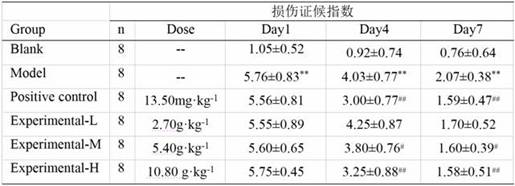
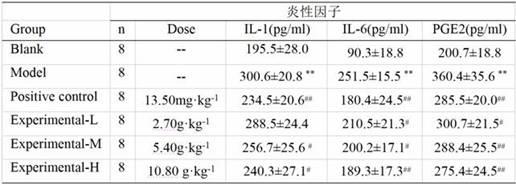
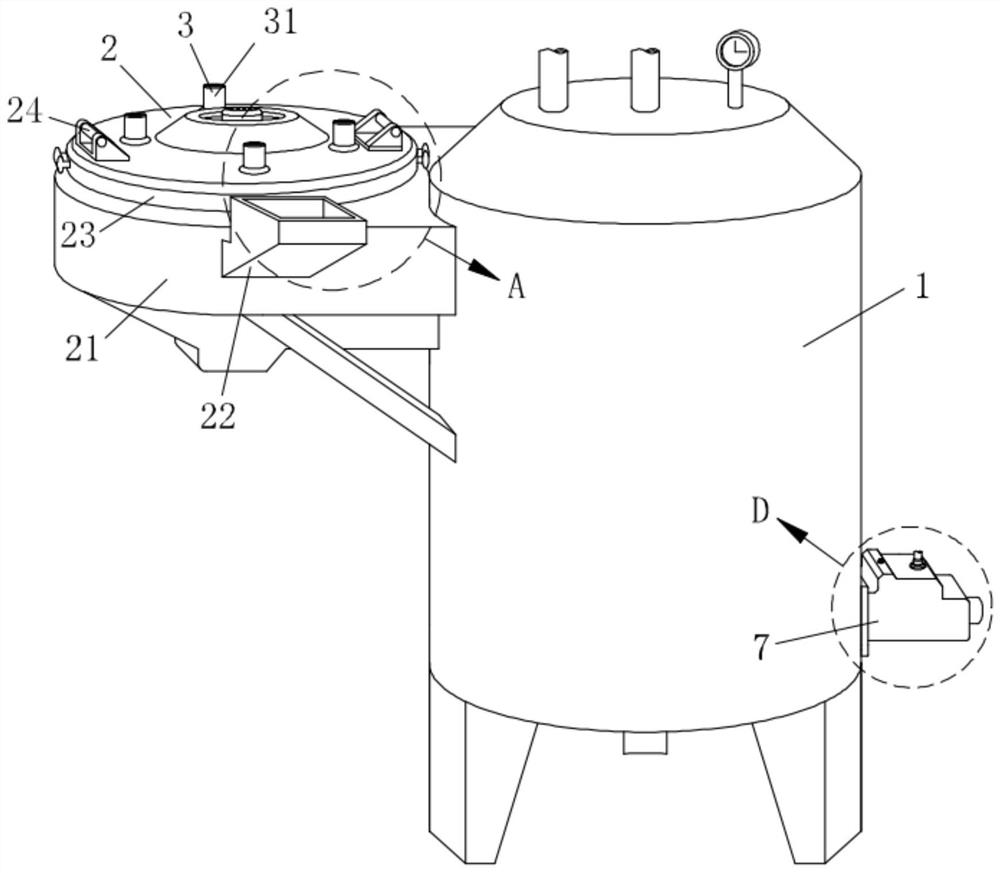
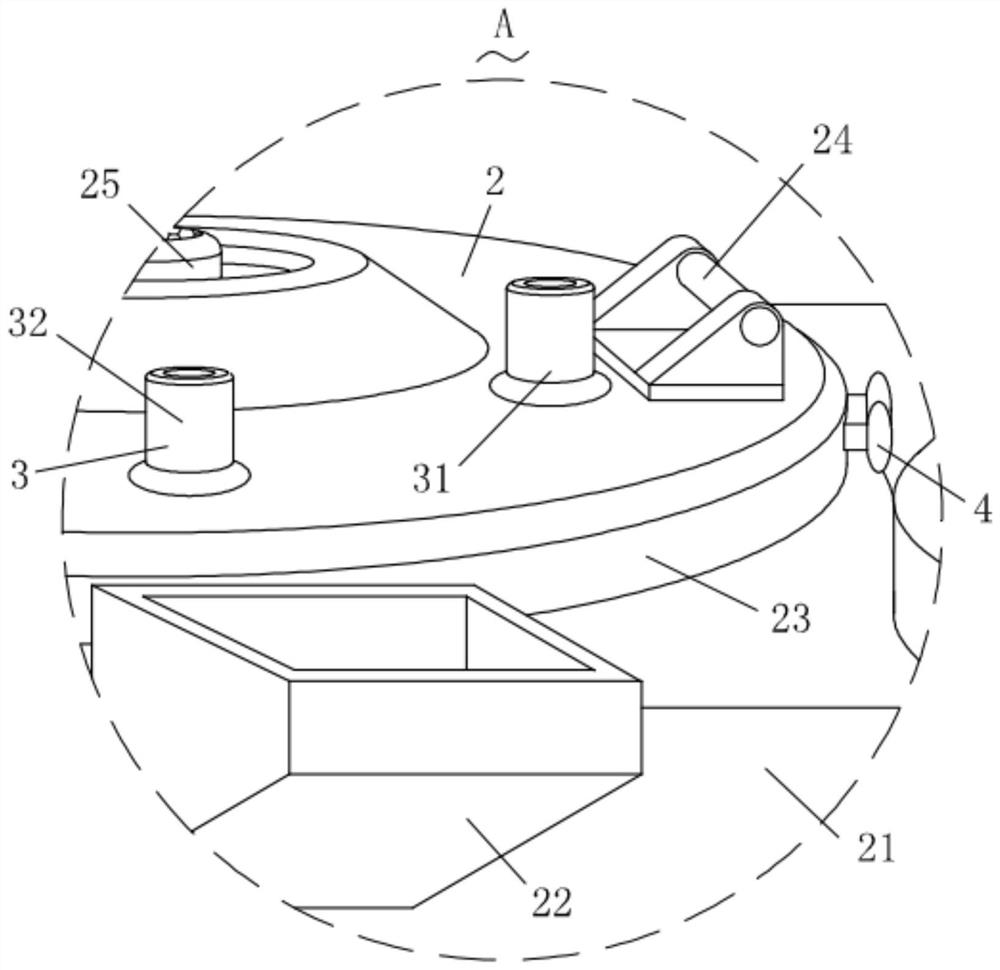
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Sodium Diclofenac" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diclofenac sodium (Voltaren®, Voltaren®-XR) belongs to a class of drugs called nonsteroidal anti-inflammatory drugs (NSAIDs). It is used to reduce pain, inflammation, swelling, and stiffness caused by several types of arthritis.
Preparation method and application of typical acidic drug multi-template molecularly imprinted polymer
InactiveCN102702428AUniquely identifiableIncreased sensitivityOther chemical processesAlkali metal oxides/hydroxidesPharmaceutical drugClofibric acid
The invention relates to a preparation method and application of a typical acidic drug multi-template molecularly imprinted polymer. The adopted template molecules are clofibric acid, diclofenac sodium, ibuprofen, naproxen and ketoprofen. The preparation method and the application of the molecularly imprinted polymer belong to the technical field of analysis, determination and removal of five typical acidic drugs in environmental samples. The preparation method is simple and reliable, and the prepared multi-template molecularly imprinted polymer has a uniform size, a large specific surface area and higher specific selectivity for acidic drugs. The five acidic drugs in the environmental samples can be separated and gathered rapidly and efficiently. The multi-template molecularly imprinted polymer can be directly applied in selective removal of the five acidic drugs in surface water and has better effects than other adsorption materials. The method is an effective method for specific separation of a series of substances with the same attributes and has broad application prospects.
Owner:TONGJI UNIV
Diclofenac sodium hydrogel microballoon with pH sensitivity, preparation method and application thereof
InactiveCN102018674ANo adverse reactionAvoid morning stiffnessOrganic active ingredientsAntipyreticStomach mucous membraneRheumatism
The invention relates to a diclofenac sodium hydrogel microballoon with pH sensitivity, a preparation method and application thereof. In the invention, chitosan and sodium alginate are used as drug carriers to prepare a diclofenac sodium hydrogel microballoon slow release preparation; the mass ratio of a used diclofenac sodium drug to the chitosan to the sodium alginate is (1-3):(5-8):(5-8); and the chitosan is N-succinyl-chitosan. The microballoon preparation can durably develop the drug effect to ensure that diclofenac sodium can be regularly and quantitatively released for a long time; after the microballoon preparation is taken, the blood concentration is stable, the fluctuation is small, and the duration time is long; the microballoon preparation has pH sensitivity, thereby irritations and toxic hazards to the stomach mucous membrane, which are caused by directly and orally taking diclofenac sodium tablets, are reduced; and the microballoon preparation has good effects of cooling down and easing pains and is suitable for various rheumatisms, rheumatoid arthritis, lupus erythematosus, ankylosing spondylitis, various pains caused after operations, fevers caused by various reasons, and the like.
Owner:TONGJI UNIV
Sodium dichlorophenolate micro-pill pharmaceutical preparation and preparation method thereof
ActiveCN101804030ASustained releaseRelease stabilityOrganic active ingredientsAntipyreticMedicineCovering system
The invention relates to the pharmaceutical preparation field and in particular to a sodium dichlorophenolate micro-pill pharmaceutical preparation and a preparation method thereof. The micro-pill pharmaceutical preparation is prepared by micro-pills, which is characterized in that the micro-pill is of enteric slow-release micro-pill, the micro-pill consists of a hollow pill core, an active layer and an outer layer lagging cover, the active layer comprises sodium dichlorophenolate, a binding-property slow-release material and a binding-property enteric material, and the outer layer comprises a slow-release material. With the particular process and formula, a release system thereof is made into a unique double slow-release system, i.e. a skeleton-type scattering system is combined with a multi-layer semi-penetration film lagging cover system, so the main medicine can be continuously and stably released in the complicated internal environment of the human body. In addition, by adopting unique auxiliary materials, the micro-pill is free from being dissolved in the stomach and almost has no harm on the gastric mucosa, the medicine is ensured to have continuous effect of 24 hours inside the intestinal tract, the bioavailability is improved, and the application effect of the medicine is ensured.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
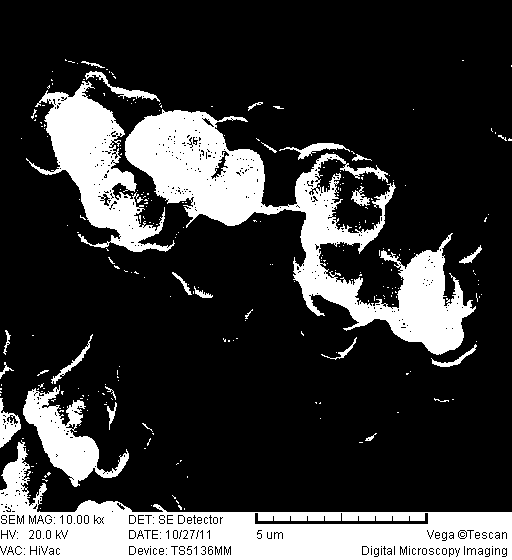
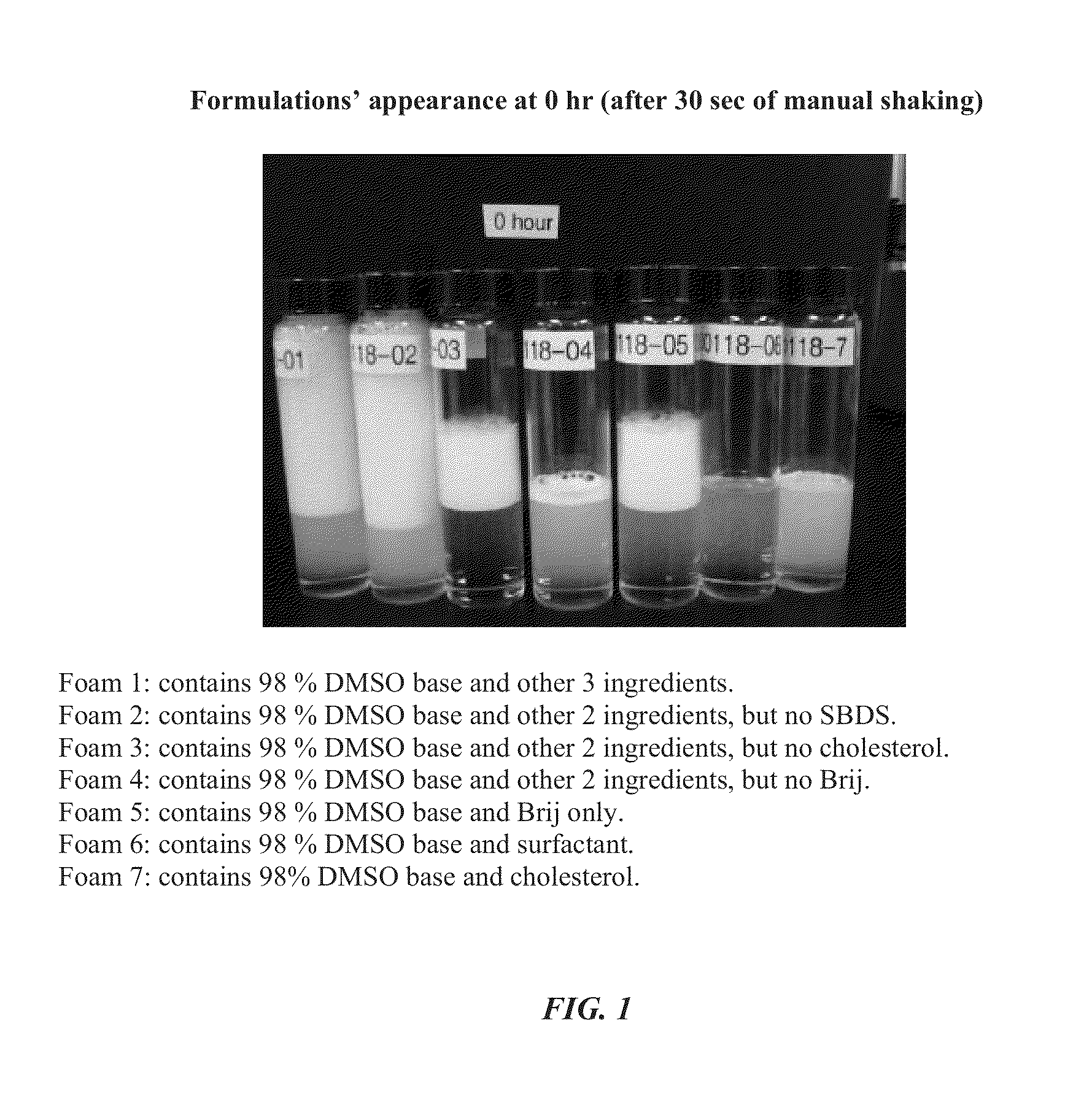
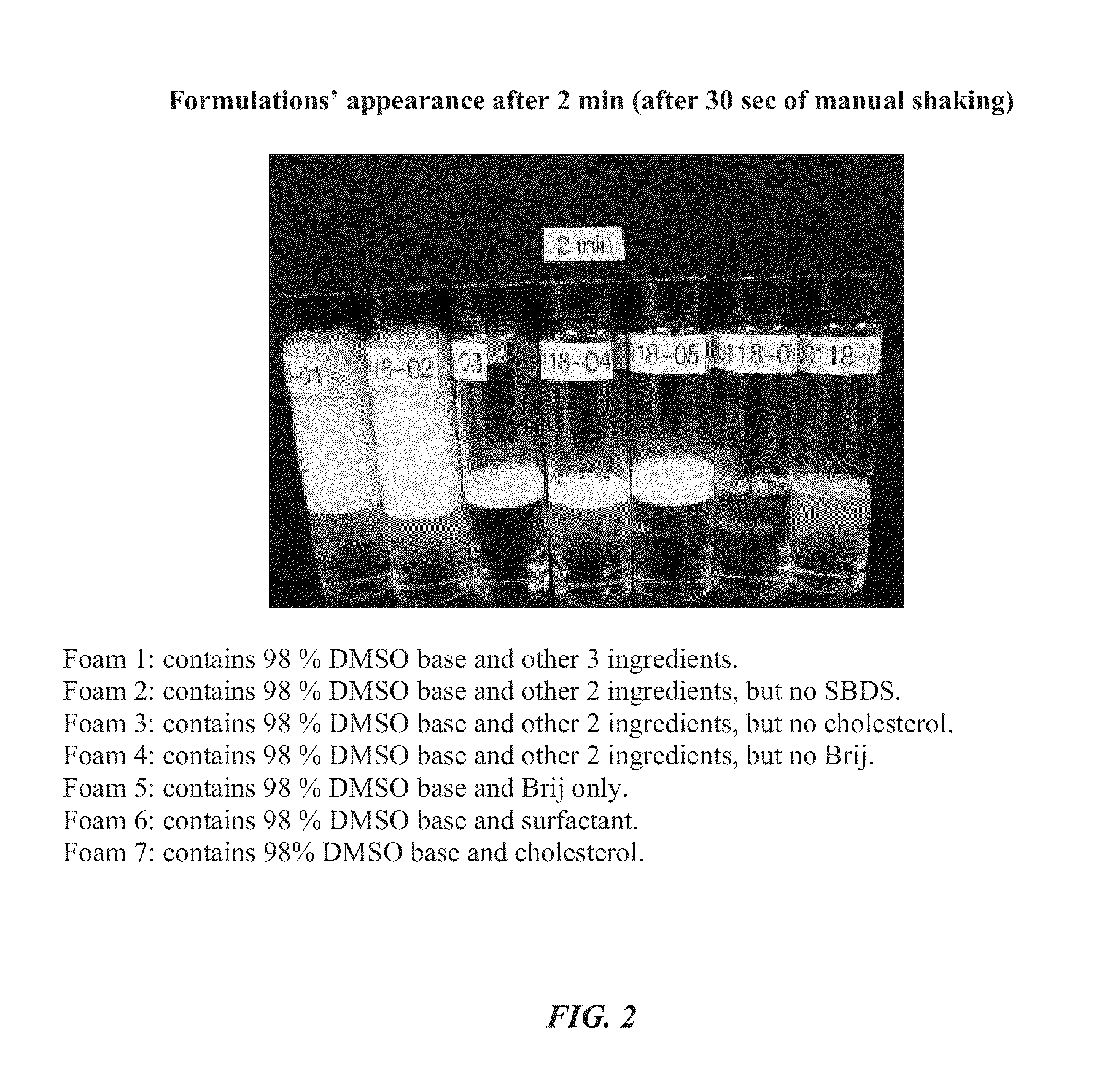
Foamable formulation
ActiveUS20130243701A1Relieve painAerosol deliverySulfur/selenium/tellurium active ingredientsAlcoholActive agent
The present invention provides DMSO-containing foamable formulations, methods for preparation, and methods of treatment. The formulations can provide good permeability and bioavailability at the target site. Preferably, the formulations are useful for treating osteoarthritis. In one embodiment, the invention provides a foamable formulation for topical use, said formulation comprising DMSO, polyalkylene glycol alkyl ether, an active agent, a monohydric lower alcohol, a diol, and water. Preferably, the active agent is a non-steroidal anti-inflammatory drug, such as diclofenac sodium or ibuprofen.
Owner:TRIBUTE PHARM CANADA INC
Diclofenac natrium slowly released capsule and its preparation method
InactiveCN1415290AStable concentrationBlood concentration fluctuates lessOrganic active ingredientsAntipyreticMedicinePlasticizer
A slowly-releasing diclofenac sodium capsule is prepared from diclofenac sodium (4.5-6.0 wt.%), excipient (60-90 wt.%), coating (4-10 wt.%), wtting agent (0-10 wt.%), surfactant (0-1 wt.%), plasticizer (0-2 wt.%) and opacifying agent (0-2 wt.%). Its advantages are high curative effect, low by-effect and high durability (12 hrs).
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Delayed-releasing compound diclofenac sodium prepn
InactiveCN1452983APain control effectEasy to useOrganic active ingredientsAntipyreticChemistryB1 Vitamin
The present invention relates to delayed-releasing compound diclofenac sodium preparation and its preparation process, and is especially the delayed-releasing compound preparation of diclofenac sodium, vitamin B1, vitamin B6 and vitamin B12 and its application in medical treatment.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Veterinary compound hydrochloric acid injection and preparation method thereof
InactiveCN102151263AGood effectEasy to prepareAntibacterial agentsOrganic active ingredientsProtozoaDisease
The invention relates to veterinary compound hydrochloric acid injection and a preparation method thereof. The veterinary compound hydrochloric acid injection consists of oxytetracycline dihydrate injection, imidocarb and diclofenac sodium; the veterinary compound hydrochloric acid injection per 100 ml comprises the required raw materials of: 10-25g of oxytetracycline dihydrate injection, 1.5-5g of diclofenac sodium, 0.1-0.2g of imidocarb, 0.2-0.6g of sodium formaldehyde sulphoxylate, 5-15g of magnesium chloride, 5-13 ml of ethanolamine and 60-81 ml of organic solvents, wherein the allowance is water for injection. The veterinary compound hydrochloric acid injection has remarkable effect when being used for treating acute respiratory infection caused by eperythrozoon suis, babesiosis and other blood protozoa diseases, porcine respiratory disease complex (PRDC) and swine influenza virus (SIV), airway inflammation induced by porcine reproductive and respiratory syndrome (PRRS), haemophilus parasuis, pasteurella, pleuropneumonia and mycoplasma diseases; and the preparation method is simple and easy to operate, and is suitable for batch production.
Owner:XUCHANG TIANYUAN BIOLOGICAL TECH CO LTD
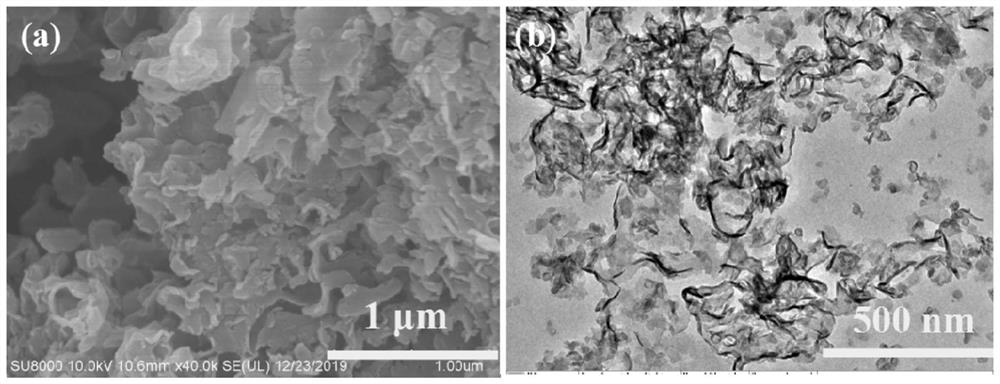
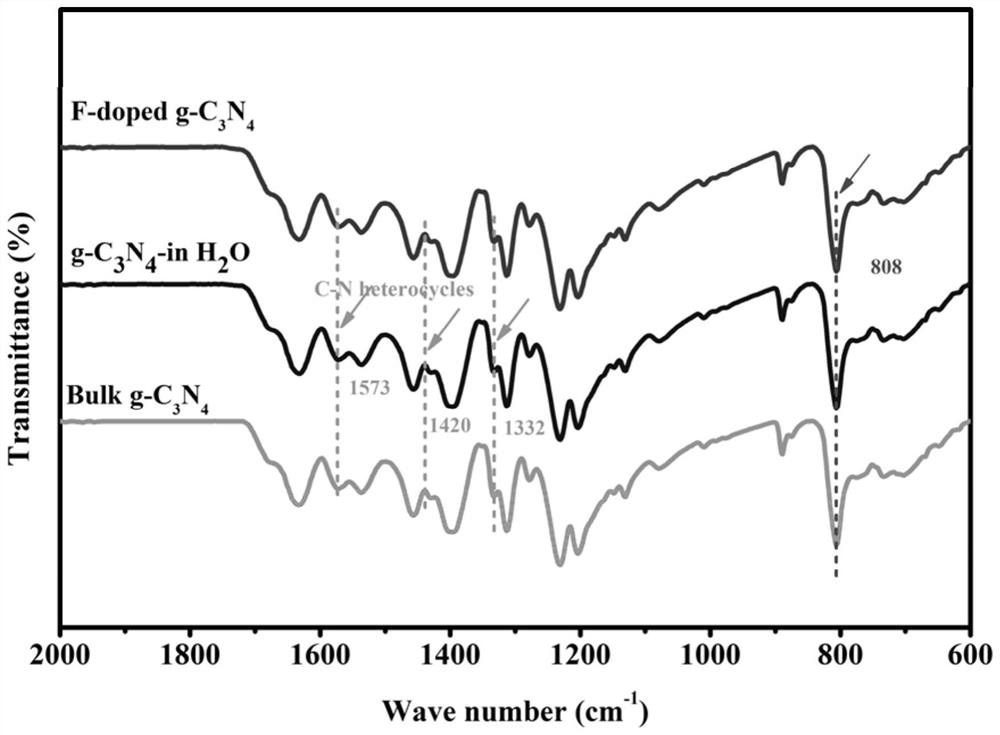
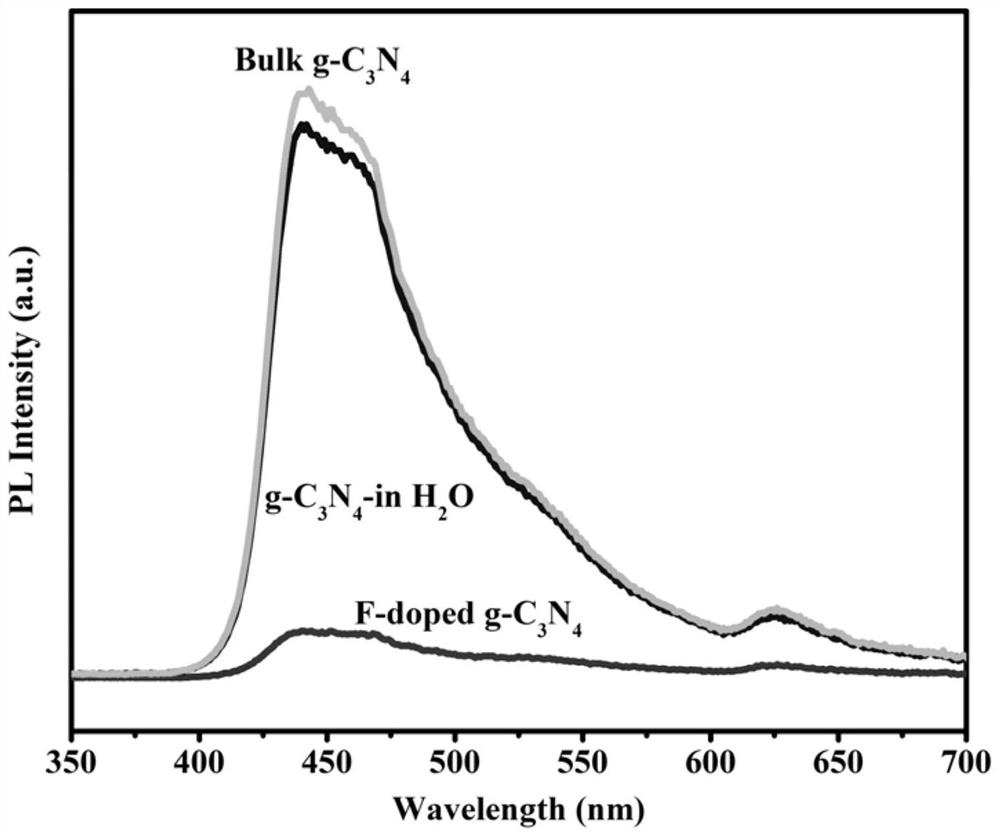
Novel F-doped g-carbon nitride photocatalytic material prepared by microwave method and application of material
ActiveCN111604076AImprove photocatalytic performanceHelp promote cross-convergenceWater/sewage treatment by irradiationWater treatment compoundsMicrowave methodPhotocatalytic degradation
The invention provides a novel F-doped g-C3N4 photocatalytic material prepared by a microwave method and the application of the novel F-doped g-C3N4 photocatalytic material. The method comprises the following steps of: preparing a body g-C3N4 material by adopting a thermal polymerization method; and carrying out microwave etching on the body g-C3N4 in a medium by using a microwave digestion instrument to form nitrogen vacancies on the surface of the body g-C3N4 material. According to the F-doped g-C3N4 material disclosed by the invention, F atoms are introduced into nitrogen vacancies and formC-F bonds with C atoms, so that electrons are distributed unevenly to form a surface polarization field, and the hole electron recombination rate of the surface of the material is reduced, and the activity of the photocatalyst is enhanced. The degradation rates of the material to diclofenac sodium, phenol and bisphenol A are 100%, 55% and 65% respectively, and are superior to those of the body g-C3N4. The material disclosed by the invention is simple in process and suitable for industrial mass production; and the photocatalytic degradation technology is applied to the field of PPCPs degradation, so that the material has very high application prospect and practical value.
Owner:北京水木宇杰环境科技有限公司
High-stability nanoscale zirconium-based metal organic framework material and preparation method and application thereof
ActiveCN107446136AGood chemical stabilityImprove thermal stabilityOrganic active ingredientsAntipyreticDiclofenac SodiumMetal-organic framework
The invention discloses a high-stability nanoscale zirconium-based metal organic framework material (Zr-MOFs) and a preparation method of the high-stability nanoscale zirconium-based metal organic framework material. Firstly, a specific organic ligand 1,4-naphthalenedi acrylic acid is obtained through a Suzuki coupling reaction; and secondly, a pure phase nanocrystal material is obtained through a solvothermal method. An anti-inflammatory drug, that is, diclofenac sodium (DS), is loaded in a hole of the activated nanocrystal material, and a drug loading system achieves the effect of controlling the release of the drug through heat. The pore diameter of the synthesized nanoscale zirconium-based metal organic framework material is well matched with the size of the diclofenac sodium (DS), so that a higher loading capacity is achieved. The material is higher in chemical stability and heat stability, and the requirements of the practical application field on local thermal therapy of cancers and inflammation tissues are greatly met. Therefore, the nanoscale metal organic framework material has a potential application prospect on the local thermal therapy and chemotherapy of the inflammation and cancer tissues.
Owner:ZHEJIANG UNIV
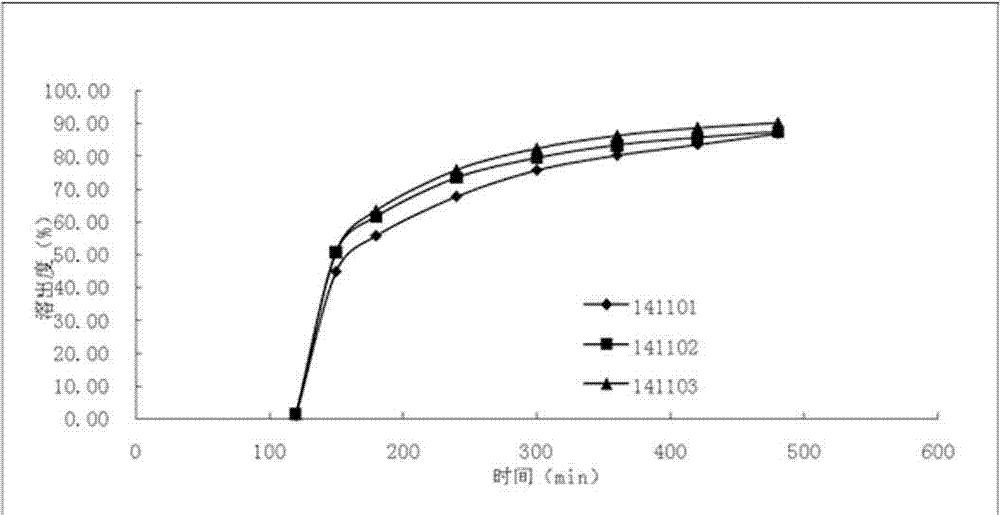
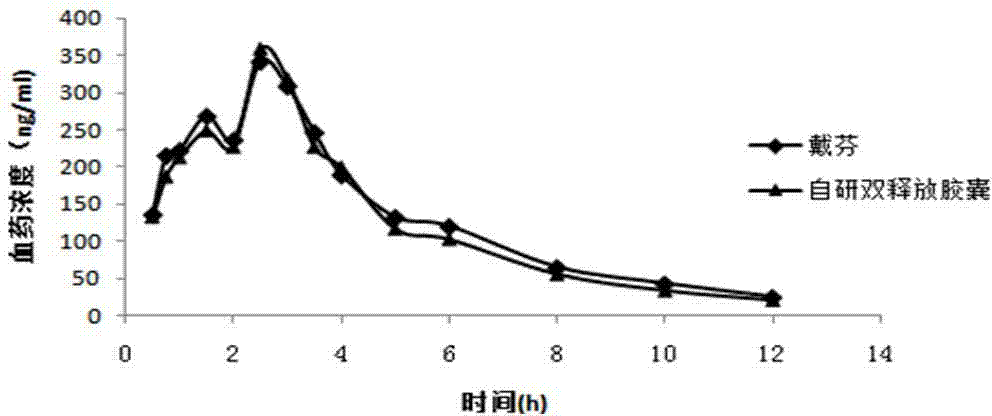
Method for evaluating in-vivo and in-vitro correlation of diclofenac sodium sustained release tablets
The invention discloses a method for evaluating in vivo and in vitro correlation (In Vivoin Vitro Correlation) of a diclofenac sodium sustained release tablet, and a novel diclofenac sodium sustained release tablet. According to the method for evaluating the in-vivo and in-vitro correlation of the diclofenac sodium sustained release tablet, the in-vivo pharmacokinetic characteristics can be well evaluated. The formula of the diclofenac sodium sustained release tablet comprises the following components: 100 mg / tablet of diclofenac sodium, 119 mg / tablet of cane sugar, 59 mg / tablet of hexadecanol, 4 mg / tablet of povidone K30, 3 mg / tablet of colloidal silicon dioxide, 3 mg / tablet of magnesium stearate and 10 mg / tablet of film coating premix. According to the diclofenac sodium sustained-release tablet disclosed by the invention, the release speed of the medicine is controlled, the irritation of the traditional diclofenac sodium sustained-release tablet to the stomach is reduced, and meanwhile, the administration mode of keeping a relatively high effective blood concentration for a long time is achieved. The medicine effect is ensured, the side effect is reduced, and the clinical use is greatly facilitated.
Owner:HQ PHARMA (SHANGHAI) CO LTD
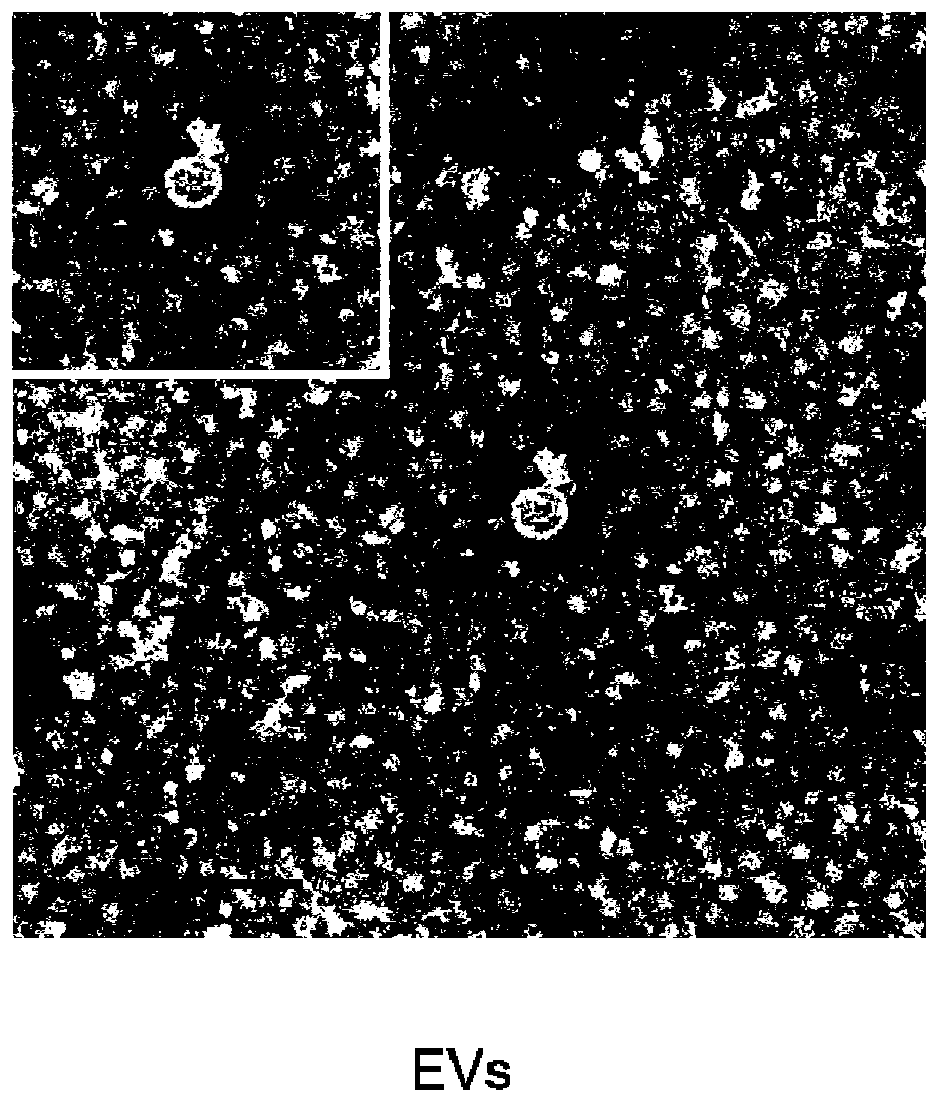
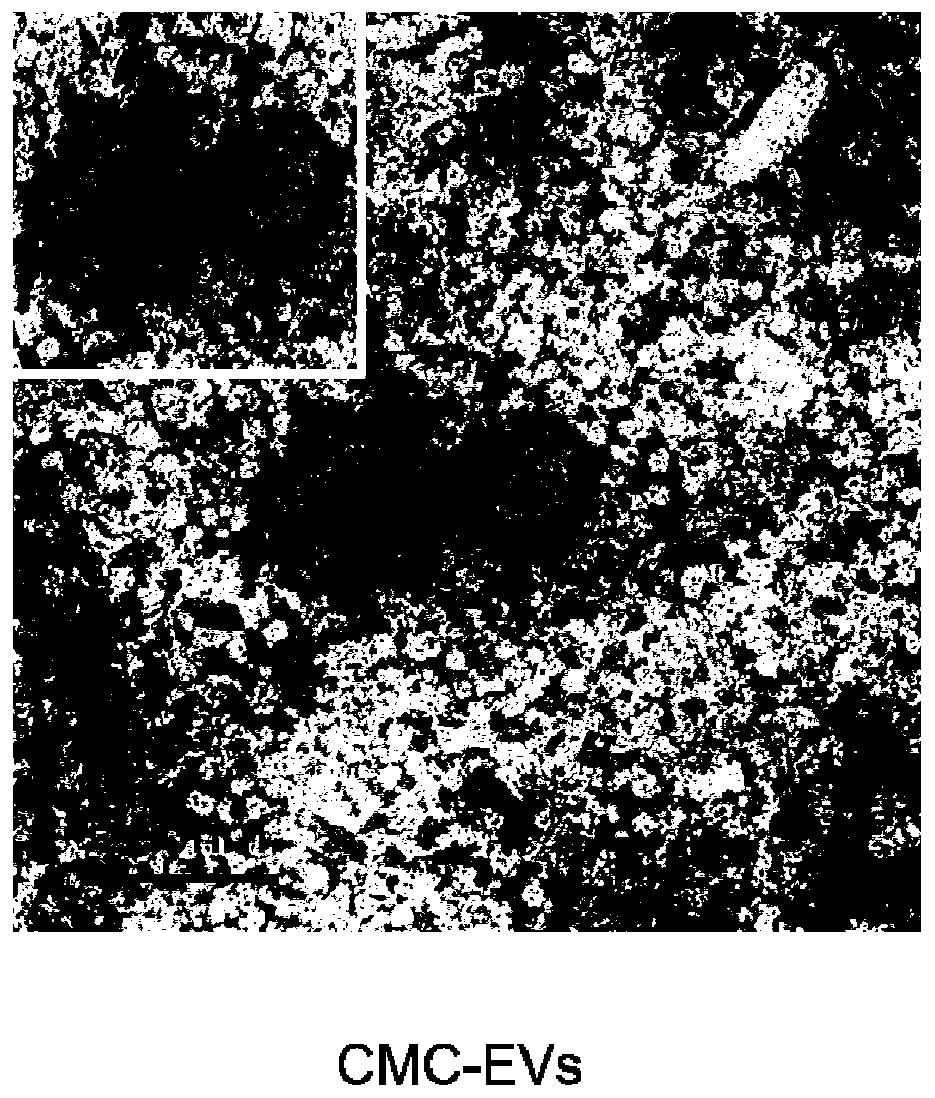
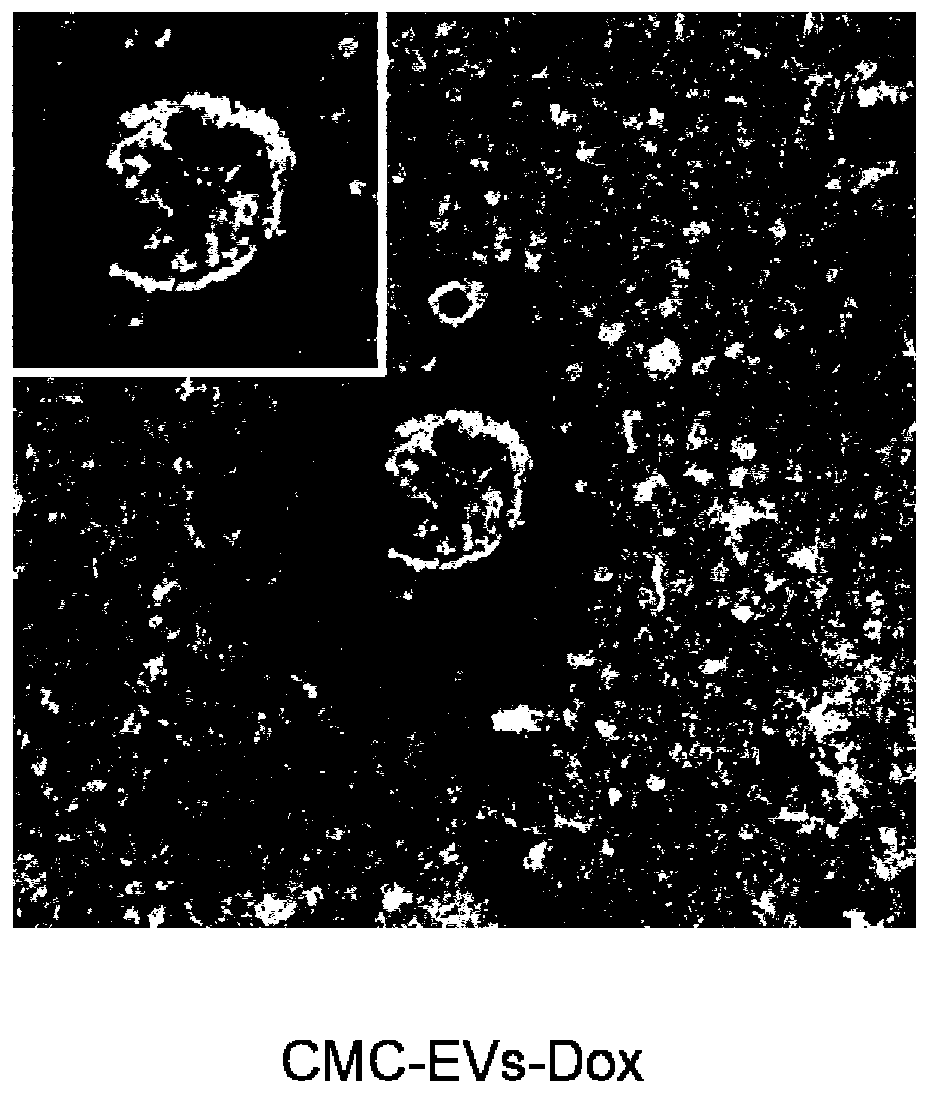
Carboxymethyl chitosan modified drug-carrying vesicle, and preparation method and application thereof
PendingCN110974976AChange surface propertiesMany vesiclesOrganic active ingredientsPeptide/protein ingredientsCell vesiclePancreatic hormone
The invention relates to a carboxymethyl chitosan modified drug-carrying vesicle. The drug-carrying vesicle is characterized in that a lipid bilayer membrane of an extracellular vesicle is coated or modified by carboxymethyl chitosan. The molecular weight of the carboxymethyl chitosan is from 165 to 119000, and the deacetylation degree is more than or equal to 50%. The drug-carrying vesicle comprises one or more medicines selected from diclofenac sodium, insulin, paclitaxel, cisplatin, doxorubicin, sipeimine and the like. According to the invention, the carboxymethyl chitosan is connected to the surface of the extracellular vesicle, and modification of the carboxymethyl chitosan changes the surface property of the extracellular vesicle and has profound influence on the drug delivery mechanism of the extracellular vesicle. Compared with a common extracellular vesicle, the drug-carrying vesicle is targeted to tumor cells. A preparation prepared from the carboxymethyl chitosan modified drug-carrying vesicle has more cell vesicles under a condition of ingestion in tumors.
Owner:TIANJIN CITY THIRD CENT HOSPITAL
Tablets containing diclofenac sodium and having analgesic effect on pets, and preparation method thereof
The invention belongs to the technical field of veterinary analgesic drugs and preparation methods thereof, particularly to tablets containing diclofenac sodium and having the analgesic effect on pets, and a preparation method thereof. The formulation comprises (a) a diclofenac sodium bulk drug, (b) a binder, (c) a wetting agent, (d) a disintegrating agent, (e) a filler, and (f) a sweetening agent. According to the present invention, the tablets containing diclofenac sodium are prepared by adopting the wet method, are the white tablets, have characteristics of good stability and good palatability, and can be used as the veterinary oral formulation.
Owner:QINGDAO KDN BIOTECH
Medicine composition for treating cervical spondylosis, and preparation method and application of medicine composition
ActiveCN111888396AOvercoming the disadvantage of slow onset of actionAvoid side effectsOrganic active ingredientsSkeletal disorderCervical spondylopathyBULK ACTIVE INGREDIENT
The invention provides a medicine composition for treating cervical spondylosis, and a preparation method and application of the medicine composition, and belongs to the technical field of medicine. Active ingredients of the medicine composition comprise the following two parts: (1) a traditional Chinese medicine active ingredient prepared from following traditional Chinese medicinal materials inparts by weight: 1 to 15 parts of radix notoginseng, 3 to 20 parts of rhizoma chuanxiong, 2 to 15 parts of rhizoma corydalis, 3 to 20 parts of radix paeoniae alba, 4 to 25 parts of radix clematidis, 3to 20 parts of radix puerariae and 4 to 25 parts of notopterygium roots; and (2) a western medicine active ingredient prepared from levetiracetam and diclofenac sodium according to a weight ratio of1:(1 to 4). The medicine composition for treating cervical spondylosis provided by the invention is medicine integrating traditional Chinese medicine and western medicine developed on the basis of Chinese patent CN1228071C, has the unique curative effect on treatment of cervical spondylosis, and has the advantages that the preparation process is simple; the curative effect is obvious; the effect taking time is short; the taking is convenient; no toxic and side effects exist; and safety and effectiveness are realized.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Novel diclofenac injection and preparation method thereof
ActiveCN112516081AImprove securityGood biocompatibilityOrganic active ingredientsAntipyreticPolythylene glycolPolymer
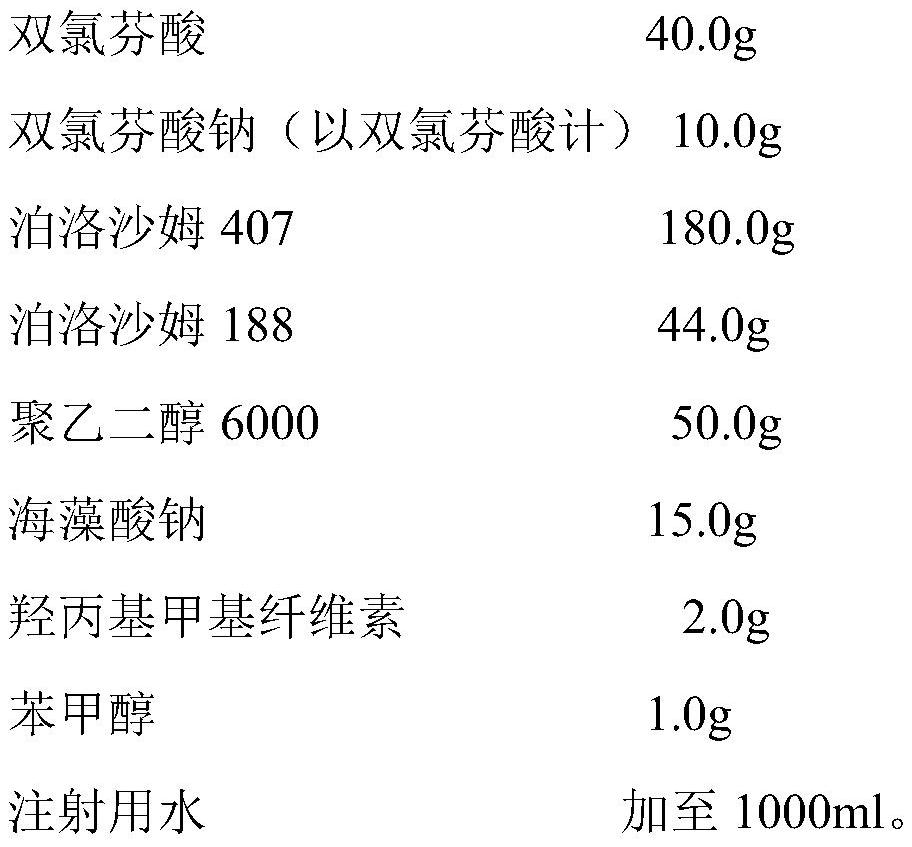
The invention belongs to the technical field of pharmaceutical preparations for animals, and discloses a novel diclofenac injection and a preparation method thereof. The diclofenac injection comprisesdiclofenac existing in a dispersed form, diclofenac sodium dissolved in a system, a temperature-sensitive in-situ gel matrix, polyethylene glycol, sodium alginate, a polymer retardant and water for injection, wherein the temperature-sensitive in-situ gel matrix consists of poloxamer 407 and poloxamer 188. The diclofenac injection specifically comprises the following components in percentage by mass of 2-18 percent of the diclofenac, 0-8 percent of the diclofenac sodium, 10-25 percent of the poloxamer 407, 0.1-16 percent of the poloxamer 188, 0.1-7 percent of polyethylene glycol, 0.02-5 percent of sodium alginate, 0.01-5 percent of the polymer retardant, 0.001-2 percent of a bacteriostatic agent and the balance of the water for injection. According to the diclofenac injection disclosed bythe invention, the gelling temperature is 31-35 DEG C, the gelling time is within 15 seconds, the diclofenac injection exists in a liquid state at room temperature, and a gel storage can be quickly formed at an injection part during intramuscular injection or subcutaneous injection administration.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
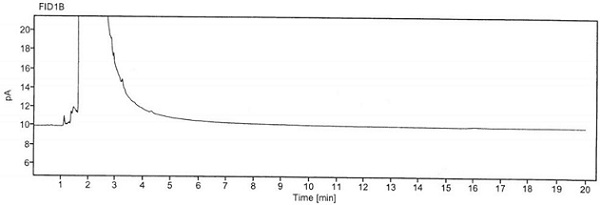
An analysis method for measuring related substances in diclofenac sodium raw materials and preparations thereof
PendingCN110687229AAccurate and effective determinationHigh recovery rateComponent separationSilanesEthylic acid
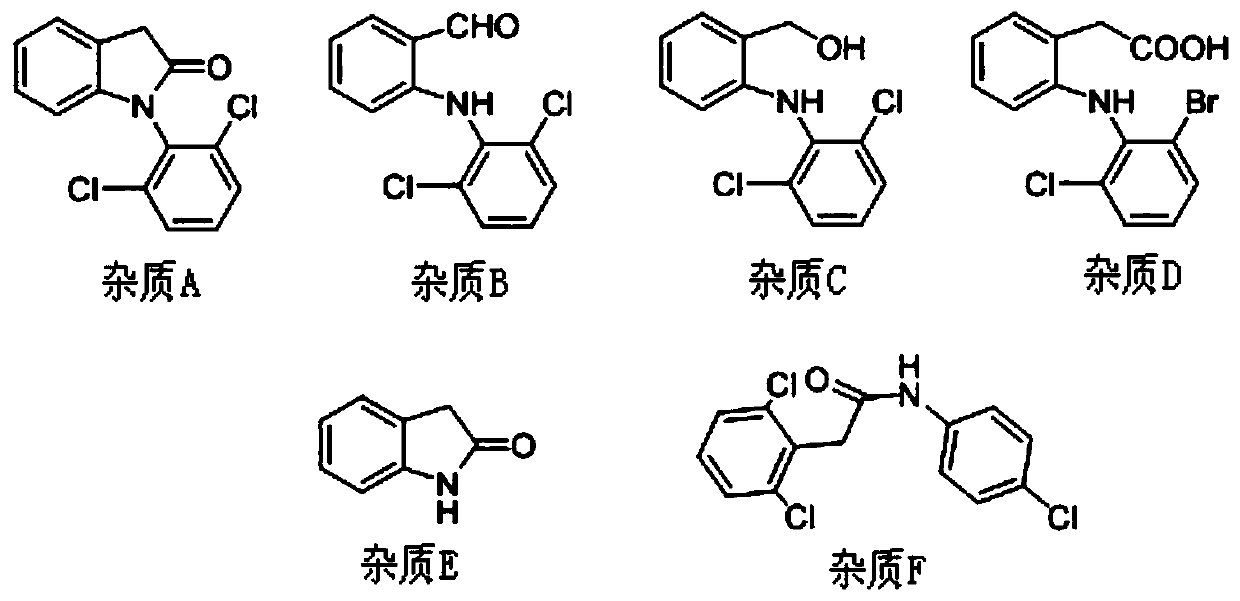
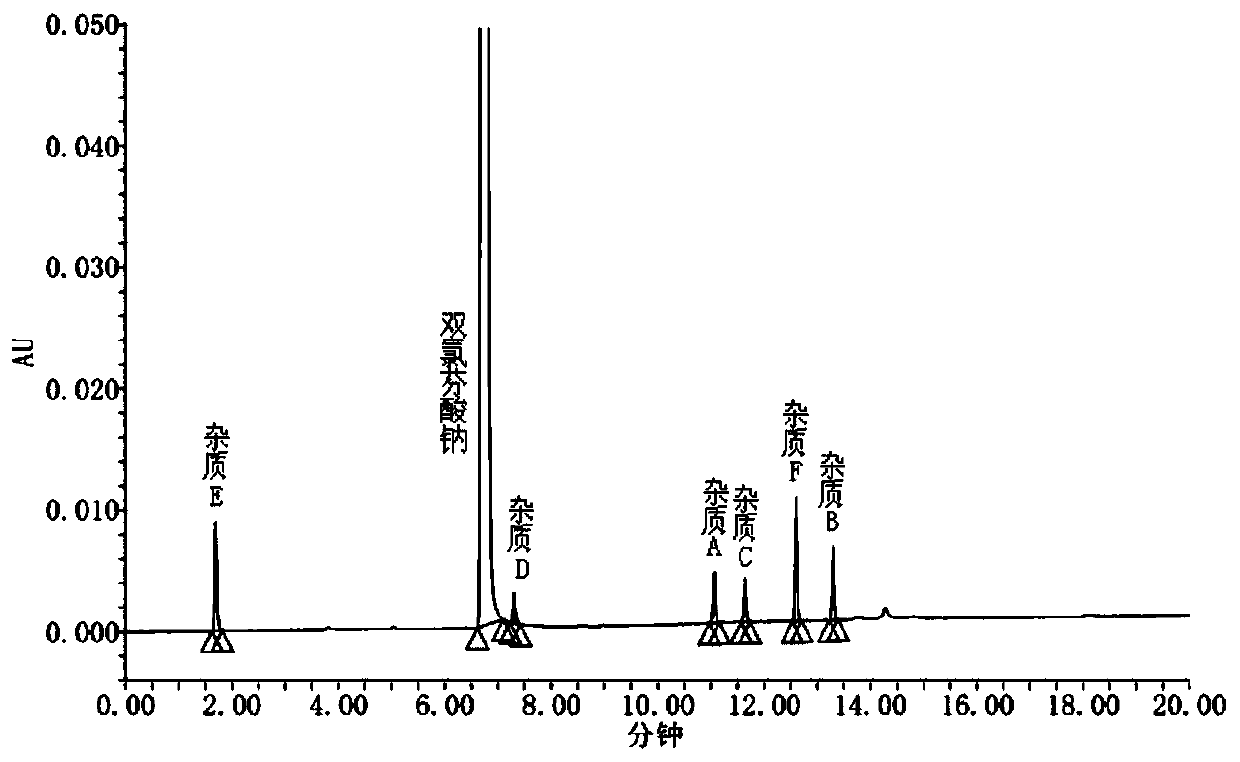
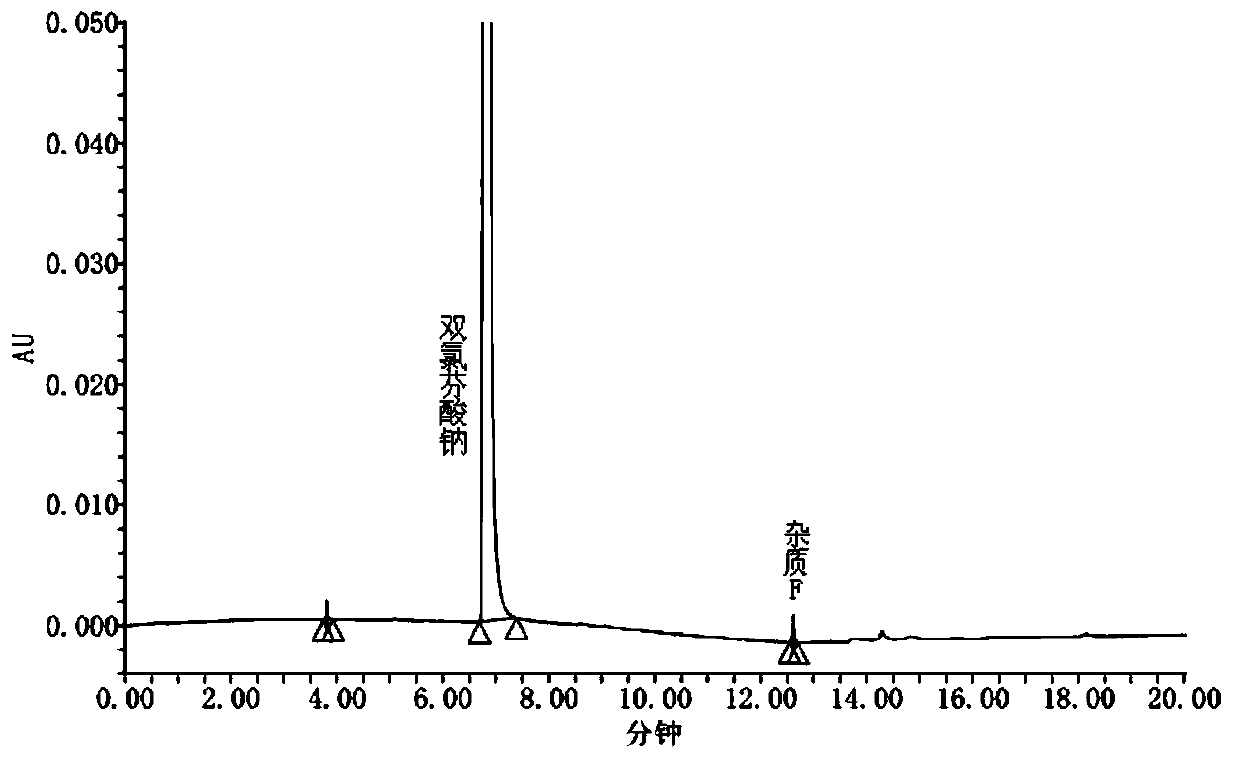
The invention discloses an analysis method for measuring related substances in diclofenac sodium raw materials and preparations thereof. The method adopts ultra-high-performance liquid chromatography;and the chromatographic conditions are as follows: a chromatographic column is an octadecylsilane chemically bonded silica gel chromatographic column; and gradient elution is carried out with acetonitrile being as a mobile phase A, an ammonium acetate solution being as a mobile phase B and the detection wavelength being 254nm. The method can accurately and efficiently measure the content of an impurity D in the diclofenac sodium raw materials and the preparations thereof, and meanwhile, can measure the existence and / or content of impurities A, B, C, E and F.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
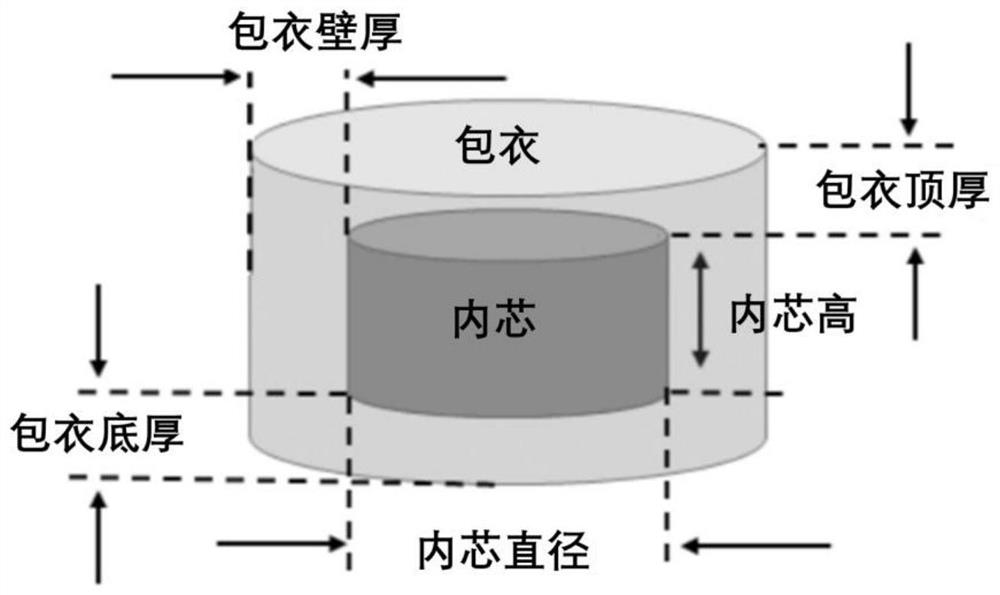
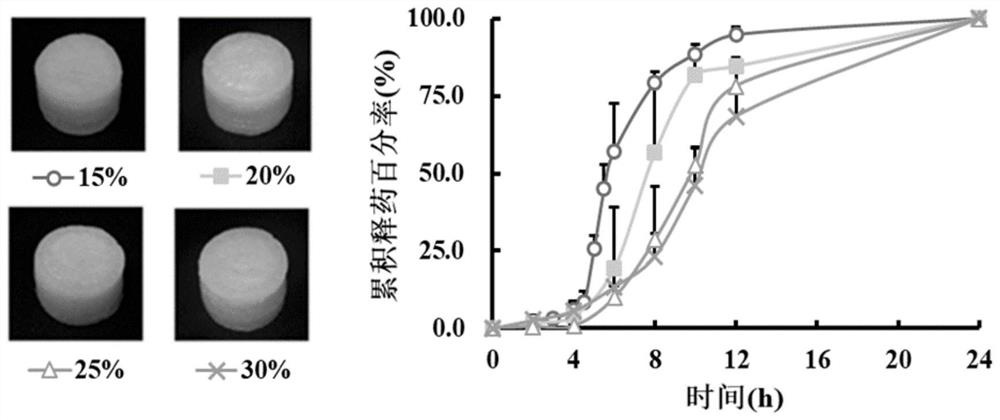
Extruded 3D printed coating type sustained release preparation and preparation method thereof
ActiveCN111450070AImprove printing effectAvoid sudden releaseAdditive manufacturing apparatusMacromolecular non-active ingredients3d printFormulary
The invention belongs to the medical field of medicines, and relates to an extruded 3D printed coating type sustained release preparation and a preparation method thereof. According to the coating type sustained release preparation disclosed by the invention, a mixture of gelatin, glycerin and water is used as a substrate, a medicine or HPMC is added, an inner core or a coating is obtained, then through combination with model slicing and printing parameters, extruding and 3D printing are performed, and a target namely the coating type sustained release preparation is obtained. The formula hasgood printing properties, and takes account of smooth extruding and quick deposition; and compared with a conventional slow release printing material, the extruded 3D printed coating type sustained release preparation has the advantages that the water content of a system is reduced, and intensity and elasticity of samples are improved. An optimized coating model structure can solve the problems existing in a conventional extruded 3D printed sustained release preparation, including low medicine loading quantity, obvious early stage sudden release and not ideal 24h slow release actions. According to the extruded 3D printed coating type sustained release preparation disclosed by the invention, diclofenac sodium, aminophylline, metoprolol tartrate and acetylsalicylic acid are further used as representatives, the application effect of the coating type sustained release preparation is disclosed, and through adjusting medicines and models, individualized treatment of various medicines can berealized.
Owner:ZHEJIANG UNIV OF TECH
Synthetizing method of farmland rodenticide
InactiveCN106106539AKeep aliveEfficient killingBiocideDead animal preservationSodium BentoniteVegetable oil
The invention discloses a synthetizing method of a farmland rodenticide. The rodenticide is prepared from, by weight, 7-12 parts of diisocyanate, 5-15 parts of tripterygium wilfordii, 5-10 parts of animal and vegetable oil, 10-20 parts of corn flour, 1-4 parts of essence, 10-20 parts of sodium alga acid, 7-15 parts of tetramethrin, 2-6 parts of bentonite, 5-10 parts of ricinine, 1-3 parts of maduramicin, 3-6 parts of indomethacin, 10-20 parts of sodium diclofenac, 2-6 parts of 4-hydroxycmarin, 10-20 parts of garden balsam stem powder, 10-15 parts of fulvic acid and 3-8 parts of citric acid. The manufacturing technology is simple, the obtained rodenticide can effectively kill rats, crops and soil will not be damaged when the rodenticide is used for a long time, and the effect of being free of toxicity and environmentally friendly is achieved.
Owner:河南中天恒信生物化学科技有限公司
Method for synthesizing dichlofenac sodium
InactiveCN1242984CHigh yieldLow costOrganic compound preparationAmino-carboxyl compound preparationPhenyl acetic acidCyclohexanone
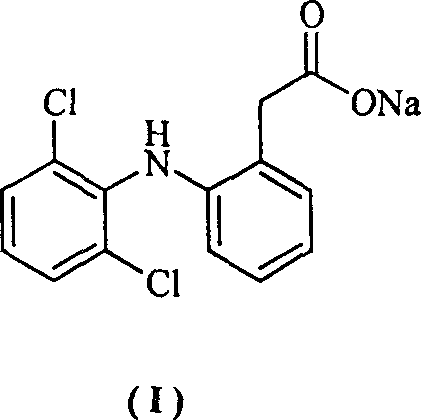
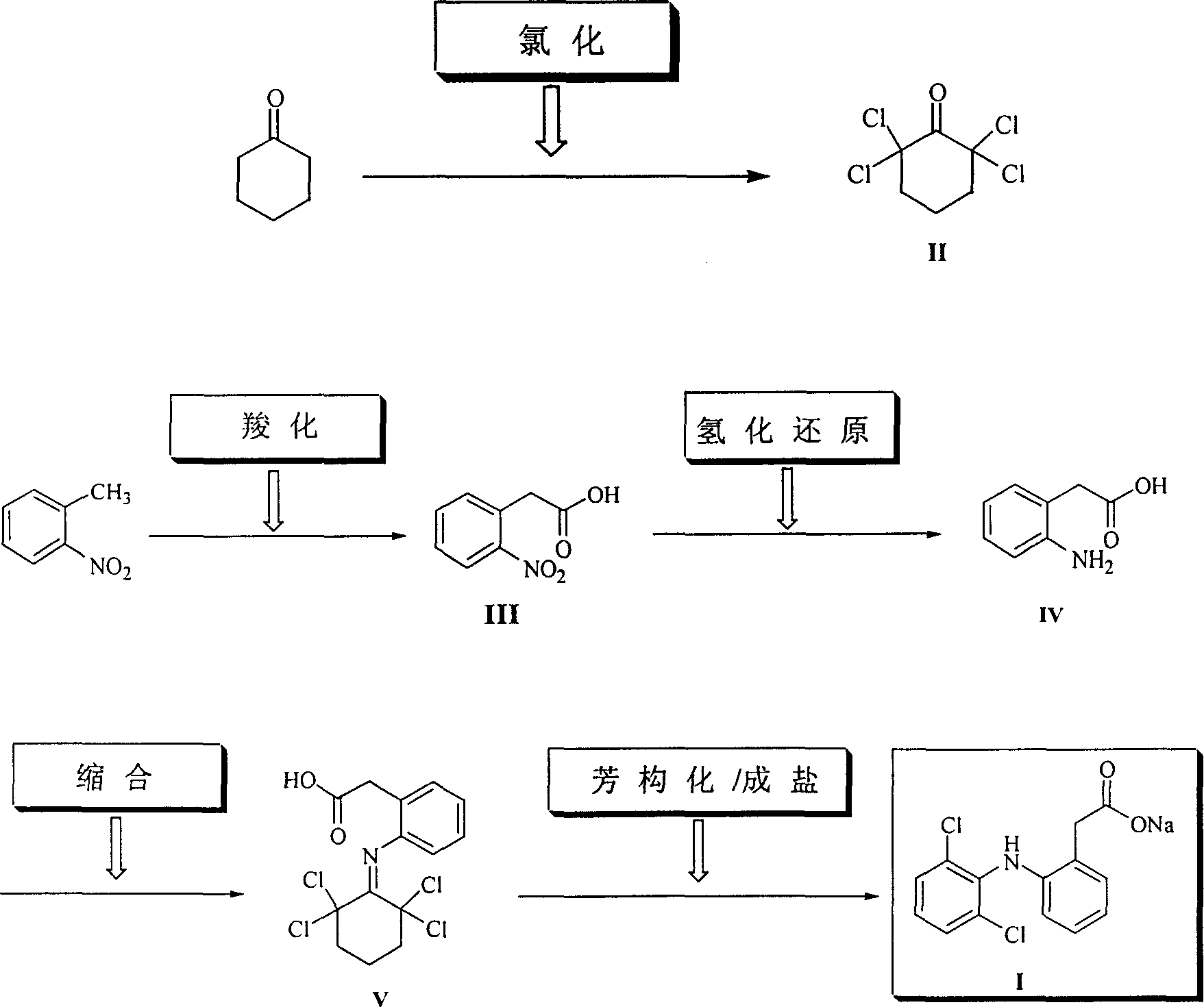
The invention provides a synthetic method of sodium diclofenac. The method includes the following steps: 1. Make the 2, 2, 6, 6-tetrachloride cyclohexanone (II) from the cyclohexanone through chlorination. 2. The carboxylation reaction occurs to the close nitrotoluene and carbon dioxide after the phase-transfer catalysis to get the close nitrophenyl acetic acid (III). 3. The hydrogenation reaction happens to the compound (III) catalysed by the polymer catalyst Pd / D-296 to produce the close aminophenyl acetic acid (IV). 4. The condensation reaction occurs to the compound IV and compound II to produce the compound of N-(2-carboxymerhy1 phenyl)-2,2,6,6-hexamethylene imine (V). 5. After the aromatization reaction and salifying happen to the compound V, we get the sodium diclofenac (I). By the close nitrotoluene, the yield is 85%. The invention is featured by facility of the raw material, simple operation, mild reation condition and easy industrialization.
Owner:FUDAN UNIV
Traumatic injury medicinal liquor and preparation method thereof
PendingCN113713066AEasy to acceptIncrease profitAnthropod material medical ingredientsDispersion deliveryLiver and kidneyMyrrh
The invention discloses a traumatic injury medicinal liquor used for improving subcutaneous congestion and muscle swelling caused by acute soft tissue injury with mildness, effectiveness and little side effect, and a preparation method thereof. The traumatic injury medicinal liquor is characterized by being prepared from the following raw materials: radix angelicae sinensis, frankincense, flos carthami, radix paeoniae rubra, radix achyranthis bidentatae, asarum, sesame, rhizoma galangae, eupolyphaga seu steleophaga, radix saposhnikoviae, caulis sargentodoxae, radix angelicae, rhizoma seu radix notopterygii, platycodon grandiflorum, radix dipsaci, lindera aggregata, semen arecae, pericarpium citri reticulatae, radix rehmanniae recen, myrrh, yellow rice wine. The traumatic injury medicinal liquor has a simple process, and complementary raw materials. The raw materials are soaked in low-alcohol yellow rice wine in a time controlling mode, so that the traumatic injury medicinal liquor is low in alcohol content, good palatability and good acceptance by patients; The traumatic injury medicinal liquor is mild, and little gastrointestinal stimulation and little injury to liver and kidneys. The reflux extraction can effectively improve the utilization rate and the efficacy of the formula drugs. The traumatic injury medicinal liquor has efficacy equivalent to that of diclofenac sodium, but fewer side effect and higher safety. The traumatic injury medicinal liquor can significantly improve subcutaneous congestion and muscle swelling caused by acute soft tissue injury, and has good effects of promoting blood circulation to remove blood stasis, diminishing swelling and resisting inflammation.
Owner:湖南省开元博物馆 +1
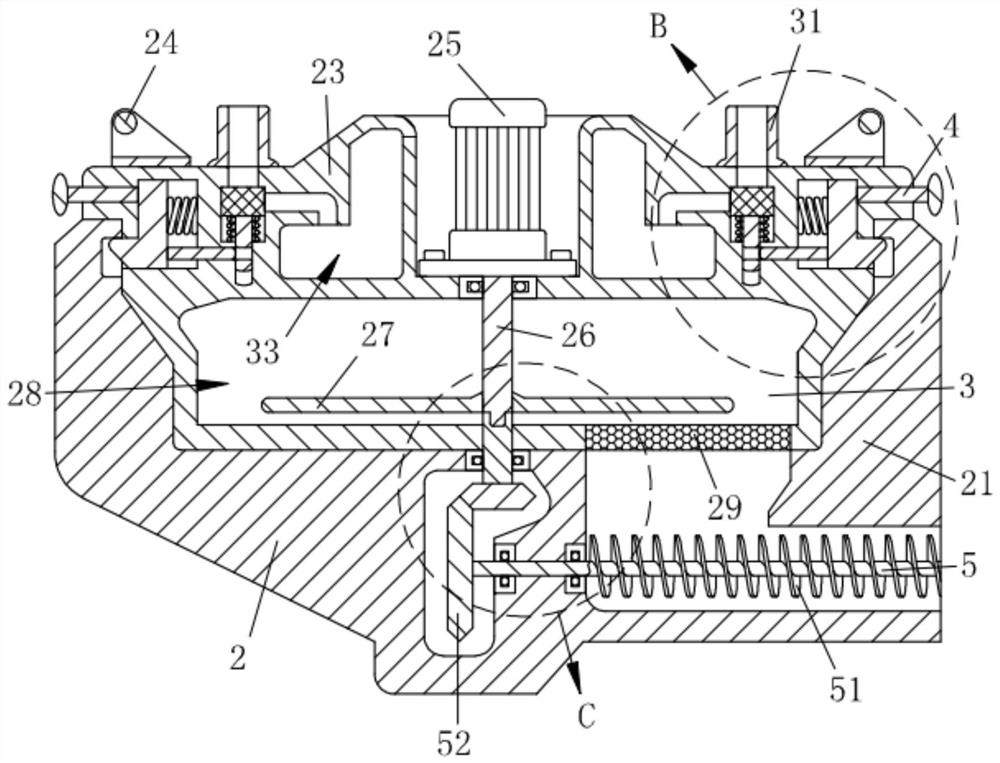
Preparation equipment for preparing diclofenac sodium sustained-release composition through melting and preparation process of diclofenac sodium sustained-release composition
ActiveCN113730375APromote meltingEasy to crushOrganic active ingredientsAntipyreticWaxPhysical chemistry
The invention relates to the technical field of preparation of diclofenac sodium sustained-release compositions and particularly relates to preparation equipment for preparing a diclofenac sodium sustained-release composition through melting and a preparation process of the diclofenac sodium sustained-release composition. The preparation equipment comprises a melter body, wherein a crushing mechanism is mounted on the melter body, a conveying mechanism is mounted in the crushing mechanism, a cooling mechanism is arranged in the crushing mechanism, two locking mechanisms are mounted in the crushing mechanism, a protection mechanism is connected into the crushing mechanism, and a discharging mechanism is connected to the melter body. The melting efficiency of a wax material is increased through the action of the crushing mechanism, material conveying is facilitated through the conveying mechanism, blockage is prevented, heat dissipation and cooling of the crushing mechanism are facilitated under the action of the cooling mechanism, the working life is prolonged, the crushing mechanism is stably mounted under the action of the locking mechanisms, follow-up maintenance and cleaning are facilitated, materials can be conveniently, rapidly and smoothly discharged through the discharging mechanism, and the blockage is prevented.
Owner:DEZHOU DEYAO PHARMA
Oral sustained-release preparation of diclofenac sodium and preparation method of oral sustained-release preparation
PendingCN112587493ALess side effectsImprove complianceOrganic active ingredientsAntipyreticUse medicationPharmaceutical Aids
The invention belongs to the technical field of pharmaceutical preparations, particularly relates to an oral sustained-release preparation containing diclofenac sodium, and discloses a preparation method of the oral sustained-release preparation. The preparation method comprises the following steps: dissolving diclofenac sodium and an inner-layer carrier in an inner-layer solvent, dissolving an outer-layer carrier in an outer-layer solvent, preparing diclofenac sodium microcapsules with coaxial electrostatic spraying method, adding a filler, carrying out uniform mixing and granulating, and adding other pharmaceutical auxiliary materials for tabletting to obtain the oral sustained-release preparation. According to the oral sustained-release preparation containing diclofenac sodium and the preparation method of the oral sustained-release preparation, the electrostatic spraying technology is creatively applied to the diclofenac sodium sustained-release preparation, the sustained-release effect is good, the gastrointestinal tract side reaction can be effectively reduced, the compliance of patients is improved, and the diclofenac sodium sustained-release preparation is suitable for therequirements of clinical medication development.
Owner:南京易亨制药有限公司
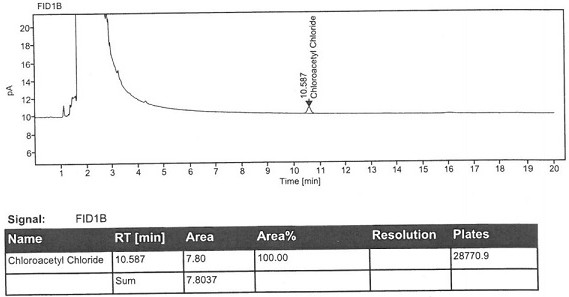
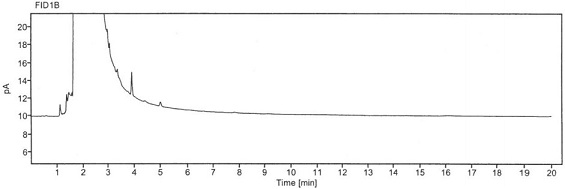
Method for detecting chloroacetyl chloride in diclofenac sodium
ActiveCN113219098AImprove quality controlSimple methodComponent separationSodium DiclofenacChloroacetyl chloride
The invention belongs to the technical field of pharmaceutical analysis, particularly relates to a method for detecting chloracetyl chloride in diclofenac sodium, and provides a convenient, efficient and accurate detection method for solving the problem of detection of chloracetyl chloride in diclofenac sodium, the method can be used for detecting the content of chloracetyl chloride in diclofenac sodium so as to effectively guarantee the medication safety, and the quality control of diclofenac sodium API can be conveniently controlled. The method is convenient, efficient and accurate, the system applicability, repeatability, specificity and accuracy completely conform to the guidance principle verified by the method in the Chinese pharmacopoeia, and the method can be used for quality control of the diclofenac sodium bulk drug.
Owner:珠海润都制药股份有限公司
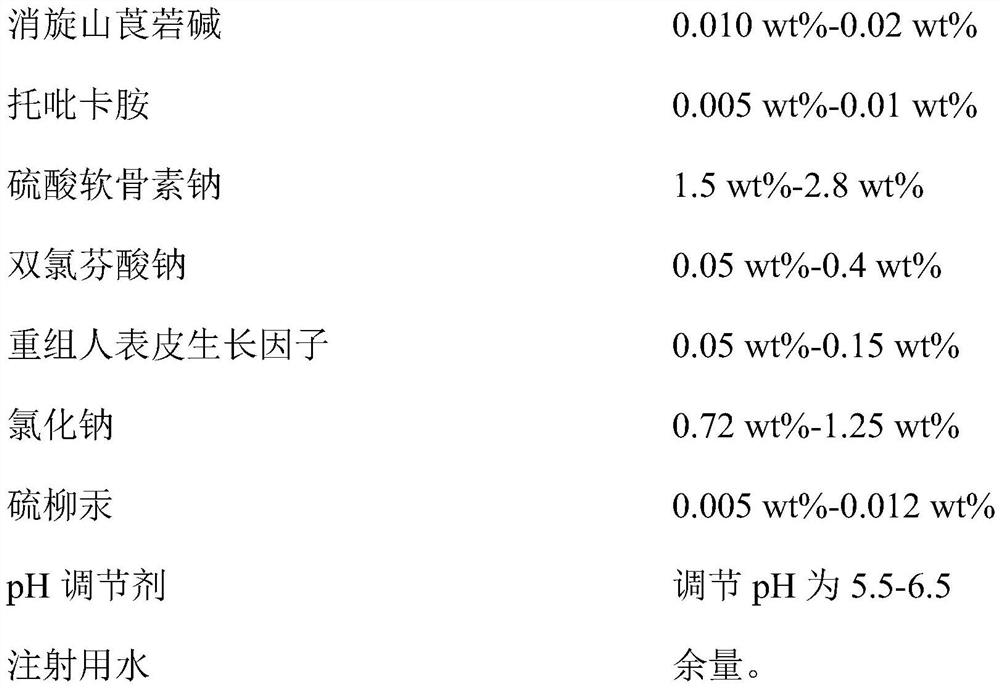
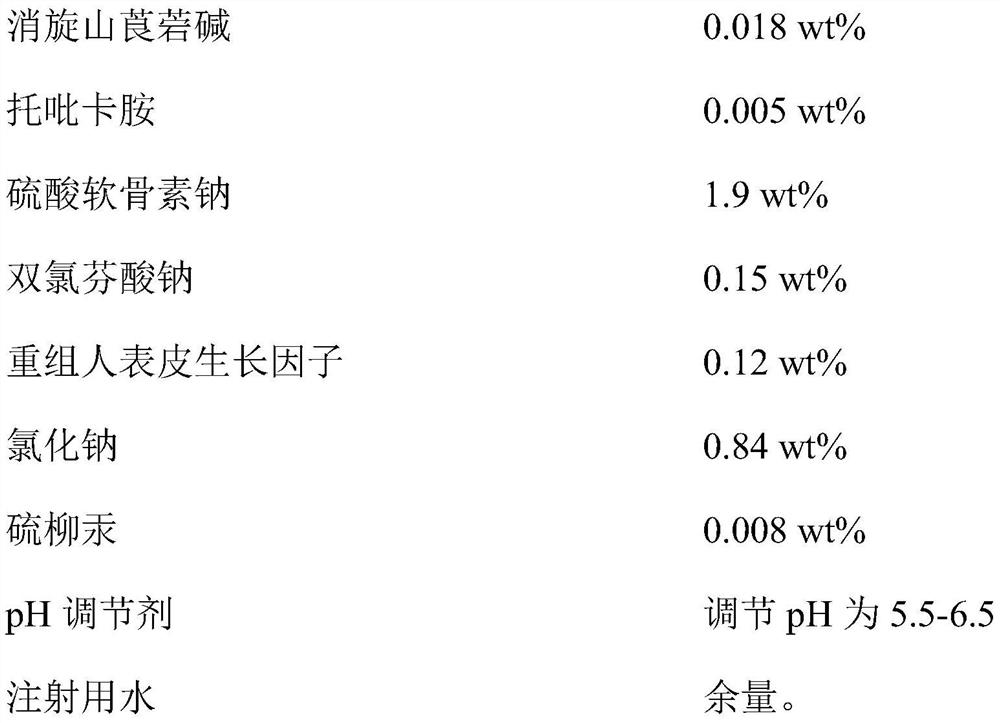
Compound racanisodamine eye drops
InactiveCN112353757ASignificant and effective delayObvious and effective control effectSenses disorderPeptide/protein ingredientsThiomersalateDrug biological activity
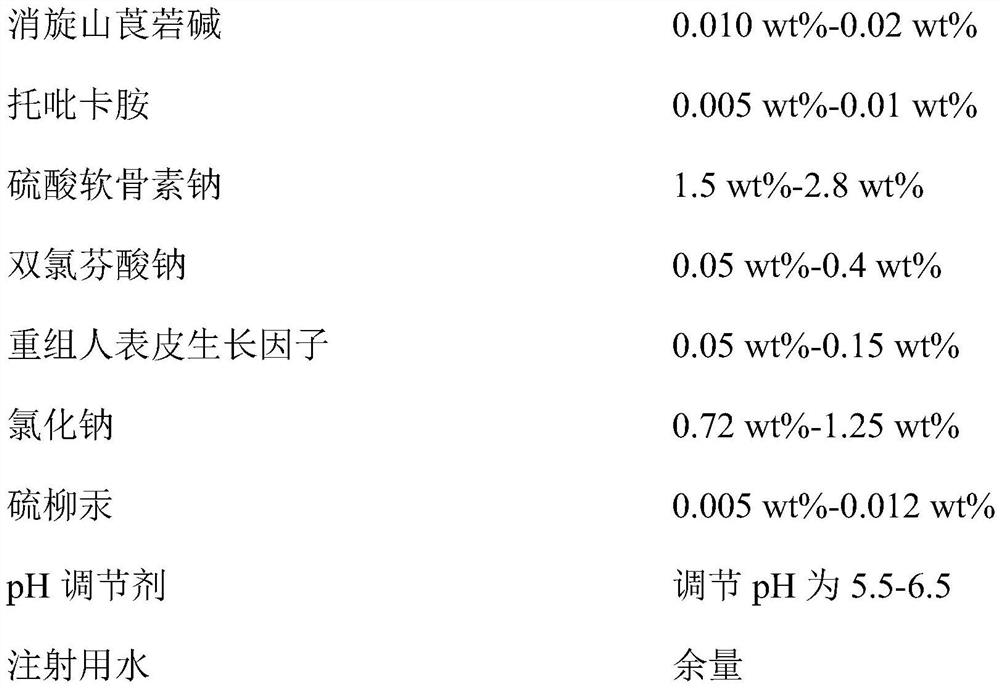
Provided is compound racanisodamine eye drops prepared from the following raw materials: 0.010-0.02 wt% of racanisodamine, 0.005-0.01 wt% of tropicamide, 1.5-2.8 wt% of sodium chondroitin sulfate, 0.05-0.4 wt% of diclofenac sodium, 0.05-0.15 wt% of a recombinant human epidermal growth factor, 0.72-1.25 wt% of sodium chloride, 0.005-0.012 wt% of thiomersalate, a pH regulator for adjusting pH to 5.5-6.5, and the balance being injection water. The compound racanisodamine eye drops disclosed by the invention are applied to adolescents, children and adults who often use eyes at a short distance, sothat the delaying and preventing effects on myopia of the adolescents and children are more obvious and effective after the compound racanisodamine eye drops are dripped for a long time, and for adults, visual fatigue can be significantly improved, and the elimination of eyeball discomfort and other symptoms can be accelerated; diclofenac sodium and racemic anisodamine are combined to achieve a good synergistic effect, diclofenac sodium is used as a ligand and matched with racemic anisodamine to generate a synergistic effect of biological activity, and meanwhile, diclofenac sodium and racemicanisodamine have the possibility of reducing toxicity, are low in price and easy to obtain and are worthy of popularization clinically.
Owner:ZHENJIANG HENGXIN PHARMA
Magnetic slow releasing diclofenac sodium prepn in supermolecular intercalation structure and its prepn
InactiveCN1973831AControl release speedImprove stabilityOrganic active ingredientsAntipyreticHydrotalciteDiclofenac Sodium
The present invention is magnetic slow releasing diclofenac sodium in supermolecular intercalation structure and its preparation process, and belongs to the field of hydrotalcite material technology. The magnetic diclofenac sodium has the chemical expression of (M2+)1-x(N3+)x(OH)2(DIK-)a(Bn-)b.mH2O.kM'Fe2O4, and is prepared through the first coprecipitation process to prepare nanometer magnetic kernel, the subsequent mixing of the magnetic kernel with corresponding salt solution, and the final coprecipitation process to assemble diclofenac sodium into the hydrotalcite layers and to obtain the diclofenac sodium intercalated hydrotalcite with magnetic kernel. The magnetic slow releasing diclofenac sodium in supermolecular intercalation structure has high stability, raised bioavailability, capable of magnetic field location and targeting location and effective control on the medicine structure, composition, etc.
Owner:BEIJING UNIV OF CHEM TECH
Diclofenac sodium and lidocaine hydrochloride injection and preparation method thereof
InactiveCN111514104AReduce manufacturing costOrganic active ingredientsNervous disorderSulfite saltDisodium Edetate
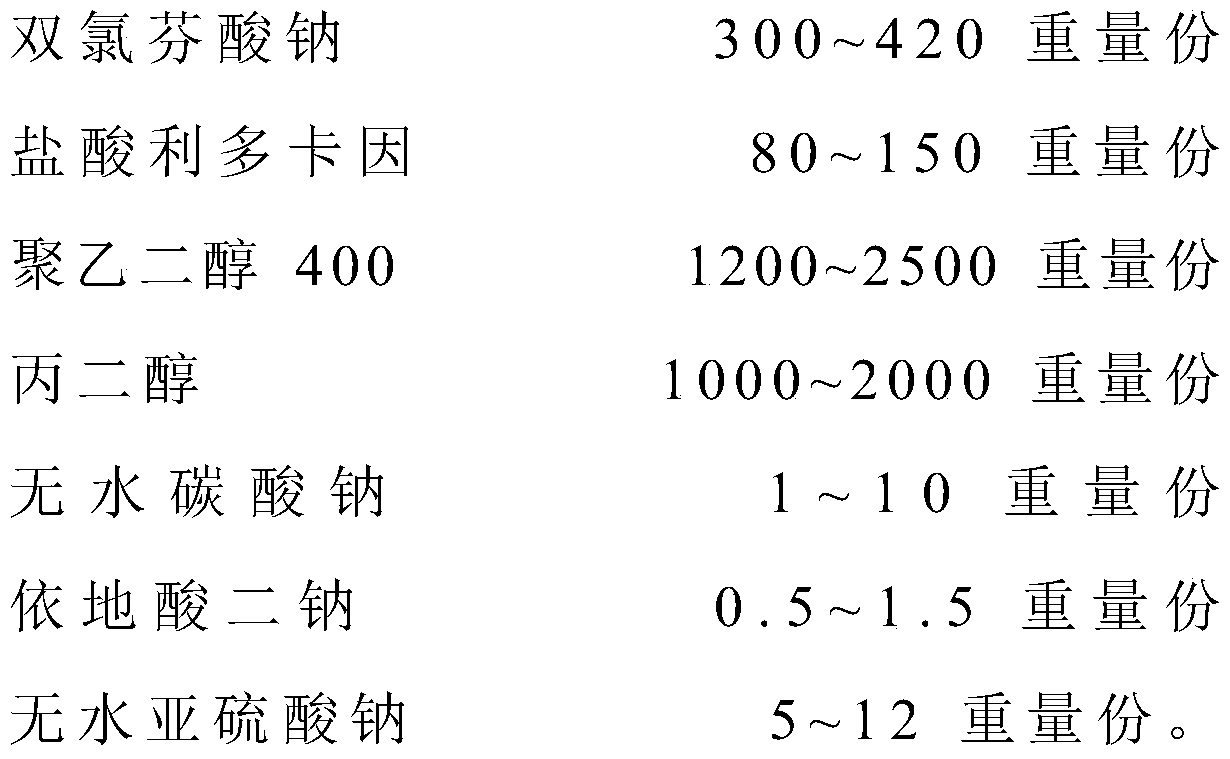
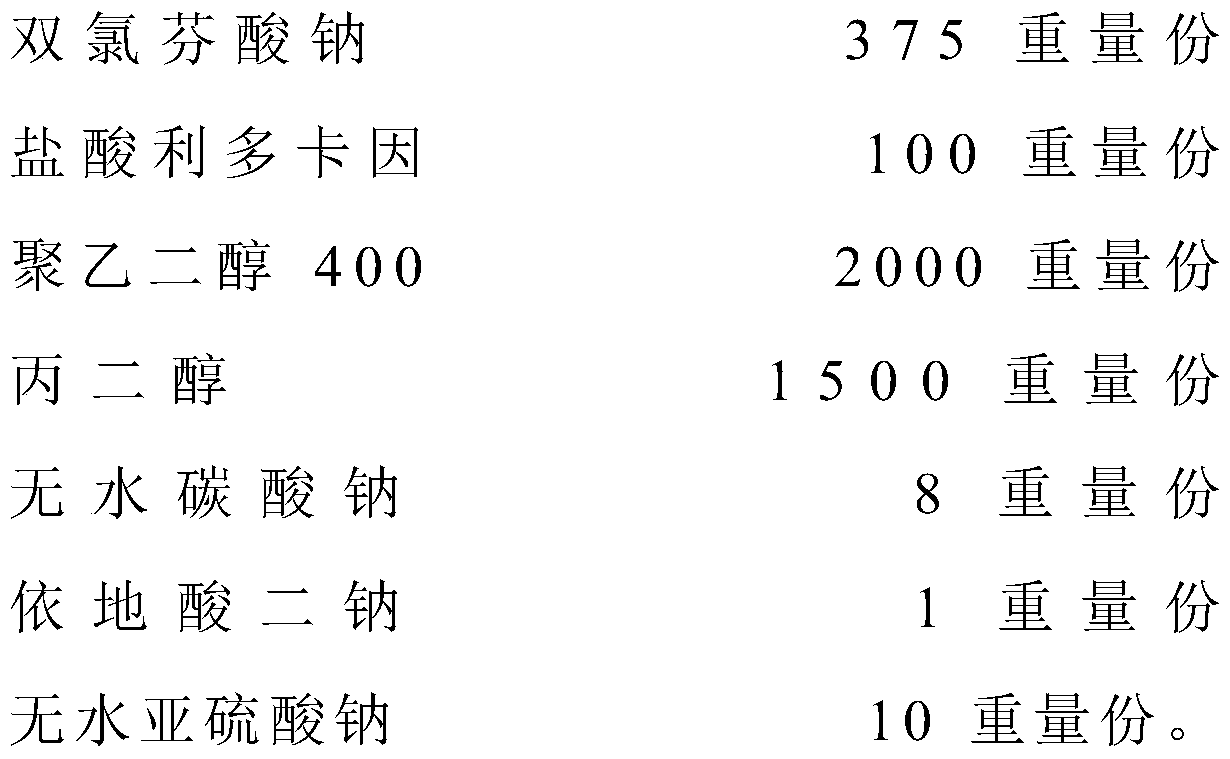
The invention relates to the technical field of red diclofenac sodium and lidocaine hydrochloride injections and preparation thereof, in particular to a diclofenac sodium and lidocaine hydrochloride injection and a preparation method thereof. The diclofenac sodium and lidocaine hydrochloride injection is prepared from the following raw materials in parts by weight: 300 to 420 parts of diclofenac sodium, 80 to 150 parts of lidocaine hydrochloride, 1200 to 2500 parts of polyethylene glycol 400, 1000 to 2000 parts of propylene glycol, 1-10 parts of anhydrous sodium carbonate, 0.5 to 1.5 parts ofedetate disodium, and 5-12 parts of anhydrous sodium sulfite. The anhydrous sodium carbonate is used as a pH stabilizer instead of the existing cysteine, thereby lowering the production cost and enhancing the economic benefit under the condition of not influencing the drug effect.
Owner:马鞍山思哲知识产权服务有限公司
Pellet composition containing diclofenac sodium and preparation method thereof
InactiveCN104510724ALess irritatingReduce dosageOrganic active ingredientsAntipyreticSustained release pelletsPharmacy
The invention belongs to the field of pharmacy, and particularly relates to a pellet composition containing diclofenac sodium and a preparation method thereof. The composition consists of pellets with different release performances, specifically namely enteric pellets with the total weight of 20-35% and sustained-release pellets with the total weight of 80-65%, wherein the enteric pellets are prepared through enteric coating, the sustained-release pellets are prepared through sustained-release coating, and finally capsules are filled with the enteric pellets and the sustained-release pellets in proportion. The preparation can maintain long blood concentration, and thus the medicine taking frequency is reduced, the adaptability of patients is improved, and the toxic and side effects are reduced. Besides, the adopted preparation technology is easy for large-scale production, little in differences among product batches and high in stability.
Owner:中盛特医食品(汕头)有限公司
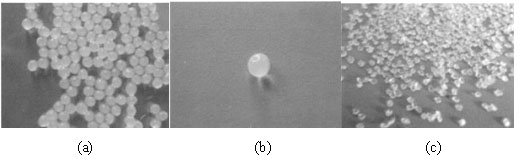
Method for preparing enteric composite microcapsules by utilizing spray drying technology
ActiveCN111514112AUniform sizeSimple processOrganic active ingredientsUnknown materialsBiotechnologyGastric juices
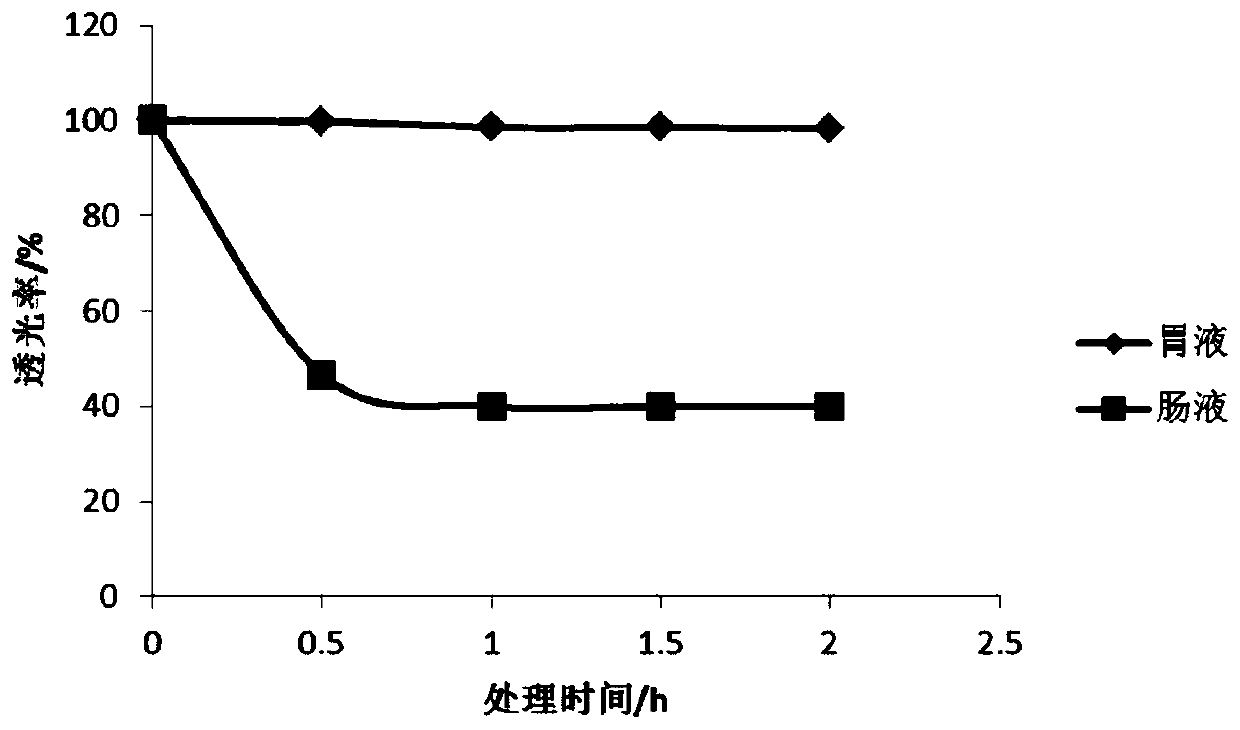
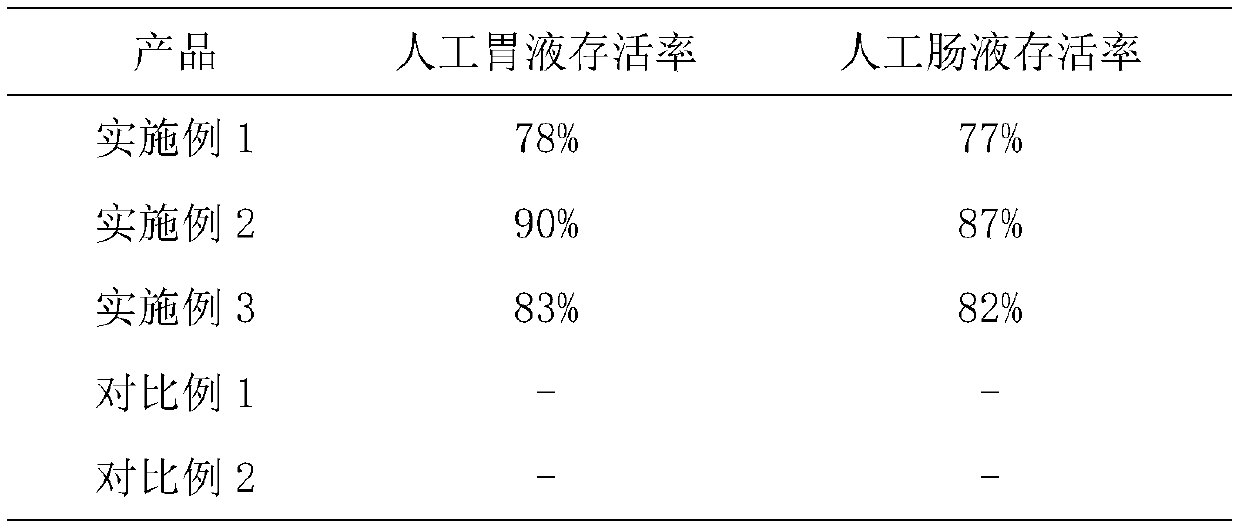
The invention discloses a method for preparing enteric composite microcapsules by utilizing a spray drying technology, and belongs to the technical field of a microcapsule preparation. The method is suitable for embedding solid medicine powder such as probiotics or diclofenac sodium and the like and uses natural macromolecular materials such as casein and collagen as enteric microcapsule wall materials, and utilizes a process of combining a complex coacervation method and spray drying at the normal temperature so as to prepare the enteric composite microcapsules which are uniform in size. According to the method, influence of a high temperature on core materials in the microcapsules in the spray drying process can be reduced, and the core materials are effectively embedded; stability of the product is improved; and the method is simple to operate and suitable for industrial production. The prepared enteric composite microcapsules are safe and non-toxic, cannot be disintegrated in 2h inartificial gastric juice, can avoid stimulus of an embedded medicine to the gastric mucosa and cover up objectionable odor, and can implement effective release in 0.5h in artificial intestinal juice.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Topical pharmaceutical gel composition of diclofenac sodium
InactiveUS20160263065A1Quick cureHydroxy compound active ingredientsAerosol deliveryDiclofenac SodiumTopical Gel
A topical pharmaceutical gel composition of diclofenac sodium suitable for twice daily application is provided. The topical gel composition contains at least about 10% w / w of diclofenac sodium and twice daily application of the composition provides relief from pain or inflammation comparable to that achieved with 4 times daily application of diclofenac sodium 1% or 3% topical gel.
Owner:GAVIS PHARMA
Extruded 3D printing coated sustained-release preparation and preparation method thereof
ActiveCN111450070BAvoid sudden releaseReasonable three-dimensional structure designAdditive manufacturing apparatusMacromolecular non-active ingredients3d printFormulary
The invention belongs to the field of pharmaceutical medicine, and relates to an extrusion 3D printing coating type sustained-release preparation and a preparation method thereof. The coated sustained-release preparation of the present invention is based on a mixture of gelatin, glycerin and water, adding drugs or HPMC to obtain an inner core or coating, and then extruding 3D printing in combination with model slices and printing parameters to obtain the target coating sustained-release preparations. The formula has good printability, taking into account "smooth extrusion" and "rapid deposition". Compared with the existing slow-release printing materials, the water content of the system is reduced, and the strength and elasticity of the sample are increased. The optimized coating model structure can solve the problems existing in the existing extrusion 3D printing sustained-release preparations, including: low drug loading, obvious early burst release and unsatisfactory 24h sustained-release behavior. The present invention is further represented by diclofenac sodium, aminophylline, metoprolol tartrate and acetylsalicylic acid, disclosing the application effect of coated sustained-release preparations, proving that by adjusting the drugs and models, the individualization of various drugs can be realized treat.
Owner:ZHEJIANG UNIV OF TECH
Diclofenac sodium lidocaine hydrochloride compound drug injection liquid and preparation method thereof
InactiveCN104274436AReduce the incidence of allergic reactionsOrganic active ingredientsAntipyreticMedication injectionFormulary
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to a diclofenac sodium lidocaine hydrochloride compound drug injection liquid and a preparation method thereof. The injection liquid is an injection preparation prepared by adding sterilization injection water into the following raw materials: 30-50mg / ml of diclofenac sodium, 5-15mg / ml of lidocaine hydrochloride, 40-60mg / ml of mannitol, and 40-60mg / ml of polyethylene glycol-12 hydroxy stearate. The compound drug injection liquid not only can reduce the patient aching feeling caused by injection, and the drug preparation is stable, non-hemolytic and low-anaphylactic.
Owner:CHENGDU LIST PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com