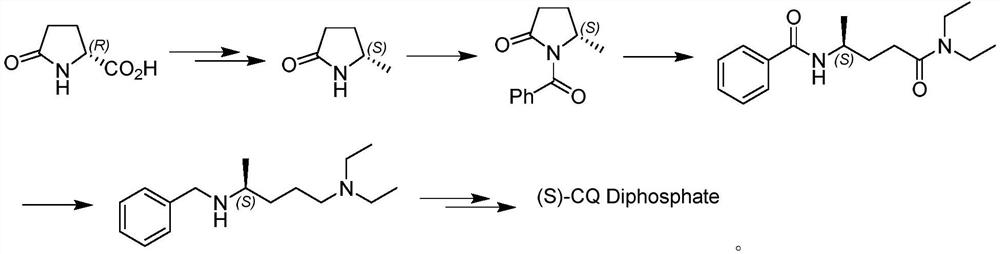
Asymmetric synthesis method of (S)-chloroquine phosphate
A chloroquine phosphate, asymmetric technology, applied in the field of drug synthesis, can solve the problems of poor nucleophilic attack ability, low yield, unsatisfactory chiral selectivity of asymmetric catalysis, etc., and achieves few reaction steps and chiral selectivity. and high yield, suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of (S, S)-5-(N'-diethylamino)-N-((1-phenyl)ethyl)-2-pentylamine
[0034] The reaction formula is as follows:
[0035]
[0036]Add methanol (150ml) into a 500mL hydrogenation reactor, start stirring, then add 5-diethylamino-2-pentanone (15.7g, 0.1mol), (S)-methylbenzylamine (12.7g, 0.105 mol) and isopropyl titanate (14.2g, 0.05mol). After stirring at room temperature for half an hour, wet Raney-Ni (25 g) was added, replaced with hydrogen three times, and hydrogen was introduced to 1.0 MPa, and the reaction was continued to stir at room temperature for 12 hours. After the reaction was over, the hydrogen in the kettle was released, the reaction kettle was opened, NaOH solution (1.0M, 100mL) was added, and stirring was continued at room temperature for 1 hour, and the reaction system became a suspension. After filtering, the filtrate evaporated about half of the solvent with a rotary evaporator, and then added dichloromethane for extraction three t...
Embodiment 2
[0037] Example 2: Synthesis of (S, S)-5-(N'-diethylamino)-N-((1-phenyl)ethyl)-2-pentylamine
[0038] The reaction formula is as follows:
[0039]
[0040] Add methanol (150ml) into a 500mL hydrogenation reactor, start stirring, then add 5-diethylamino-2-pentanone (15.7g, 0.1mol), (S)-methylbenzylamine (12.7g, 0.105 mol) and isopropyl titanate (28.4g, 0.1mol). After stirring at room temperature for half an hour, wet Raney-Ni (25 g) was added, replaced with hydrogen three times, and hydrogen was introduced to 1.0 MPa, and the reaction was continued to stir at room temperature for 12 hours. After the reaction was over, the hydrogen in the kettle was released, the reaction kettle was opened, NaOH solution (1.0M, 100mL) was added, and stirring was continued at room temperature for 1 hour, and the reaction system became a suspension. After filtering, the filtrate evaporated about half of the solvent with a rotary evaporator, and then added dichloromethane for extraction three t...
Embodiment 3
[0041] Example 3: Synthesis of (S, S)-5-(N'-diethylamino)-N-((1-phenyl)ethyl)-2-pentylamine
[0042] The reaction formula is as follows:
[0043]
[0044] Add methanol (150ml) into a 500mL hydrogenation reactor, start stirring, then add 5-diethylamino-2-pentanone (15.7g, 0.1mol), (S)-methylbenzylamine (12.7g, 0.105 mol) and isopropyl titanate (42.6g, 0.15mol). After stirring at room temperature for half an hour, wet Raney-Ni (25 g) was added, replaced with hydrogen three times, and hydrogen was introduced to 1.0 MPa, and the reaction was continued to stir at room temperature for 12 hours. After the reaction was over, the hydrogen in the kettle was released, the reaction kettle was opened, NaOH solution (1.0M, 100mL) was added, and stirring was continued at room temperature for 1 hour, and the reaction system became a suspension. After filtering, the filtrate evaporated about half of the solvent with a rotary evaporator, and then added dichloromethane for extraction three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com