GRP NANTONG
Organic composite photocatalyst for degrading drugs and pathogenic bacteria and preparation method thereof
ActiveCN113058648BPromote absorptionImprove adsorption capacityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsSuperoxide radicalTrimethoprim
The invention discloses an organic composite photocatalyst for degrading medicines and pathogenic bacteria and a preparation method thereof, and belongs to the technical field of water pollutant treatment. In the method of the present invention, a simple green method is used to load cercosporin on the macroporous adsorption resin to obtain a supported photocatalyst. It can generate singlet oxygen, superoxide radicals, etc. under the irradiation of fluorescent lamps and even sunlight. These oxygen free radicals can react with drugs and pathogenic bacteria, so as to achieve the purpose of degrading drugs and pathogenic bacteria. The method can completely degrade drugs within 3 hours of reaction under light, these drugs include: antibacterial drugs: quinolones ciprofloxacin, gatifloxacin, moxifloxacin, ofloxacin, enrofloxacin , sparfloxacin, trimethoprim sulfonamides, and antiviral drugs: chloroquine phosphate. In addition, the photocatalyst can also effectively inhibit the pathogenic bacteria Staphylococcus aureus, realizing the simultaneous removal of drugs and pathogenic bacteria in water pollution.
Owner:JIANGNAN UNIV
Preparation method of responsive drug carrier for spinal cord injury repair
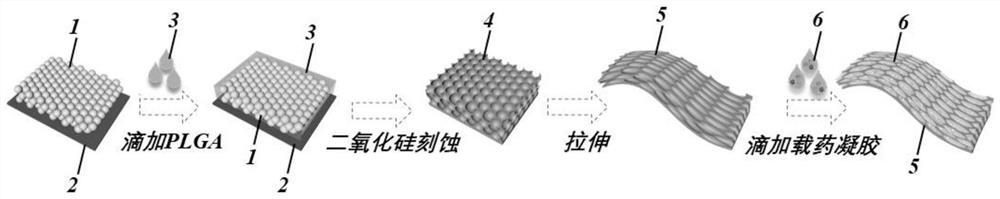
ActiveCN114425047AReduce releaseLarge specific surface areaOrganic active ingredientsNervous disorderSilica nanoparticlesO-Phosphoric Acid
The invention provides a preparation method of a responsive drug carrier for spinal cord injury repair, which comprises the following steps: S1, depositing monodisperse silicon dioxide nanoparticles on a glass slide, dropwise adding poly (lactic acid-glycolic acid) on the glass slide, and etching the monodisperse silicon dioxide nanoparticles after the poly (lactic acid-glycolic acid) is cured, so as to obtain poly (lactic acid-glycolic acid); a flexible inverse opal film is obtained; s2, dissolving agarose in a phosphoric acid buffer salt solution to form a solution A, dissolving gelatin and hyaluronic acid in the phosphoric acid buffer salt solution to form a solution B, mixing the solution A and the solution B to form a pre-gel solution, then adding fibroblast growth factors 10, chloroquine phosphate and black phosphorus quantum dots into the pre-gel solution, and mixing to form a drug-loaded pre-gel solution; s3, dropwise adding the drug-loaded pre-gel solution prepared in the step S2 into the inverse opal membrane prepared in the step S1, and obtaining the drug-loaded inverse opal membrane after the drug-loaded pre-gel solution is gelatinized.
Owner:WENZHOU INST UNIV OF CHINESE ACAD OF SCI
Application of L-chiral chloroquine phosphate in preparation of medicine for treating coronavirus
PendingCN113350344AGood inhibitory effectLow inhibitory concentrationOrganic active ingredientsOrganic chemistry methodsMedicineVirology
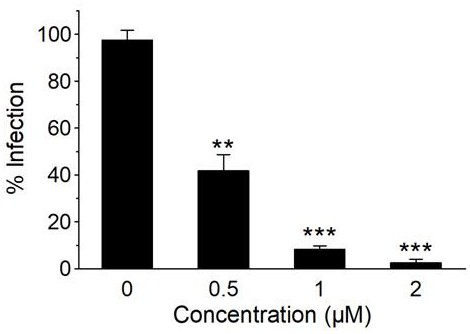
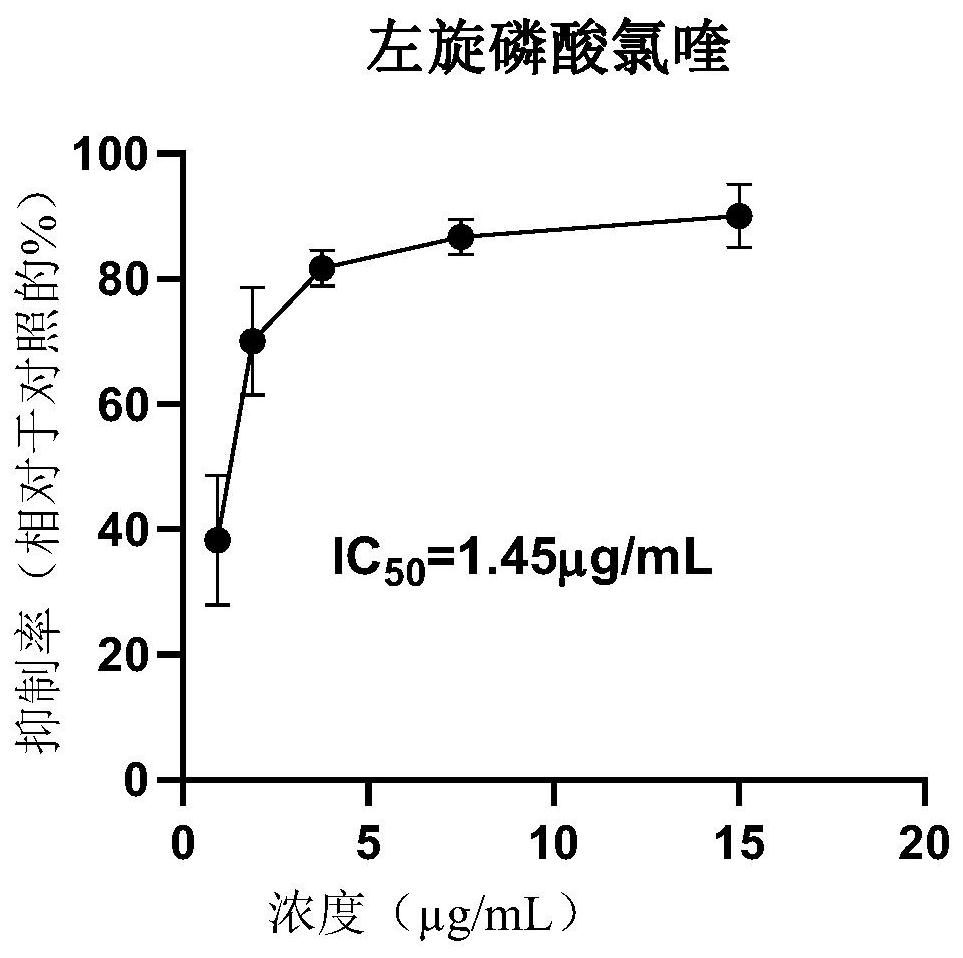
The invention discloses an application of L-chiral chloroquine phosphate in preparation of a medicine for treating coronavirus. Based on the fact that in an in-vitro cell experiment, L-chiral chloroquine phosphate has a remarkable inhibition effect on cytopathy caused by infection of Vero E6 cells by novel coronavirus SARS-CoV-2, the IC50 value is 1.45 mu g / mL, the inhibition concentration is remarkably lower than that of D-chloroquine phosphate and racemate chloroquine phosphate. The important significance is realized on the prevention, control and treatment of novel coronavirus infection.
Owner:SOUTH CHINA UNIV OF TECH
Composite medicine for reducing blood pressure and clearing fat
InactiveCN107823427ASignificant effectShort duration of actionOrganic active ingredientsMetabolism disorderBlood pressure kitOyster
The invention provides a composite medicine for reducing blood pressure and clearing fat. The composite medicine comprises the following raw materials in parts by weight: radix ophiopogonis, irbesartan, fructus liquidambaris, bendazol, chloroquine phosphate, telmisartan, radix paeoniae alba, bone fossil, oysters, radix cynanchi panicullati and reserpine. Compared with the prior art, the compositemedicine has the beneficial effects that the composite medicine is applicable to early-stage and medium-stage hypertension and particularly applicable to late-stage hypertension as well, and has a treatment effect which is remarkably prior to that of a composite hypertension pill on medium and serious hypertension. In addition, compared with the composite hypertension pill, the composite medicineis rapid in effect taking, long in effect lasting time, relatively stable in blood pressure and applicable to patient suffering from hypertension in accompany with cankers.
Owner:GUANGDONG YIMING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com