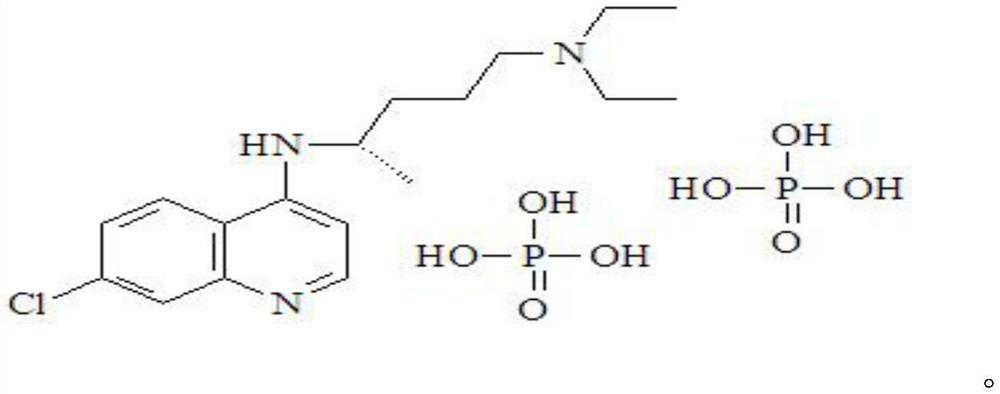
Application of dextro-chiral chloroquine phosphate in preparation of medicine for treating coronavirus
A kind of d-chloroquine phosphate, coronavirus technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 chiral chloroquine phosphate
[0016] (1) Preparation of S-(+)-chloroquine binaphthol phosphate
[0017] Take 8.3g of chloroquine racemate, add 9.7g of (S)-(+)-binaphthol phosphate, add 90g of isopropanol and 30g of acetone, heat to dissolve at 70°C, keep warm for 1h, stir and cool to room temperature, then cool down to 0~ Stand at 4°C for 18 hours, crystallize out, filter, drain, and save the filtrate as R-rich mother liquor. The filter cake is a light yellow solid. Dry at 60°C to obtain 11.8g. This solid is S-(+)-chloroquine binaphthol Phosphate salt.
[0018] (2) Recrystallization
[0019] Add 75g of isopropanol and 25g of acetone to 11.8g (17.7mmol) of the solid obtained in step (1), heat to dissolve at 70°C, stir slowly to room temperature, cool down to 0-4°C, and let stand for 6h to precipitate large crystals. Filtrate, drain to obtain a glossy loose solid, and dry at 60°C to obtain 8.6g.
[0020] (3) Preparation of S-(+)-chloro...
Embodiment 2
[0030] The preparation of embodiment 2 chiral chloroquine phosphate
[0031] (1) Preparation of R-(-)-chloroquine binaphthol phosphate
[0032] Take 4.6g of chloroquine racemate (CASNo.54-05-7), add 5.4g of (R)-(-)-binaphthol phosphate, add 50g of isopropanol and 16g of acetone, heat to dissolve at 60°C, keep warm for 1h, Stir and cool to room temperature, lower the temperature to 0-4°C, let stand for 12 hours, precipitate crystals, filter, and drain, the filtrate is stored as S-rich mother liquor, the filter cake is a light yellow solid, and 6.2g of solid is obtained by drying at 60°C. R-(-)-chloroquine binaphthol phosphate.
[0033] (2) Recrystallization
[0034] Add 6.2 g of the solid obtained in step (1) to 30 g of isopropanol and 9 g of acetone, heat to dissolve at 60 ° C, slowly stir to room temperature, cool to 0-4 ° C, let stand for 6 h, precipitate large crystals, filter, and drain It was a glossy and loose solid, and it was dried at 60°C to obtain 4.7 g of solid. ...
Embodiment 3
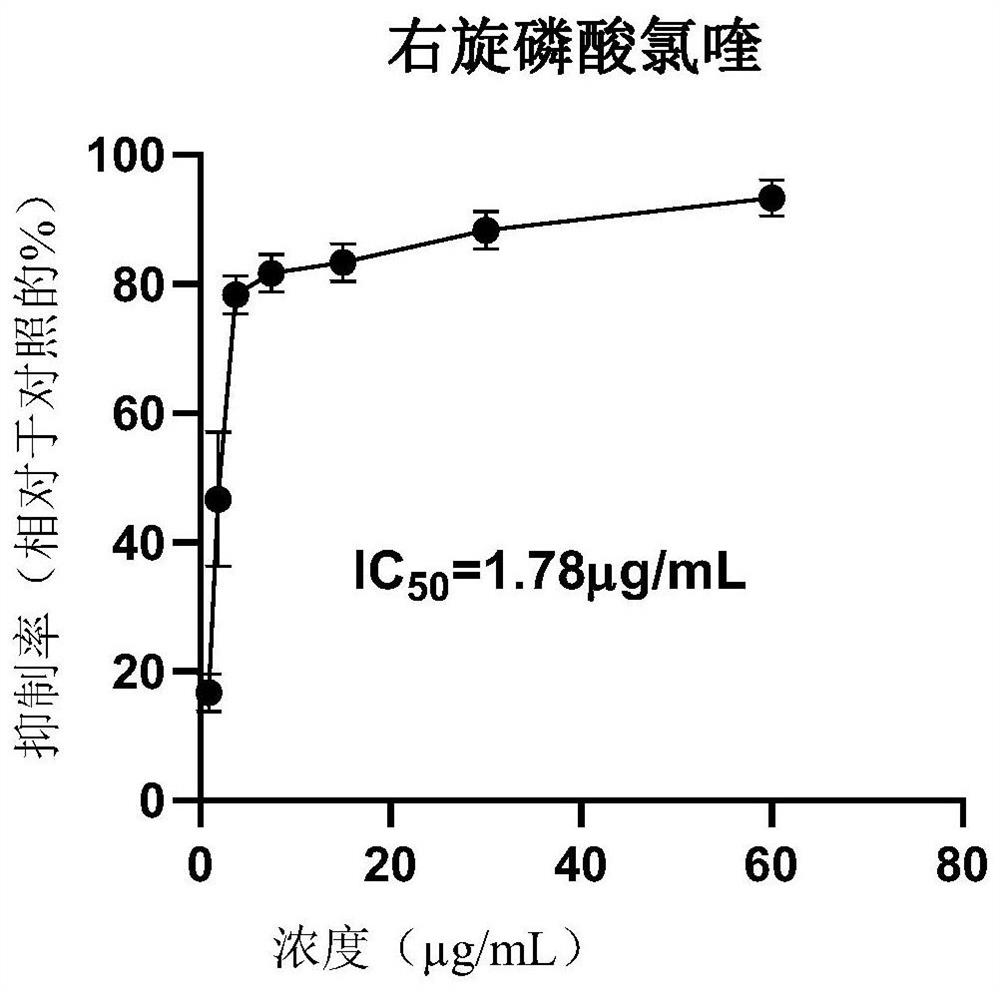
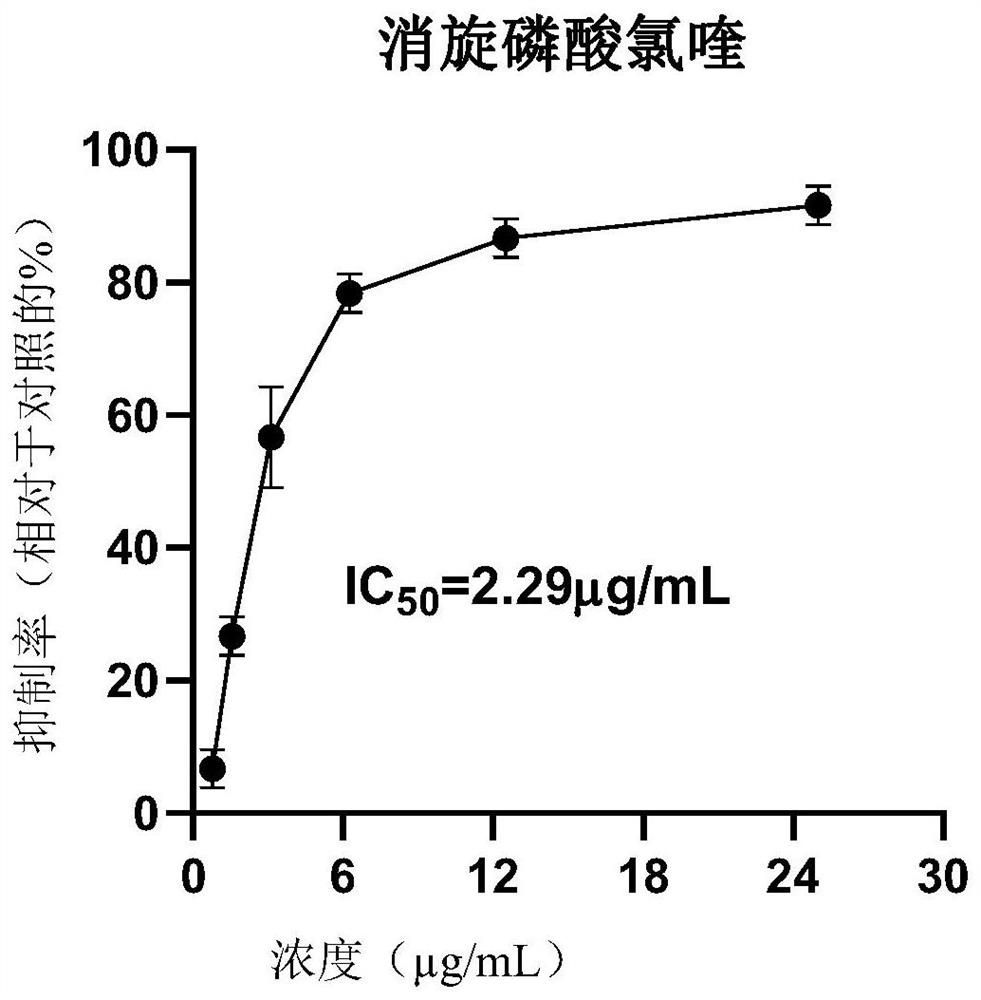
[0045] Embodiment 3 cell level evaluation D-, racemic chloroquine phosphate anti-new coronavirus (SARS-COV-2) effect
[0046] The experiment to evaluate the effect of drugs against the new coronavirus (SARS-COV-2) at the cellular level was conducted by the Guangzhou Institute of Respiratory Health. Among them, the cells used are VeroE6 cells, which are preserved by the virus room of the State Key Laboratory of Respiratory Diseases, Guangzhou Institute of Respiratory Health; the virus SARS-CoV-2, the titer is TCID 50 =10 -6 / 100μL, stored at -80°C by BSL-3 Laboratory of Guangzhou Customs Technology Center (Highly Pathogenic Pathogenic Microorganism Research Laboratory of State Key Laboratory of Respiratory Diseases). Use a viral titer of 100TCID 50 .
[0047] Right-handed chiral chloroquine chlorate is prepared by embodiment 1 step (1)
[0048] 2.1 Test drug antiviral experimental operation
[0049] (1) Test drug:
[0050] Table 1 Drug name, experimental concentration and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com