Method for evaluating in-vivo and in-vitro correlation of diclofenac sodium sustained release tablets
A technology of diclofenac sodium and sustained-release tablets, which is applied in the field of medicine and can solve problems such as high costs
Inactive Publication Date: 2022-02-25
HQ PHARMA (SHANGHAI) CO LTD
View PDF0 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, clinical trials have high cost constraints, so in the testing process of new drug development or generic drug consistency evaluation, it is generally necessary to first evaluate through in vitro testing
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
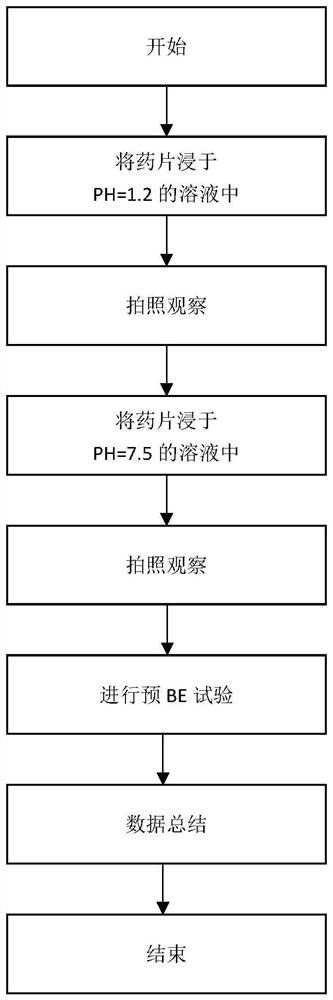
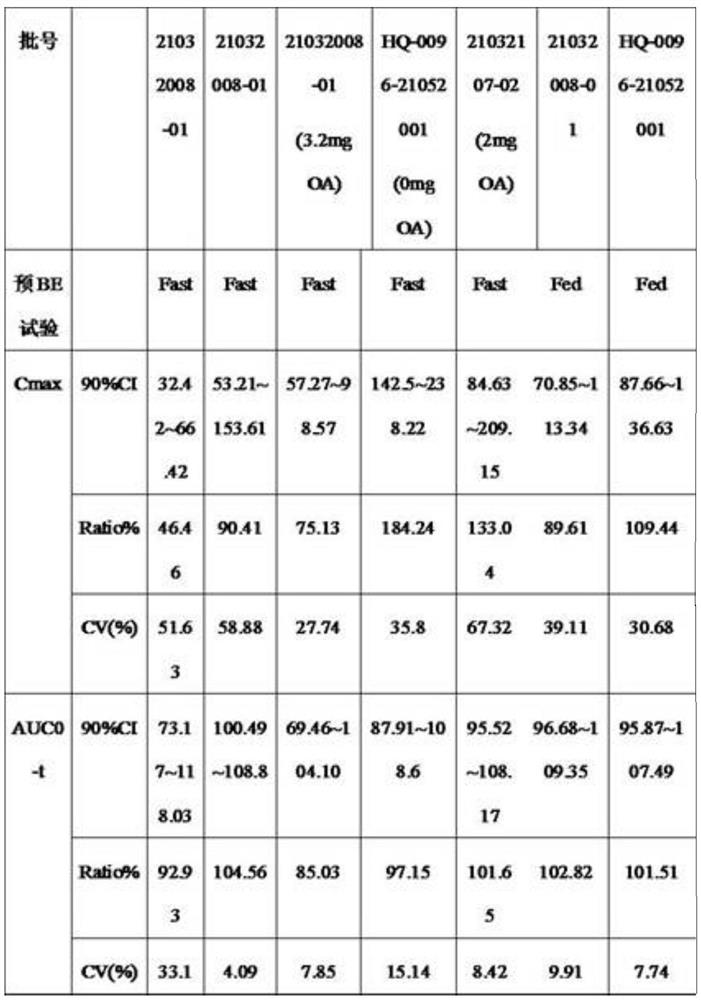
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0018] HQ-0096-21052001 batch prescription composition:
[0019]
Embodiment 2
[0021] 21032107-01 Batch prescription composition:
[0022]
Embodiment 3
[0024] 21032107-02 Batch prescription composition:
[0025]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for evaluating in vivo and in vitro correlation (In Vivoin Vitro Correlation) of a diclofenac sodium sustained release tablet, and a novel diclofenac sodium sustained release tablet. According to the method for evaluating the in-vivo and in-vitro correlation of the diclofenac sodium sustained release tablet, the in-vivo pharmacokinetic characteristics can be well evaluated. The formula of the diclofenac sodium sustained release tablet comprises the following components: 100 mg / tablet of diclofenac sodium, 119 mg / tablet of cane sugar, 59 mg / tablet of hexadecanol, 4 mg / tablet of povidone K30, 3 mg / tablet of colloidal silicon dioxide, 3 mg / tablet of magnesium stearate and 10 mg / tablet of film coating premix. According to the diclofenac sodium sustained-release tablet disclosed by the invention, the release speed of the medicine is controlled, the irritation of the traditional diclofenac sodium sustained-release tablet to the stomach is reduced, and meanwhile, the administration mode of keeping a relatively high effective blood concentration for a long time is achieved. The medicine effect is ensured, the side effect is reduced, and the clinical use is greatly facilitated.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to a method for evaluating the in vivo and in vitro correlation of diclofenac sodium sustained-release tablets, and provides a novel prescription for diclofenac sodium sustained-release tablets. Background technique [0002] Diclofenac sodium, also known as diclofenac, has a structural formula such as figure 1 , with a molecular weight of 318.13, is a non-Yong body anti-inflammatory drug. Its properties are white, odorless, hygroscopic crystalline powder. Diclofenac sodium is used to relieve the acute attack or persistent joint swelling and pain symptoms of various chronic arthritis such as rheumatoid arthritis, osteoarthritis, spondyloarthropathy, gouty arthritis, and rheumatoid arthritis. Diclofenac sodium will have a stronger irritating effect on the gastrointestinal tract during use, so pharmaceutical preparations are generally used as sustained-release preparations now. How...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): G01N33/15G01N21/84A61K9/28A61K31/196A61K47/32A61K47/26A61K47/04A61P19/02A61P19/06A61P29/00
CPCG01N33/15G01N21/84A61K31/196A61K9/2806A61K9/2027A61K9/2018A61K9/2009A61P29/00A61P19/02A61P19/06
Inventor 贺敦伟秦杰子张艳
Owner HQ PHARMA (SHANGHAI) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com