Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Reversible hydrogen electrode" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
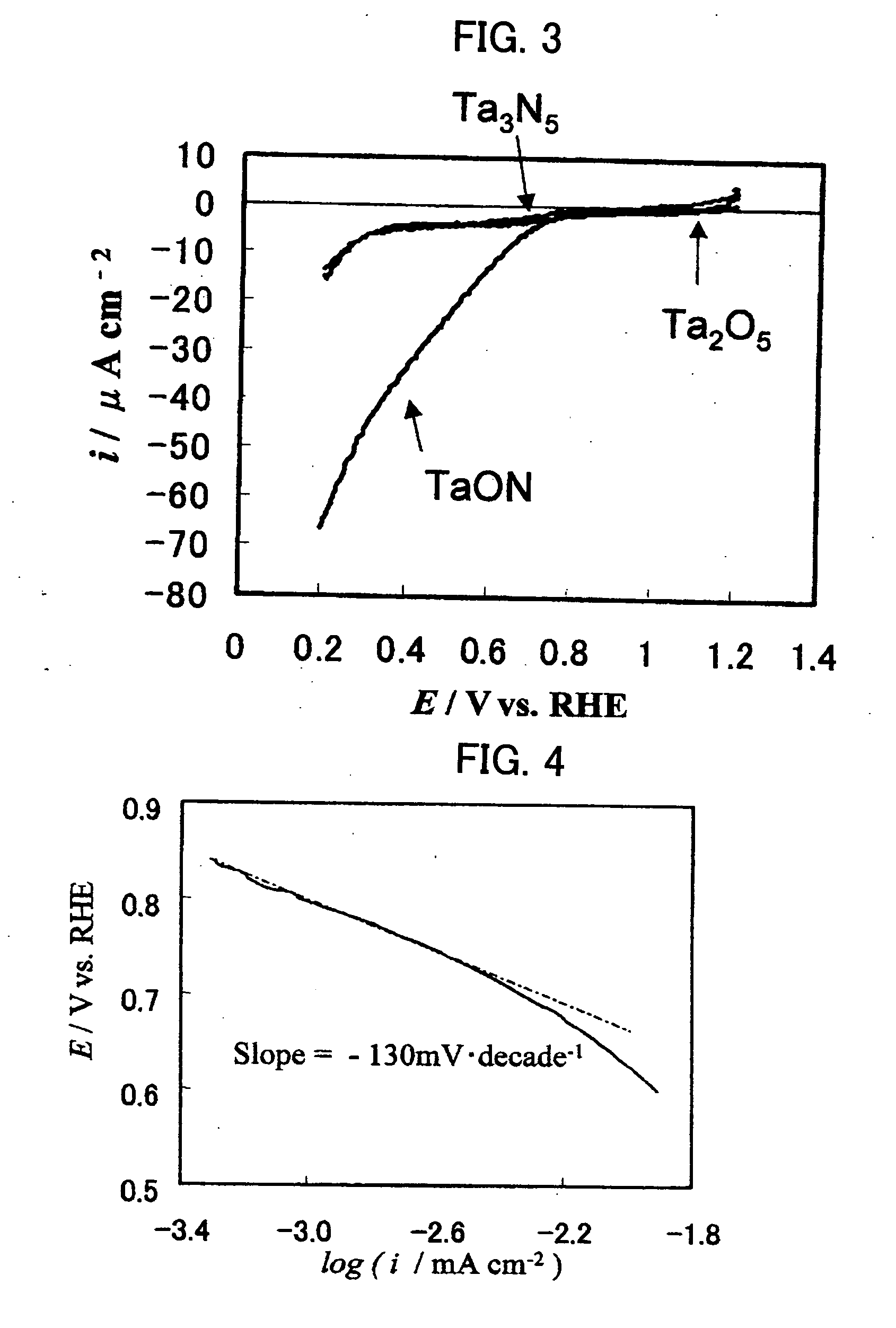
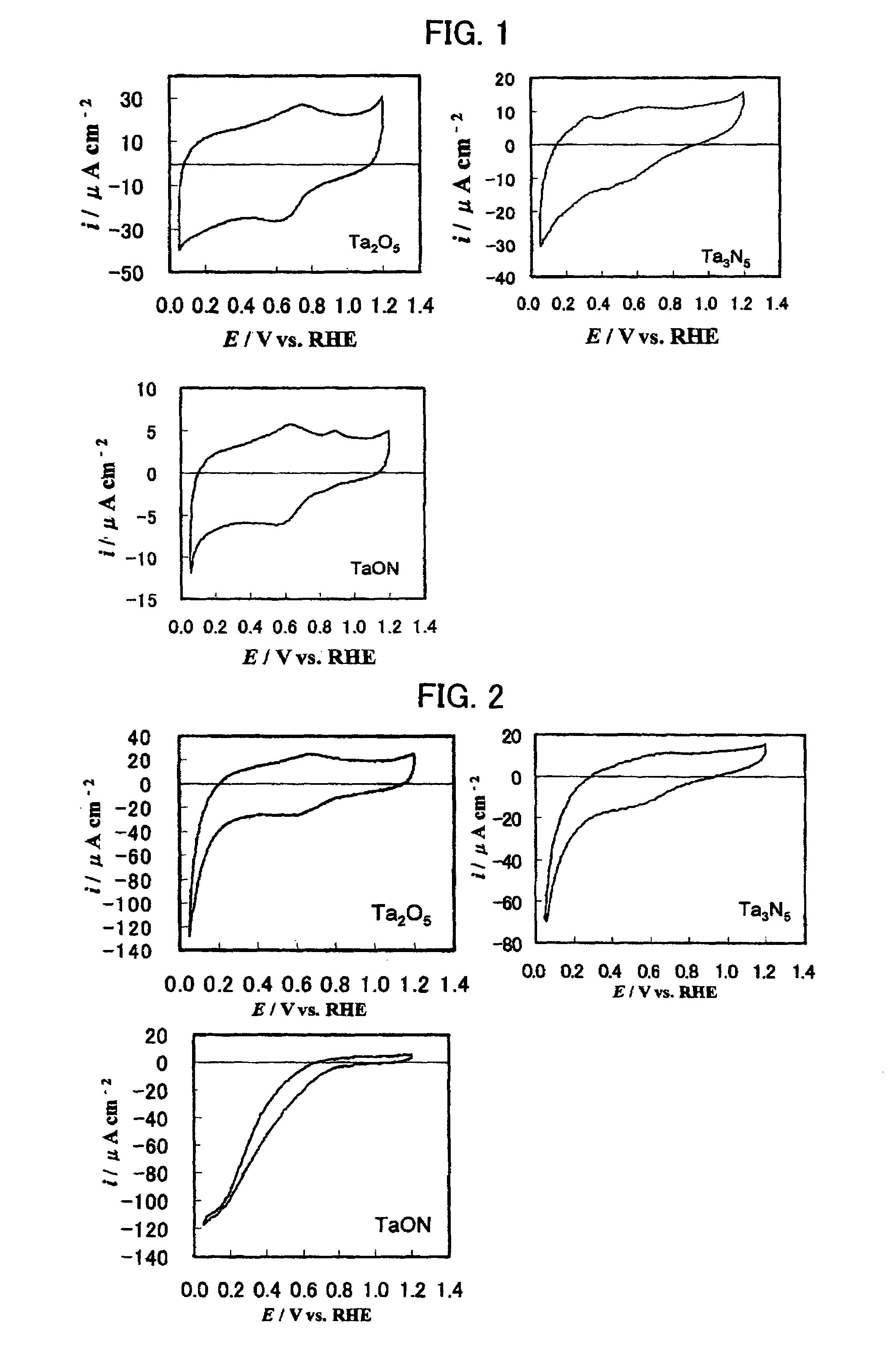
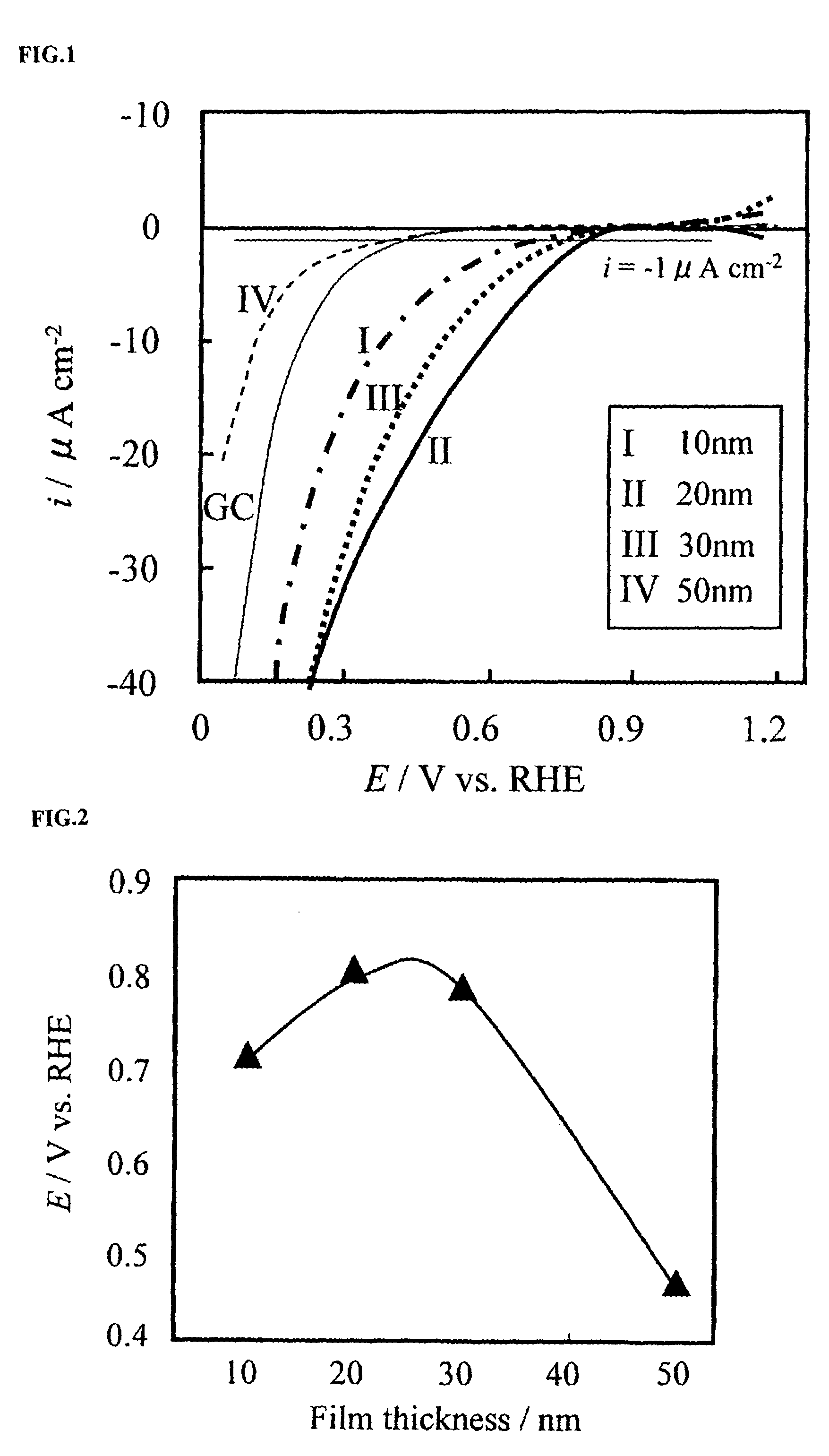
A reversible hydrogen electrode (RHE) is a reference electrode, more specifically a subtype of the standard hydrogen electrodes, for electrochemical processes. Unlike the standard hydrogen electrode, its measured potential does not change with the pH, so it can be directly used in the electrolyte. The name refers to the fact that the electrode is in the actual electrolyte solution and not separated by a salt bridge.
Method for preparing porous alpha-Fe2O3 photo-anode
ActiveCN105601124AEasy to prepareSimple and fast operationElectrolysis componentsCoatingsClean energyPhotocurrent
The invention discloses a method for preparing a porous alpha-Fe2O3 photo-anode and belongs to the technical field of photoelectric water decomposition. The method uses fluorine doped SnO2 conductive glass (FTO) as a substrate, a FeOOH film uniformly grows on an FTO surface through hydrothermal reaction, and then a photo-anode material of an alpha-Fe2O3 film with pores formed in the surface is prepared through microwave assisted heating. The method is simple in operation, mild in reaction condition, short in reaction period and high in repeatability, raw materials are cheap and are easy to obtain, complicated and expensive devices are not needed, the preparation cost is low, the prepared material has excellent oxygen production performance when being applied to photoelectric water decomposition, the photoelectric current density can be up to 2.4 mA / cm<2> under the simulated sunlight irradiation compared with a reversible hydrogen electrode 1.23 V, and the method has wide application prospect on the aspect of development of sustainable clean energy.
Owner:FUZHOU UNIV
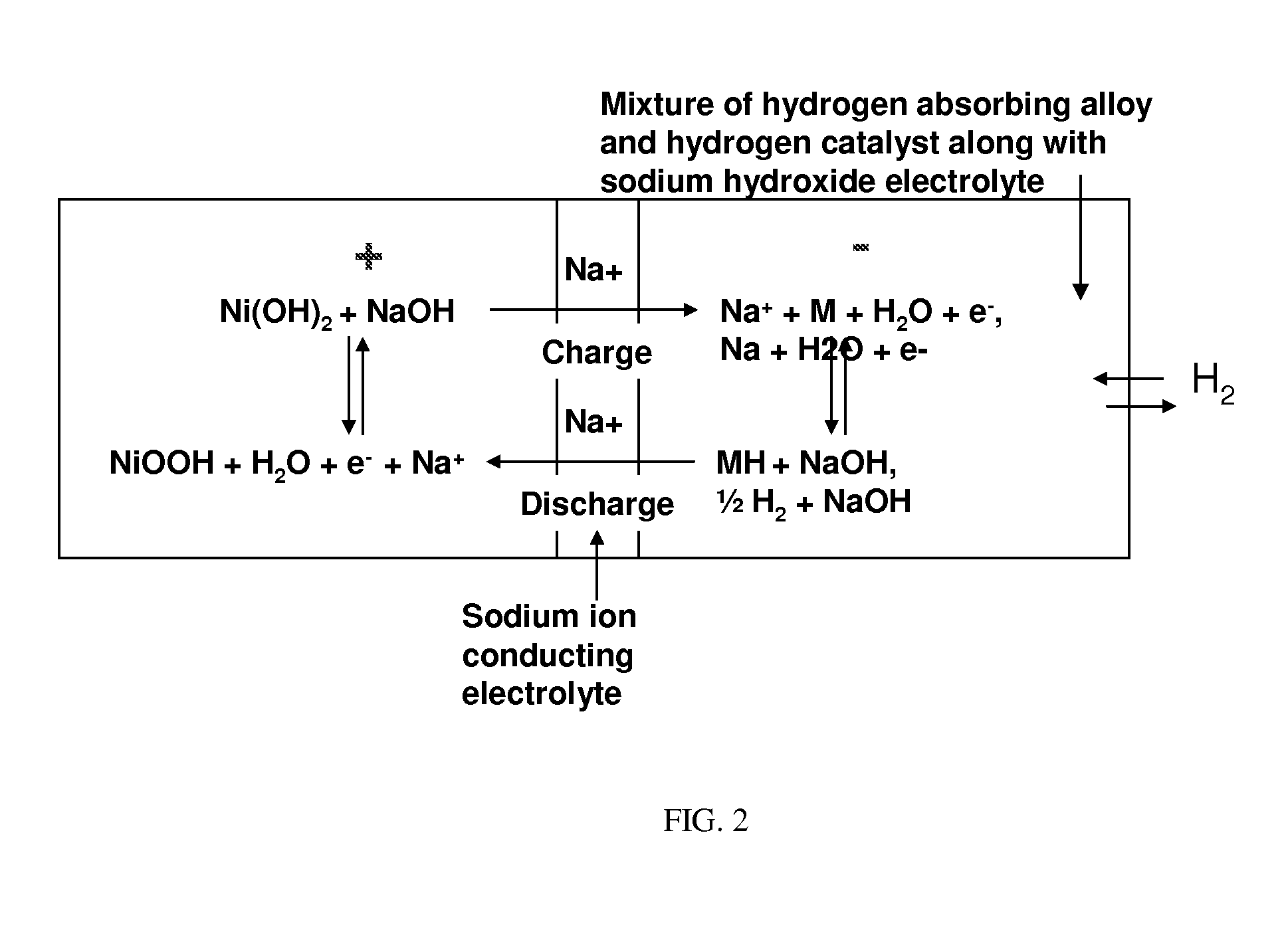
Nickel-metal hydride/hydrogen hybrid battery using alkali ion conducting separator
ActiveUS20120126752A1Batteries circuit arrangementsAlkaline accumulatorsAlkali ionsNickel oxide hydroxide
A nickel-metal hydride (hydrogen) hybrid storage battery comprising a positive electrode containing nickel hydroxide, a combination negative electrode containing a hydrogen storage alloy electrode and a reversible hydrogen electrode, an alkaline electrolyte, and an alkali conducting separator disposed between the positive electrode and the negative electrode. The alkali conducting separator may be a substantially non-porous ion conducting material wherein the alkali conducted is Na, K, or Li. A method of charging and discharging such a hybrid battery is also disclosed.
Owner:FIELD UPGRADING USA INC
Evaluation device and evaluation method an electrocatalyst property used for a solid polymer electrolyte fuel cell and water electrolysis
ActiveCN106596686AHigh simulationReduce wasteMaterial analysis by electric/magnetic meansElectricityPolymer electrolytes
The invention relates to an evaluation device and evaluation method of an electrocatalyst property used for a solid polymer electrolyte fuel cell and water electrolysis, and belongs to the technical field of electrocatalyst activity evaluation. The problem that environment at which the electrocatalyst is located is different from actual application in a currently widely adopted liquid three-electrode system and the technical problem that evaluation the technology is tedious, the amount the needed catalyst is high, the cost is high and the influence factor is complex in an assembled battery evaluation method. The evaluation device microminiaturizes a membrane electrode assembly, a reversible hydrogen electrode is added as a reference electrode to test an electrocatalytic property, the advantages of the currently widely adopted liquid three-electrode system and a membrane electrode catalytic property are combined, and the catalyst is made to be located in the solid polymer electrolyte fuel cell to test the electrocatalyst property of the solid polymer electrolyte and better simulates the catalytic property in an actual using environment. The evaluation method can accurately and effectively evaluate the electrocatalyst property used for the polymer electrolyte fuel cell and the water electrolysis.
Owner:SHANDONG SAIKESAISI HYDROGEN ENERGY
Metal Oxide Electrode Catalyst
InactiveUS20070259267A1Improve corrosion resistanceActivity of catalystActive material electrodesAqueous electrolyte fuel cellsElectrolysisGold particles
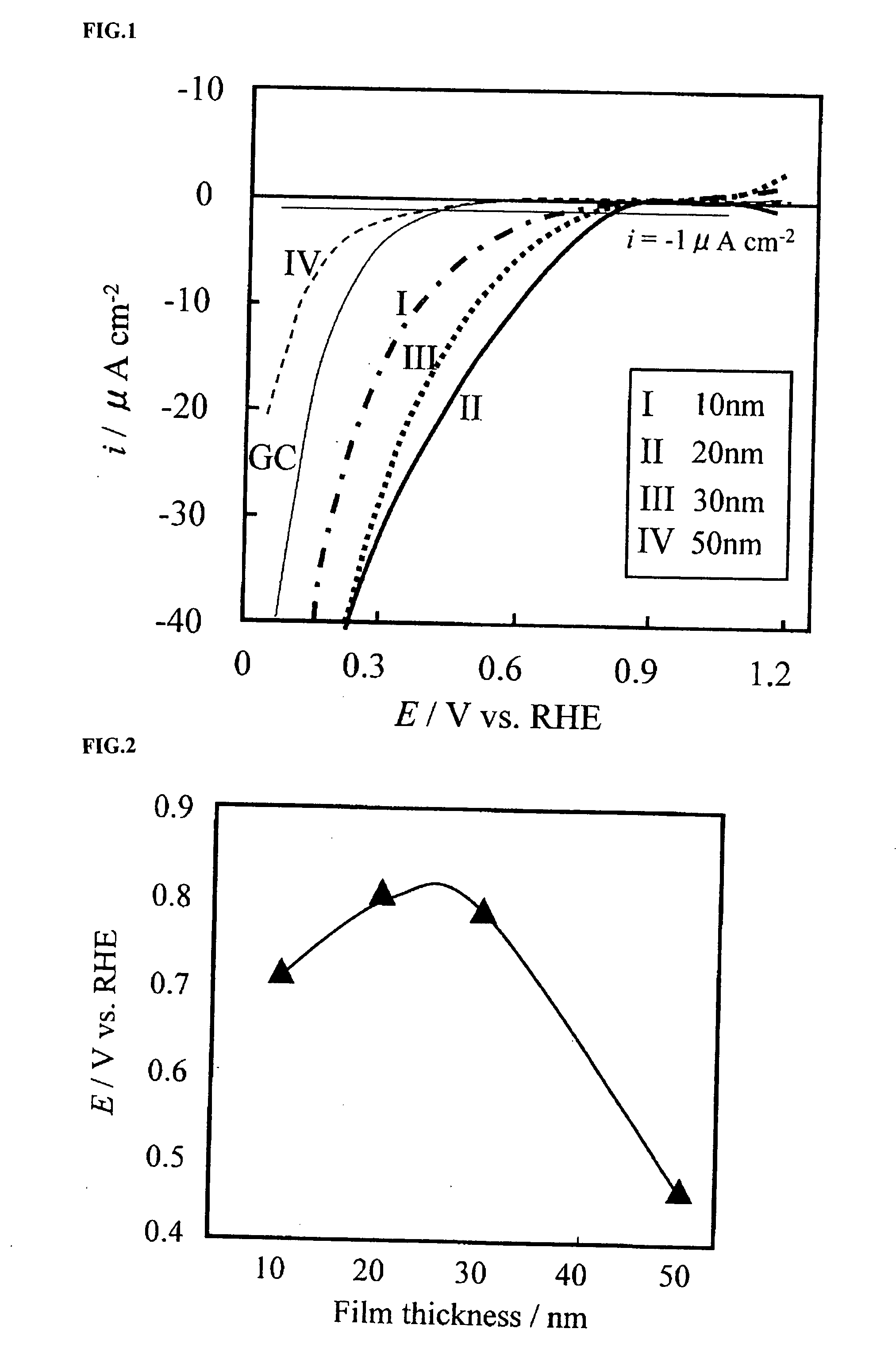
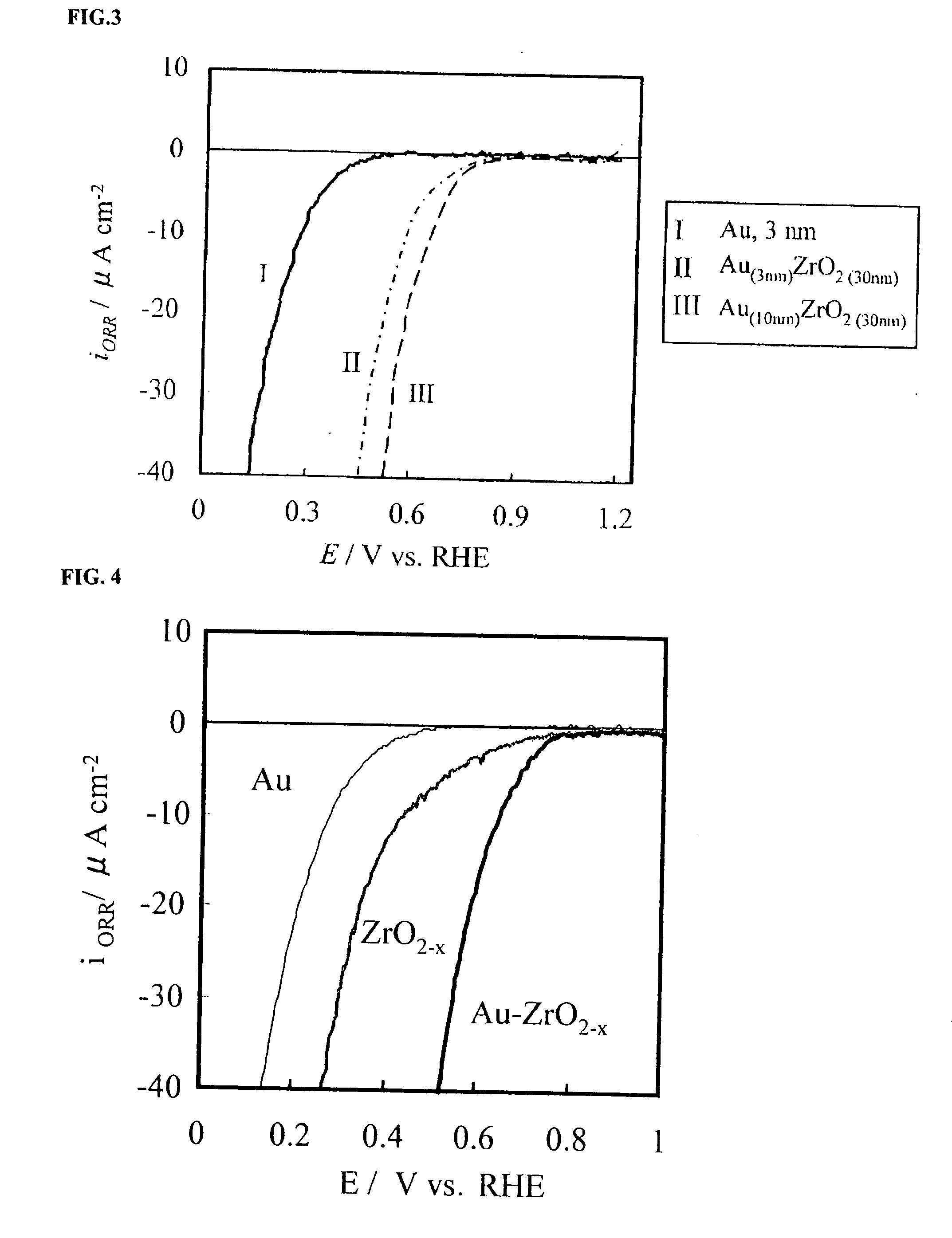
A corrosion-resistant electrode catalyst for oxygen reduction includes a main catalyst composed of at least one transition metal oxide selected from oxygen-deficient ZrO2, Ta2O5, Nb2O5, TiO2, V2O5, MoO3, and WO3 and a co-catalyst composed of gold. The electrode catalyst is used in contact with an acidic electrolyte at a potential at least 0.4 V higher than the reversible hydrogen electrode potential. The catalyst may be used, for example, in such a form that the transition metal oxide in the form of fine particles and gold in the form of fine particles, or fine particles including fine gold particles coated with the transition metal oxide are dispersed on a catalyst carrier which is an electron conductive powder. This electrode catalyst is suitable as an electrode catalyst for an electrochemical system using an acidic electrolyte in the fields of water electrolysis, inorganic / organic electrolysis, fuel cells, etc.
Owner:JAPAN SCI & TECH CORP
Metal oxynitride electrode catalyst
InactiveUS20070128884A1Cell electrodesSemiconductor/solid-state device manufacturingElectrode potentialElectrolysis
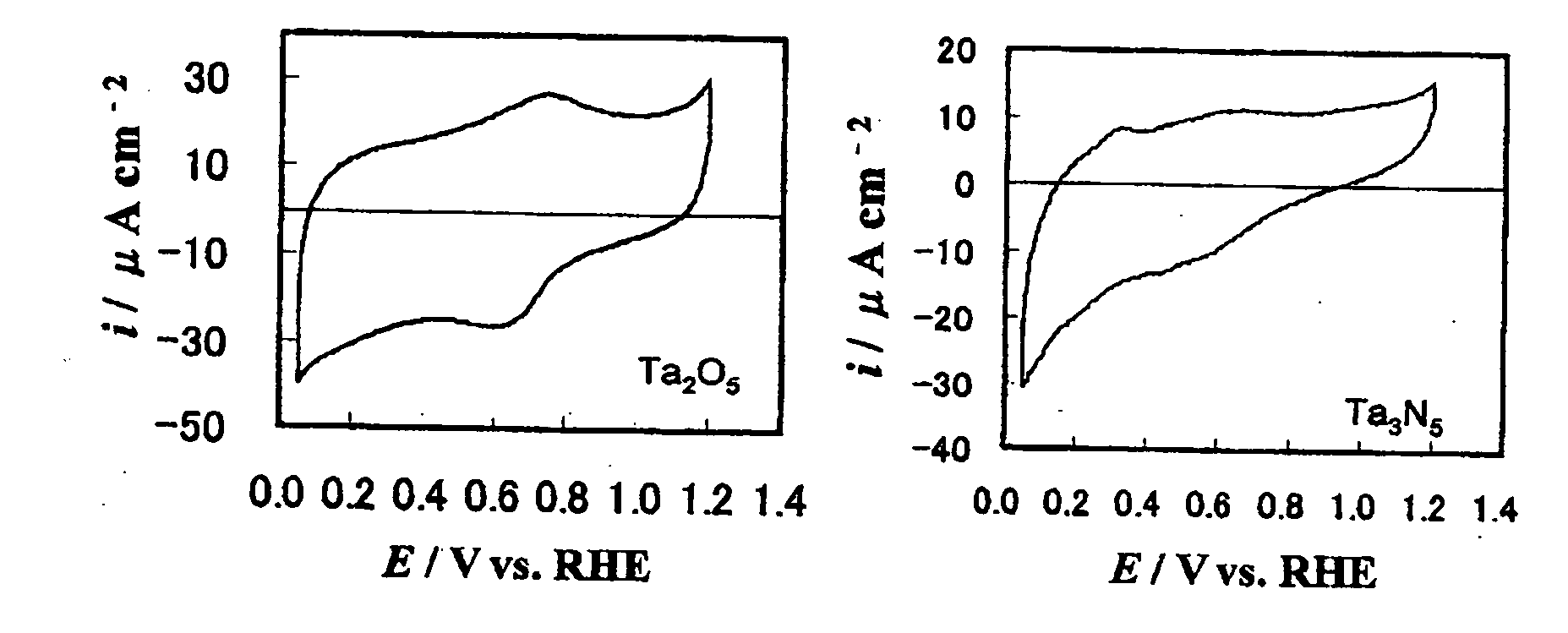
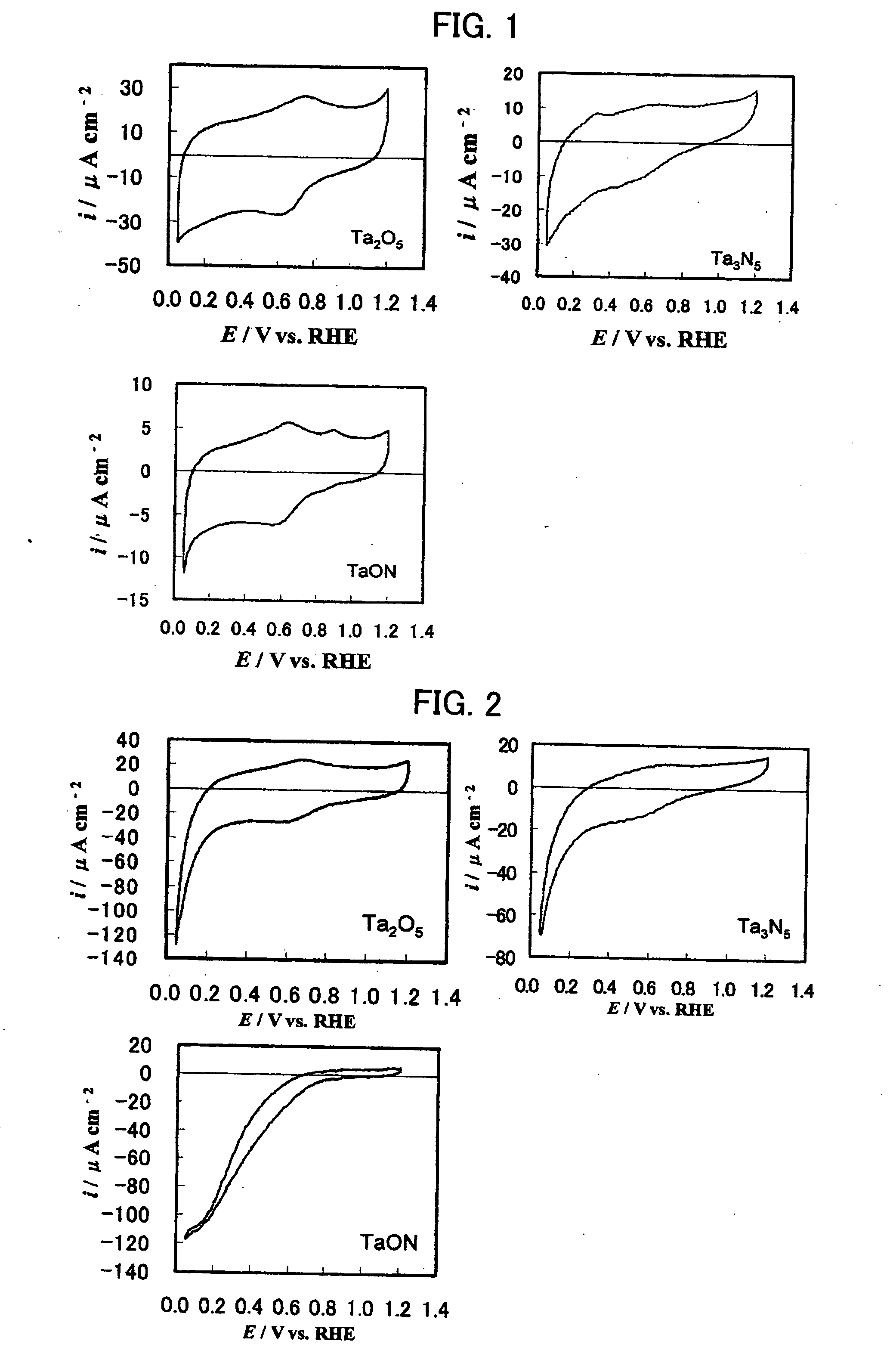
[Problems] Carbides and many other non-platinum-based compounds are activated and dissolved and cannot be stably present in an acidic electrolyte under conditions of an electrode potential as high as 0.4 V or above, and thus, the application range of these compounds as an electrode catalyst is limited to low electrode potentials. There has been need for development of an electrode catalyst that maintains catalytic activity under these conditions and exhibits improved stability. [Means for Solving Problems] To provide a metal oxynitride electrode catalyst composed of an oxynitride containing at least one transition metal element selected from the group consisting of La, Ta, Nb, Ti, and Zr, the metal oxynitride electrode catalyst being used at a potential of 0.4 V or higher relative to the reversible hydrogen electrode potential in an acidic electrolyte. The metal oxynitride electrode catalyst is useful as an electrode catalyst for electrochemical systems used in acidic electrolytes in the fields of water electrolysis, organic electrolysis, fuel cells, etc.
Owner:JAPAN SCI & TECH CORP
Metal oxynitride electrode catalyst
An electrode catalyst that maintains catalytic activity under conditions of an electrode potential as high as 0.4 V or above and exhibits improved stability. The metal oxynitride electrode catalyst is composed of an oxynitride containing at least one transition metal element selected from the group consisting of La, Ta, Nb, Ti, and Zr, the metal oxynitride electrode catalyst being used at a potential of 0.4 V or higher relative to the reversible hydrogen electrode potential in an acidic electrolyte. The metal oxynitride electrode catalyst is useful as an electrode catalyst for electrochemical systems used in acidic electrolytes in the fields of water electrolysis, organic electrolysis, fuel cells, etc.
Owner:JAPAN SCI & TECH CORP
Catalyst for preparing formic acid through efficient electrocatalytic reduction of carbon dioxide and preparation method thereof
ActiveCN110396701AKeep verticalMaintain sheet shapeMaterial nanotechnologyCatalyst activation/preparationChemical vapor depositionEnergy conversion efficiency
The invention relates to a catalyst for preparing formic acid through efficient electrocatalytic reduction of carbon dioxide and a preparation method thereof, and belongs to the technical field of electrocatalysis. According to the preparation method, SnS2 nanosheets are used as precursors, and the black porous SnO2 nanosheet catalyst for preparing formic acid through efficient electrocatalytic reduction of carbon dioxide is prepared through high-temperature calcination and annealing processes. The method specifically comprises the following steps: (1) preparing a SnS2 nanosheet precursor through chemical vapor deposition; (2) calcining the SnS2 precursor in air to obtain a white porous SnO2 nanosheet; and (3) annealing the white SnO2 nanosheet in a mixed atmosphere of hydrogen and argon to obtain the black porous SnO2 nanosheet. The catalyst for electrocatalytic reduction of carbon dioxide shows excellent performance of preparing formic acid by electrocatalytic reduction of carbon dioxide. Under the overpotential of 0.51 V, the voltage of the capacitor is reduced; the Faraday conversion efficiency of converting carbon dioxide into formic acid reaches 92.4%; the Faraday conversionefficiency of formic acid within the voltage range of -0.60 V to -1.10 V (relative to a reversible hydrogen electrode, RHE) can be stabilized at 90+ / -2%; and obtaining a current density of 11.4 + / - 0.3 mA / cm2 and a formic acid Faraday conversion efficiency of 90+ / -0.5% at -1.10 V (relative to the reversible hydrogen electrode, RHE) for at least 10 hours.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of Fe/N/C codoped electrocatalyst for efficient oxygen reduction reaction and application of Fe/N/C codoped electrocatalyst for efficient oxygen reduction reaction
The present invention belongs to the technical field of electro-catalysis, and especially relates to a preparation method of a Fe / N / C codoped electrocatalyst for efficient oxygen reduction reaction and application of a Fe / N / C codoped electrocatalyst for efficient oxygen reduction reaction. The composition of the Fe / N / C codoped electrocatalyst for efficient oxygen reduction reaction mainly comprises two steps consisting of a hydrothermal reaction step and a high-temperature pyrolysis step. The step 1 is that: carbon black, glucose, sodium dodecyl sulfate and a ferric salt dissolved into secondary water for ultrasonic treatment to form a uniform suspension liquid, and the mixed solution is put into a reaction kettle to perform the hydrothermal reaction to obtain black solid powder, namely aprecursor; and the step 2 is that: the precursor is fully mixed with a nitrogen source in a certain proportion for high-temperature calcinations in a nitrogen atmosphere to obtain a Fe / N / C codoped catalyst. According to the prepared catalyst, it can be seen that the obvious active particles are uniformly doped into a carbon skeleton, a related electrochemical testing result shows that the catalystcan effectively catalyze the oxygen reduction reaction (ORR) in an alkaline medium, the half-wave potential of the Fe / N / C codoped electrocatalyst reaches 821 mV (compared to a reversible hydrogen electrode) which is slightly lower than a commercial Pt / C with 857mV, and the Fe / N / C codoped electrocatalyst has the better stability and methanol tolerance performance than the commercial Pt / C.
Owner:QUFU NORMAL UNIV
Electrochemical reduction device and method for manufacturing hydride of aromatic hydrocarbon compound or nitrogen-containing heterocyclic aromatic compound
ActiveUS20150090602A1Improve efficiencyCellsPhotography auxillary processesGeneration ratePotential difference
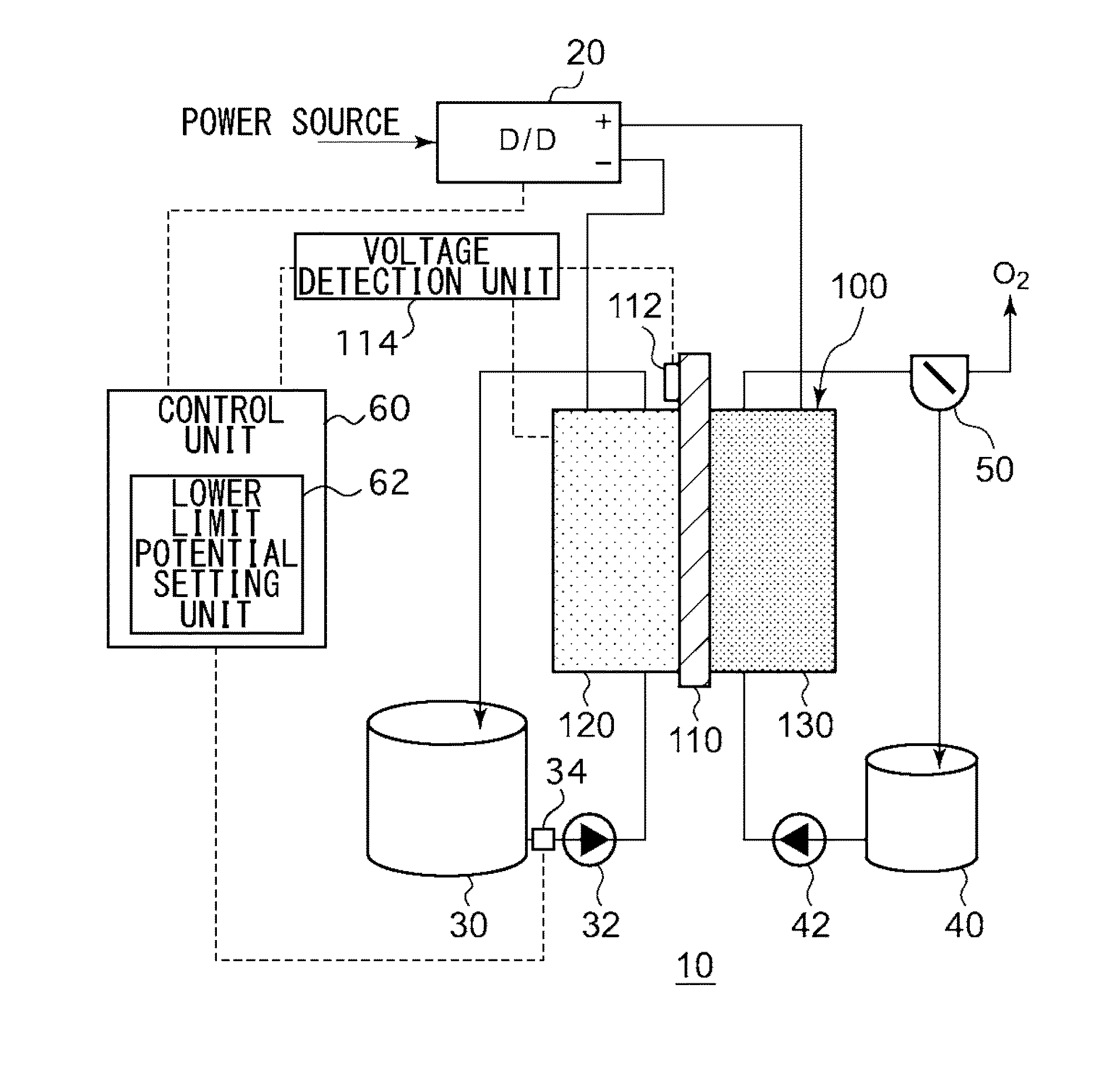
An electrochemical reduction device comprises an electrode unit including an electrolyte membrane, a reduction electrode, and an oxygen evolving electrode; a power control unit that applies a voltage Va between the reduction electrode and the oxygen evolving electrode; a hydrogen gas generation rate measurement unit that measures a hydrogen gas generation rate F1; and a control unit that controls the power control unit so as to gradually increase the Va within a range that satisfies a relationship of F1≦F0 and VCA>VHER−acceptable potential difference (APD), when the potential at a reversible hydrogen electrode is VHER, the potential of the reduction electrode is VCA, the acceptable upper limit of the hydrogen gas generation rate is F0, and the APD is a potential difference that defines an upper limit of a potential difference between VCA and VHER.
Owner:JXTG NIPPON OIL & ENERGY CORPORATION
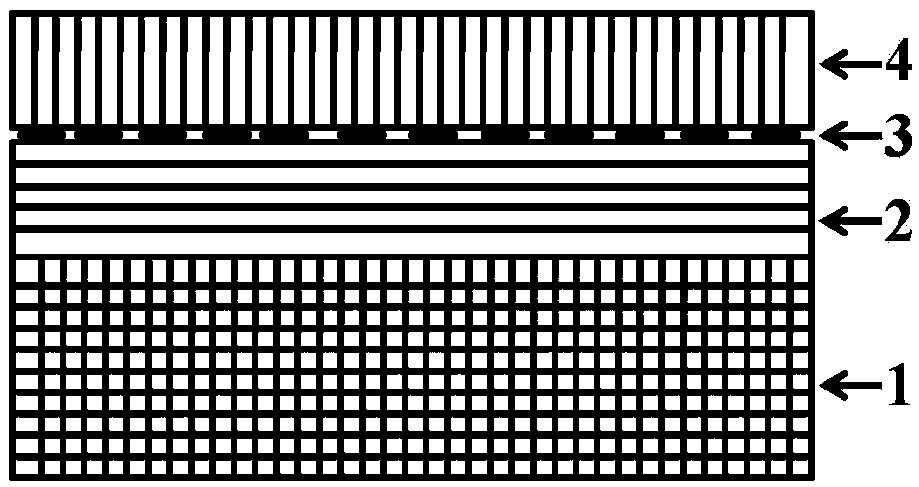
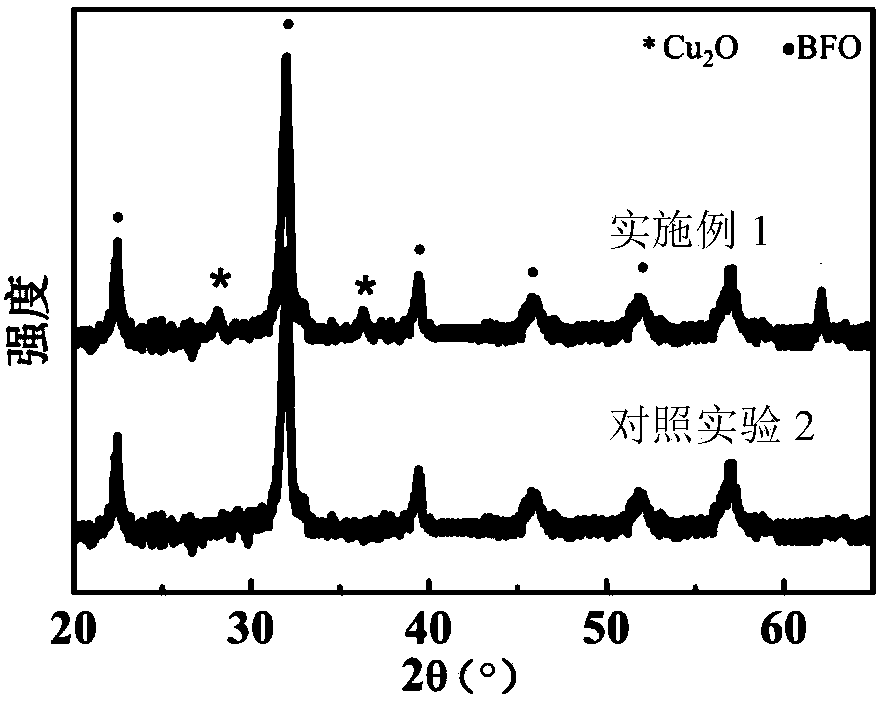
Ferroelectric composite Cu2O visible light water photolysis hydrogen production photocathode and preparing method thereof
ActiveCN108385131APrevent oxidationImprove stabilityVacuum evaporation coatingSputtering coatingHeterojunctionPhotocathode
The invention relates to a ferroelectric composite Cu2O visible light water photolysis hydrogen production photocathode and a preparing method thereof. The photocathode is sequentially composed of a BiFeO3 ferroelectric film layer, a gold nano-rod granular layer, a Cu2O film layer and a silicon wafer substrate from top to bottom, and heterojunctions are formed between the Cu2O film layer and the BiFeO3 ferroelectric film layer. According to the ferroelectric composite Cu2O visible light water photolysis hydrogen production photocathode and the preparing method of the photocathode, adverse effects of energy band barriers between the Cu2O film layer and the BiFeO3 ferroelectric film layer are eliminated through gold nano-rod granules by means of the LSPR effect, absorption of visible light with the wave length ranging from 650 nm-750 nm is strengthened, the Cu2O film layer is protected by the BiFeO3 ferroelectric film layer, residual polarization electric fields are used for eliminatingupwarp barriers on photoelectrode / electrolyte interfaces, the water photolysis efficiency and the hydrogen production efficiency are improved, the light current density reaches 91 microampere / cm<2>, and the threshold voltage relative to a reversible hydrogen electrode reaches 1.01 V.
Owner:SUZHOU TAIHU ELECTRIC ADVANCED MATERIAL CO LTD
Alpha-Fe2O3 photo-anode and preparation method thereof
ActiveCN104617355APromotes rapid oxidationTightly boundPhotoelectrochemical storage cellsDecompositionNon doped
The present invention relates to an alpha-Fe2O3 photo-anode and a preparation method thereof, the alpha-Fe2O3 photo-anode uses alpha-Fe2O3 as a light absorption layer, and uses Fe3O4, FeO and Fe as a conductive substrate. A direct high temperature fast oxidation method is used to prepare the photo-anode which comprises the conductive substrate and doped or non-doped alpha-Fe2O3, the doped or non-doped alpha-Fe2O3 as a light active layer is closely contacted with the conductive substrate, has good photoelectrocatalytic activity, under the AM1.5 standard test conditions, and relative to the reversible hydrogen electrode 1.23V, 0.63mA / cm2 can be achieved. Unlike publicly reported methods, the raw material of the method is easy to get, the whole photo-anode preparation process is fast, and large scale preparation is easy to realize. The photo-electrode can be used for the use of the decomposition of water for preparation of hydrogen, reduction of carbon dioxide, and the photocatalytic degradation of organic compounds, and the like.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI +1
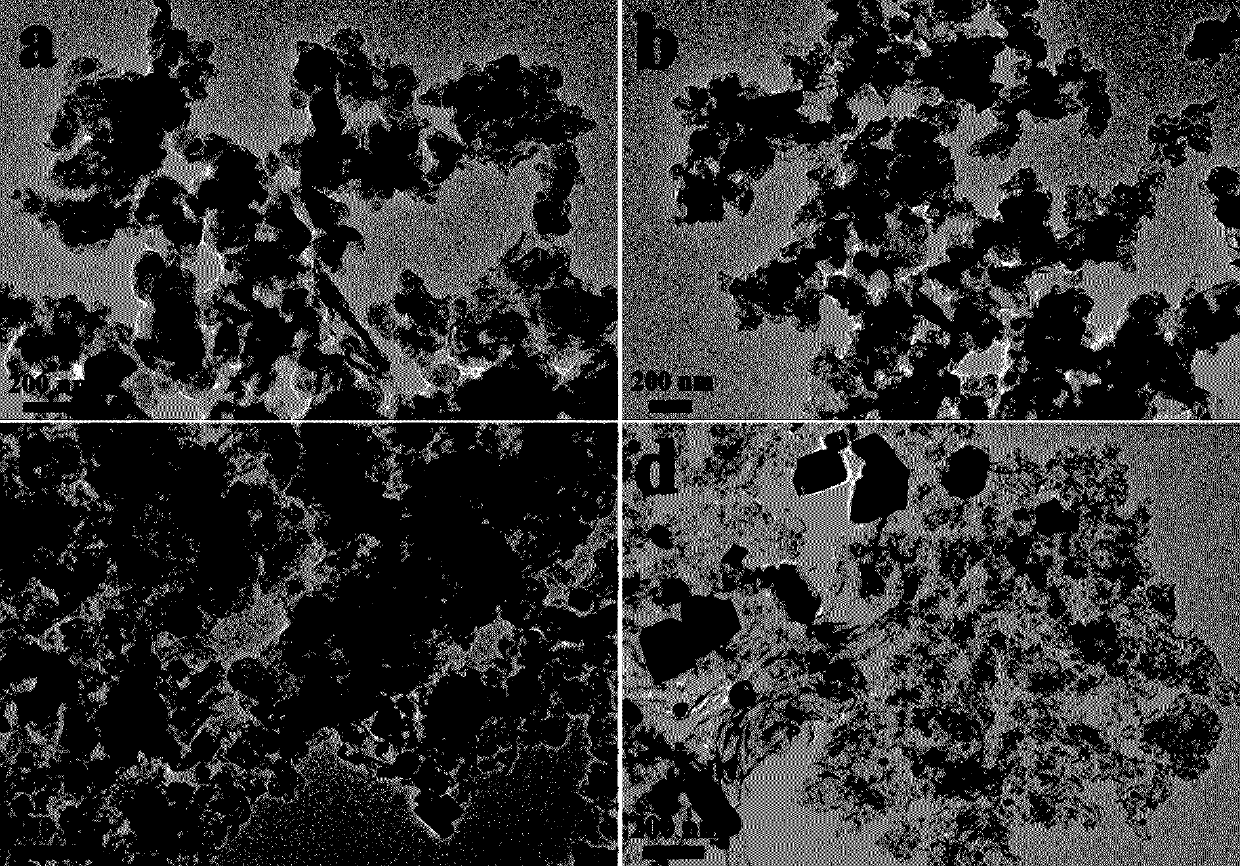
CuO nanosheet as well as preparation method and application thereof from top to bottom
The invention discloses a CuO nanosheet as well as a preparation method and application thereof. CuO nanosheets are prepared from top to bottom, that is to say , CuO with a nano structure is preparedfrom a copper material with a large size through ultrasonic etching and other technologies, and the specific operation method comprises: carrying out oil removal, acid pickling and water washing on the copper raw material, adding the copper raw material into a mixed solution of ammonium persulfate and sodium hydroxide, carrying out ultrasonic treatment, filtering, carrying out centrifugal separation on the filtrate, taking the precipitate, washing and drying to obtain a precursor; and grinding the precursor into powder, heating, and roasting to obtain the CuO nanosheet. The preparation methodprovided by the invention is simple in process and relatively low in cost, and the obtained product is uniform in morphology, good in repeatability and relatively high in electrocatalytic performance.The CuO nanosheet is used as a catalyst for electrocatalytic reduction of carbon dioxide, a reversible hydrogen electrode is used as a standard, the Faraday efficiency of a C<2<+>> product obtained through reduction at-0.97 V exceeds 60%, and high C<2<+>> product selectivity of a CuO electrocatalyst in carbon dioxide reduction is shown.
Owner:广东智达伊诺为科技有限公司
Preparation method for CQDs/GCNNs/Fe(2-x)TixO3/FTO photo-anode
The invention discloses a preparation method for a CQDs / GCNNs / Fe(2-x)TixO3 / FTO photo-anode. The preparation method comprises the steps that soluble trivalent molysite and urea are put in a hydrothermal environment with an organic titanium solution as a titanium source and FTO conducting glass as a carrier, so that Fe(2-x)TixO3 / FTO is prepared; and then, the Fe(2-x)TixO3 / FTO is treated through thetreatment method as specified, so that the CQDs / GCNNs / Fe(2-x)TixO3 / FTO photo-anode is obtained. The photoelectricity test indicates that after the prepared photo-anode is treated through the treatmentprocess, the photoelectric current density of CQDs / GCNNs / Fe(2-x)TixO3 / FTO is 3.38 mA / cm<2> at the voltage of 1.23 V relative to a reversible hydrogen electrode, the photoelectric current density of the Fe(2-x)TixO3 / FTO is 1.66 mA / cm<2> at the voltage of 1.23V relative to the reversible hydrogen electrode, and the photoelectric current density is increased by two times or more; and therefore, thephoto-anode and the treatment method thereof are one of advanced technologies through which water can be efficiently decomposed in a low-energy-consumption manner; the good industrial application prospect is achieved; and the preparation method can be used for easing the current situation that energy supply is getting tense day by day globally.
Owner:JILIN UNIV
Electrochemical reduction device and method for manufacturing hydride of aromatic compound
An electrochemical reduction device includes an electrode unit, a power control unit, an organic material storage tank, a concentration measurement unit, a water storage tank, a gas-water separation unit, and a control unit. The electrode unit includes an electrolyte membrane, a reduction electrode, and an oxygen evolving electrode. The control unit controls the power control unit so as to satisfy a relation of VHER−Vallow≦VCA≦VTRR when the potential at a reversible hydrogen electrode, the standard redox potential of the aromatic compound, and the potential of the reduction electrode are expressed as VHER, VTRR, and VCA, respectively. Vallow is adjusted according to the concentration of the aromatic compound measured by the concentration measurement unit.
Owner:ENEOS CORP
Catalytic oxygen evolution electrode and preparation method and application thereof
The invention belongs to the technical field of electrolysis water catalyzation, and discloses a catalytic oxygen evolution electrode and a preparation method and application thereof. The catalytic oxygen evolution electrode is prepared in a manner that the voltage equivalent to -0.8 V--0.4 V of the voltage of a saturated Ag / AgCl reference electrode is applied in an electrolyte and the constant-pressure treatment time ranges from 30 s to 1800 s. According to the method that the catalytic oxygen evolution electrode can be used for reducing electrolysis water energy consumption, the catalytic activity of a noble metal catalyst (RuO2, IrO2), a metal catalyst (Co3O4) and a double perovskite material PrBa0.5Sr0.5Co1.5Fe0.5O5+alpha nanowire is improved; the treatment method is simple and practicable, and in the electrode preparation process, the shape and size of the electrode can be controlled; and the oxygen evolution performance of the catalytic oxygen evolution electrode prepared by themethod under the voltage ranging from 1 V to 2 V (relative to a reversible hydrogen electrode) is greatly improved, and application prospects are good.
Owner:SOUTH CHINA UNIV OF TECH
Electrochemical reduction device, and method for producing hydrogenated product of aromatic hydrocarbon compound or nitrogen-containing heterocyclic aromatic compound
InactiveCN104204304AEfficient Electrochemical Hydrogenation of GeonucleiCellsElectrolytic organic reductionWater storage tankOxygen
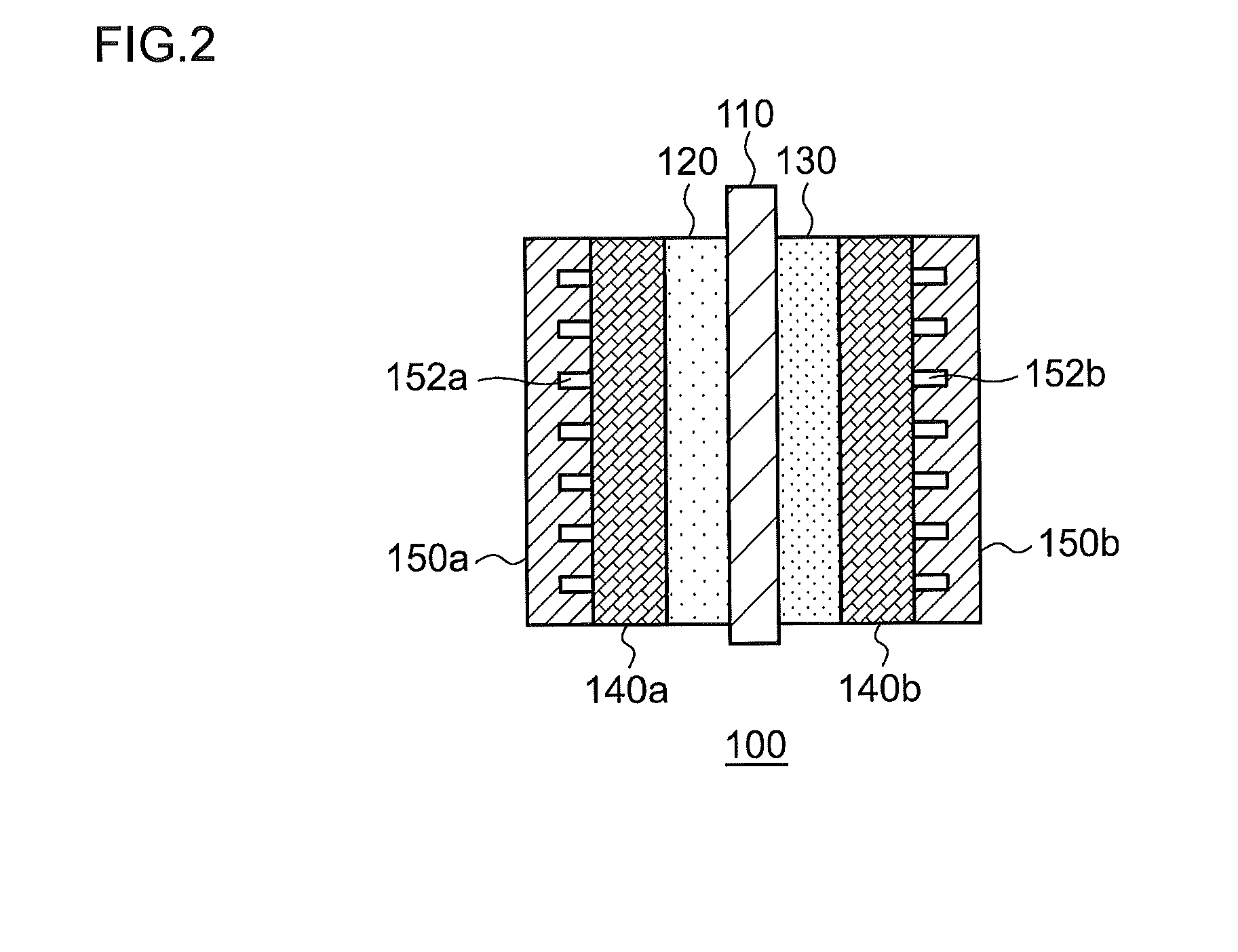
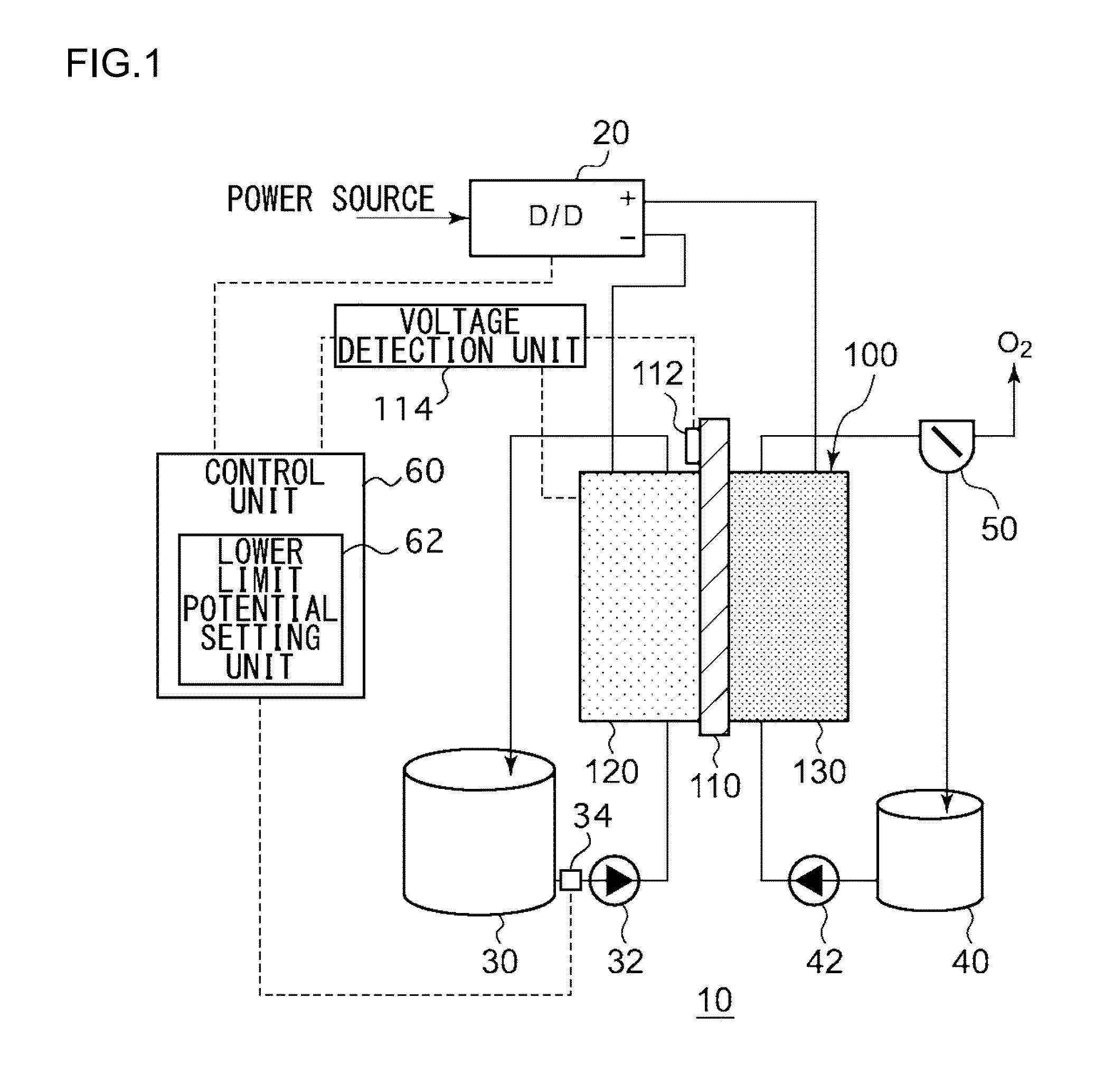
This electrochemical reduction device (10) is provided with an electrode unit (100), a power control unit (20), an organic matter storage tank (30), a water storage tank (40), a steam separator (50) and a control unit (60). This electrode unit (100) has an electrolyte film (110), a reduction electrode (120) and an oxygen-generating electrode (130). The electrolyte film (110) is formed from ionomers. The reduction catalyst used in the reduction electrode (120) contains at least one of Pt and Pd. This oxygen-generating electrode (130) contains a noble metal oxide catalyst such as RuO2, IrO2, etc. Representing the potential in the reversible hydrogen electrode as VHER, the standard redox potential of an aromatic hydrocarbon compound or nitrogen-containing heterocyclic aromatic compound as VTRR, and the potential of the reduction electrode (120) as VCA, the control unit (60) controls the power control unit (20) such that the relation VHER-20mV @ VCA @ VTRR is satisfied.
Owner:JX NIPPON OIL & ENERGY CORP
Electrochemical reduction device and method for manufacturing hydride of aromatic hydrocarbon compound or n-containing heterocyclic aromatic compound
InactiveUS20150008138A1Improve efficiencyCellsMachining electric circuitsWater storage tankElectrochemistry
An electrochemical reduction device is provided with an electrode unit, a power control unit, an organic material storage tank, a water storage tank, a gas-liquid separator, and a control unit. The electrode unit has an electrolyte membrane, a reduction electrode, and an oxygen evolving electrode. The electrolyte membrane is formed of an ionomer. A reduction catalyst used for the reduction electrode contains at least one of Pt and Pd. The oxygen evolving electrode contains catalysts of noble metal oxides such as RuO2, IrO2, and the like. The control unit controls the power control unit such that a relationship, VHER−20 mV≦VCA≦VTRR, can be satisfied when the potential at a reversible hydrogen electrode, the standard redox potential of an aromatic hydrocarbon compound or an N-containing heterocyclic aromatic compound, and the potential of the reduction electrode are expressed as VHER, VTRR, and VCA, respectively.
Owner:JX NIPPON OIL & ENERGY CORP
Corrosion resistant metal oxide electrode catalyst for oxygen reduction
InactiveUS7919215B2Reduced activityImprove corrosion resistanceActive material electrodesAqueous electrolyte fuel cellsElectrolysisFuel cells
A corrosion-resistant electrode catalyst for oxygen reduction includes a main catalyst composed of at least one transition metal oxide selected from oxygen-deficient ZrO2, Ta2O5, Nb2O5, TiO2, V2O5, MoO3, and WO3 and a co-catalyst composed of gold. The electrode catalyst is used in contact with an acidic electrolyte at a potential at least 0.4 V higher than the reversible hydrogen electrode potential. The catalyst may be used, for example, in such a form that the transition metal oxide in the form of fine particles and gold in the form of fine particles, or fine particles including fine gold particles coated with the transition metal oxide are dispersed on a catalyst carrier which is an electron conductive powder. This electrode catalyst is suitable as an electrode catalyst for an electrochemical system using an acidic electrolyte in the fields of water electrolysis, inorganic / organic electrolysis, fuel cells, etc.
Owner:JAPAN SCI & TECH CORP
Electrode catalyst for fuel cell, method of preparing the same, and membrane electrode assembly and fuel cell, each including the same
InactiveUS20140162169A1High catalytic activityActive material electrodesCatalyst activation/preparationFuel cellsCerium
Owner:SAMSUNG ELECTRONICS CO LTD
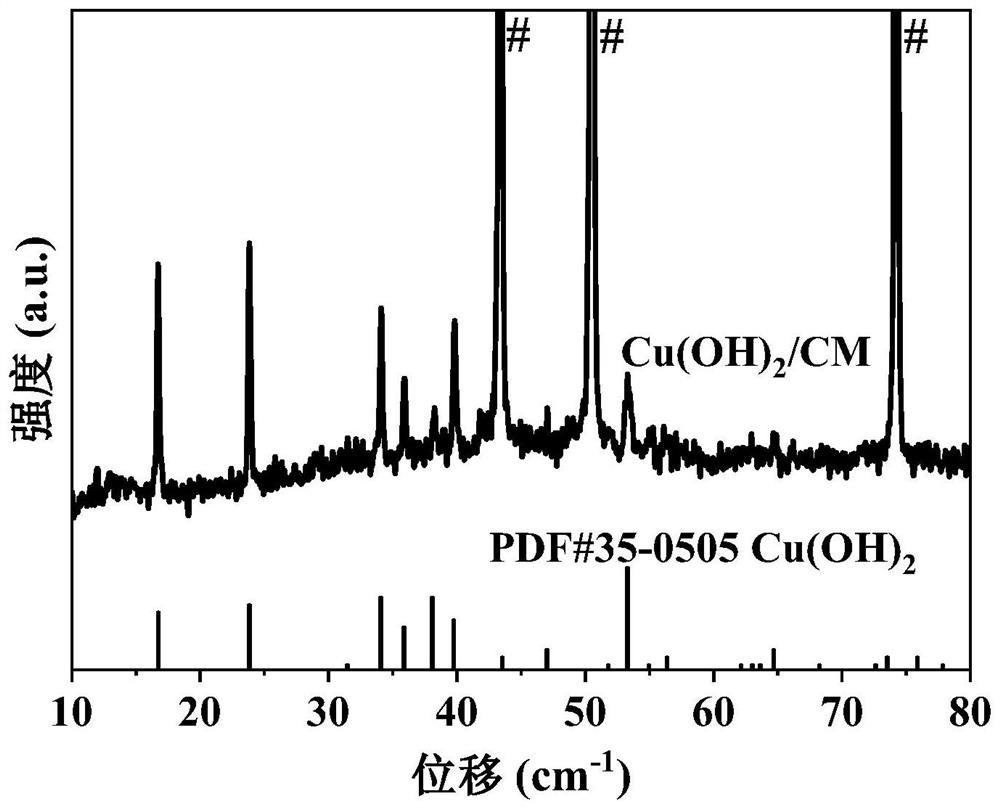
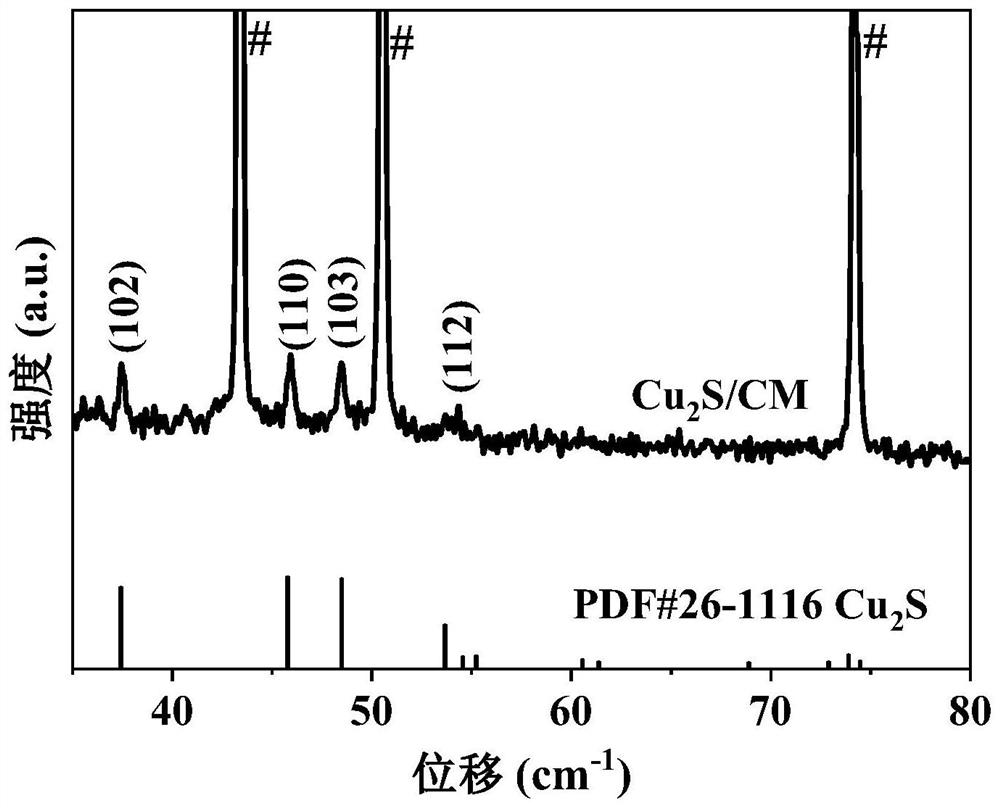
Nanowire structure copper/cuprous sulfide/copper mesh electrode material and preparation method and application thereof
ActiveCN114381750AFacilitates dimerizationLower energy barrierElectrolytic organic productionElectrodesCapacitanceCuprous sulfide
The invention provides a preparation method and application of a nanowire-structured copper / cuprous sulfide / copper mesh electrode material, the method takes a copper mesh as a substrate, cuprous sulfide nanorods grow on the copper mesh substrate by chemical oxidation and gas phase vulcanization methods to obtain an electrode material, and the electrode material is subjected to electrochemical reduction to obtain the nanowire-structured copper / cuprous sulfide / copper mesh electrode material. A copper / cuprous sulfide nanowire / copper mesh electrode is formed, and the surface chemistry is shown as Cu / Cu2S / CM; wherein Cu / Cu2S is of a nanowire net structure. The material is characterized in that the copper / cuprous sulfide nanowires grown on the copper mesh are uniformly distributed, and the copper / cuprous sulfide nanowires have abundant elemental copper and cuprous sulfide interfaces. The electrode material is used for generating ethanol through electrocatalytic reduction of carbon dioxide and has good catalytic performance, and under the overpotential of-0.8 V (vs RHE, relative to a reversible hydrogen electrode), the initial current can reach 12.8-13.5 mA cm <-2 >, the selectivity of ethanol reaches 9.8-13.8%, the yield of ethanol reaches 982.5-987.3 [mu] mol.L <-1 >. H <-1 >, and the double-electric-layer capacitance value of the electrode reaches 19.18-20.50 mF cm <-1 >.
Owner:BEIJING UNIV OF CHEM TECH
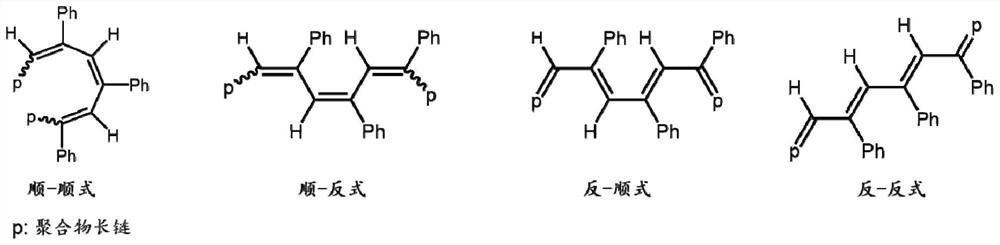
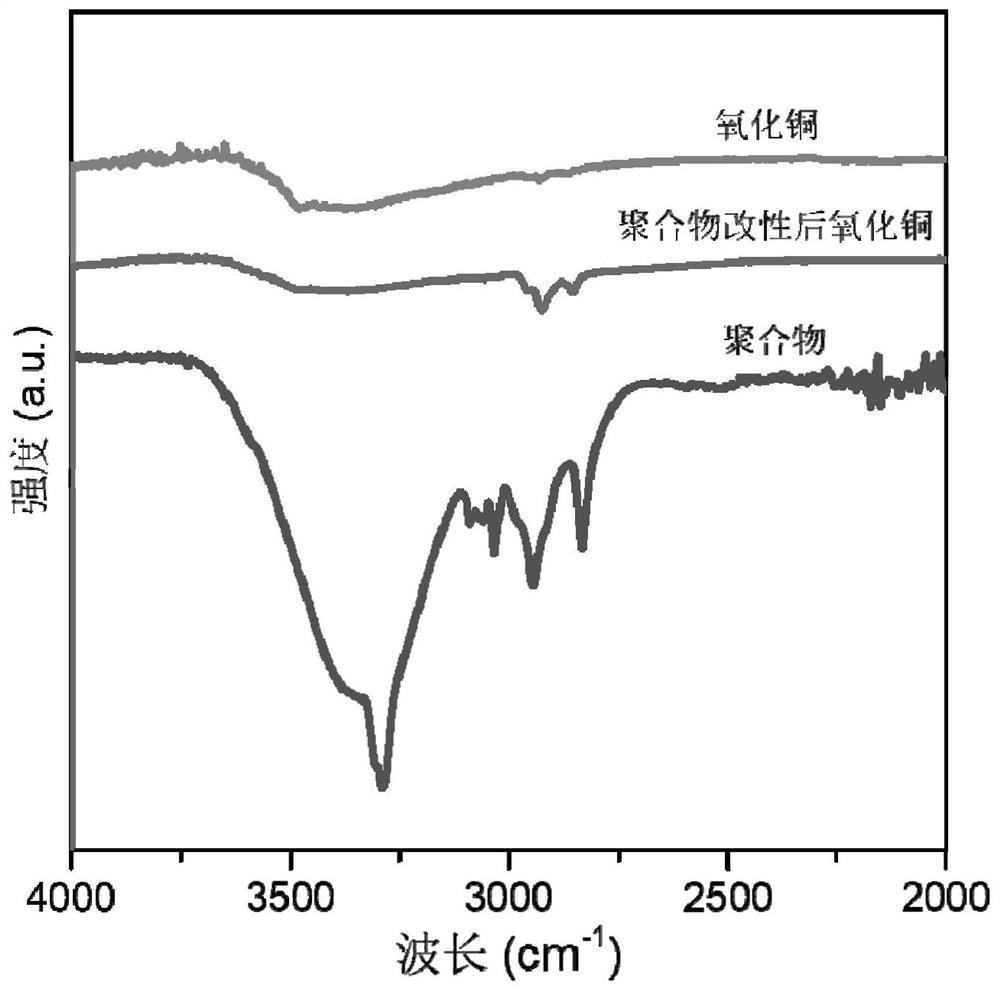
Conjugated conductive polymer modified copper-based catalyst as well as preparation method and application thereof
ActiveCN114277402AUniform modificationGood repeatabilityElectrolytic organic productionElectrodesElectrolysisPtru catalyst
The invention provides a conjugated conductive polymer modified copper-based catalyst and a preparation method and application thereof.The surface of the conjugated conductive polymer modified copper-based catalyst is covered with a polymer layer with the thickness of 2-5 nm, and the hydrophobicity and conductivity of an electrode material can be remarkably improved; the obtained catalyst is good in stability, a polymer layer is not prone to falling off, the Faraday efficiency of electrocatalysis is larger than 82% and the Faraday efficiency of a C2 + product is larger than 70% when the catalyst serves as the catalyst for electrocatalysis of CO2 reduction and a reversible hydrogen electrode serves as the standard under the electrolysis voltage of-0.93 volts, and excellent product selectivity and high conversion efficiency are shown.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method and application of nitrogen and oxygen co-doped carbon material containing 3d metal single atom
ActiveCN114180549ASimple processLow costCarbon preparation/purificationElectrodesPtru catalystHigh activity
The invention relates to a preparation method and application of a nitrogen and oxygen co-doped carbon material containing 3d metal monatomic, and the method comprises the following steps: mixing ethylenediaminetetraacetic acid or derivatives and hydroxides of ethylenediaminetetraacetic acid and 3d metal salts, calcining at high temperature, centrifugally washing, and drying to obtain the nitrogen and oxygen co-doped carbon material containing 3d metal monatomic. The method is simple and easy to implement, saves energy and is low in cost, the prepared carbon material is uniform in morphology and good in repeatability, the carbon material shows good electro-catalysis performance, when the carbon material serves as an electro-catalyst for producing hydrogen peroxide through oxygen reduction, the electro-catalysis initial potential of the carbon material reaches 0.75 V compared with a reversible hydrogen electrode, the molar selectivity exceeds 95%, and the carbon material has a good application prospect. And the catalyst shows high activity and high selectivity in a reaction for producing hydrogen peroxide through oxygen reduction.
Owner:SOUTH CHINA UNIV OF TECH
An evaluation device and evaluation method for the performance of solid polymer electrolyte fuel cells and water electrolysis electrocatalysts
ActiveCN106596686BHigh simulationReduce wasteMaterial analysis by electric/magnetic meansElectricityPolymer electrolytes
The invention relates to an evaluation device and evaluation method of an electrocatalyst property used for a solid polymer electrolyte fuel cell and water electrolysis, and belongs to the technical field of electrocatalyst activity evaluation. The problem that environment at which the electrocatalyst is located is different from actual application in a currently widely adopted liquid three-electrode system and the technical problem that evaluation the technology is tedious, the amount the needed catalyst is high, the cost is high and the influence factor is complex in an assembled battery evaluation method. The evaluation device microminiaturizes a membrane electrode assembly, a reversible hydrogen electrode is added as a reference electrode to test an electrocatalytic property, the advantages of the currently widely adopted liquid three-electrode system and a membrane electrode catalytic property are combined, and the catalyst is made to be located in the solid polymer electrolyte fuel cell to test the electrocatalyst property of the solid polymer electrolyte and better simulates the catalytic property in an actual using environment. The evaluation method can accurately and effectively evaluate the electrocatalyst property used for the polymer electrolyte fuel cell and the water electrolysis.
Owner:SHANDONG SAIKESAISI HYDROGEN ENERGY
Copper porphyrin complex for electro-catalysis oxygen evolution reaction and preparation method of copper porphyrin complex
InactiveCN107118217ASimple reaction conditionsRapid responseOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsSpace groupCopper nitrate
The invention discloses a copper porphyrin complex for electro-catalysis oxygen evolution reaction and a preparation method of the copper porphyrin complex, and in particular discloses a metal-organic complex which is concise, efficient and green at relatively low temperature and is prepared on the basis of a 5,10,15,20-tert-cyan phenyl porphyrin organic ligand, a preparation method, and application of the complex in electro-catalysis oxygen evolution reaction. The 5,10,15,20-tert-cyan phenyl porphyrin organic ligand and copper nitrate trihydrate are adopted as raw materials, the chemical formula of the obtained copper porphyrin complex is [C108Cu2N20O4H0.25], the complex belongs to a monoclinic system and a space group of P2 / a, the cell parameters includes the axial length a being 15.3680(2) angstroms, b being 17.1634(2) angstroms, c being 33.5945(6) angstroms, the axial angle alpha being 90.00 degrees, beta being 93.8488(13) degrees, and gamma being 90.00 degrees, and the unit cell volume V is 8841.2(2) angstrom 3. The copper porphyrin complex disclosed by the invention is good in catalysis property in the electro-catalysis oxygen evolution reaction, the oxygen evolution overpotential of the copper porphyrin complex is 1.66v (vs.RHE is relative to a reversible hydrogen electrode) when the current density is 10mA / cm<2>. The preparation method is simple in process, only organic solvents are used, the copper porphyrin complex can be recycled, and no wastes are discharged.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
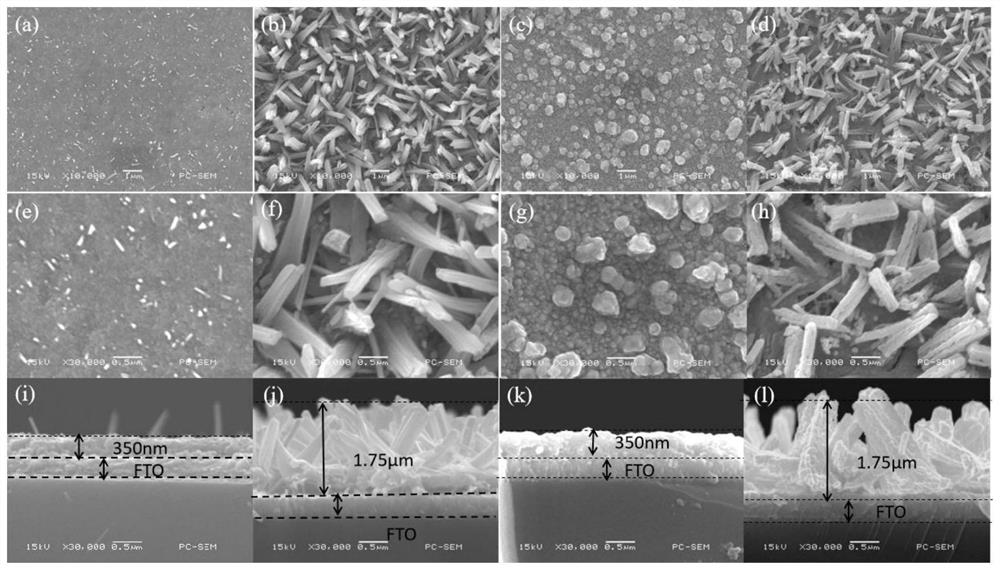
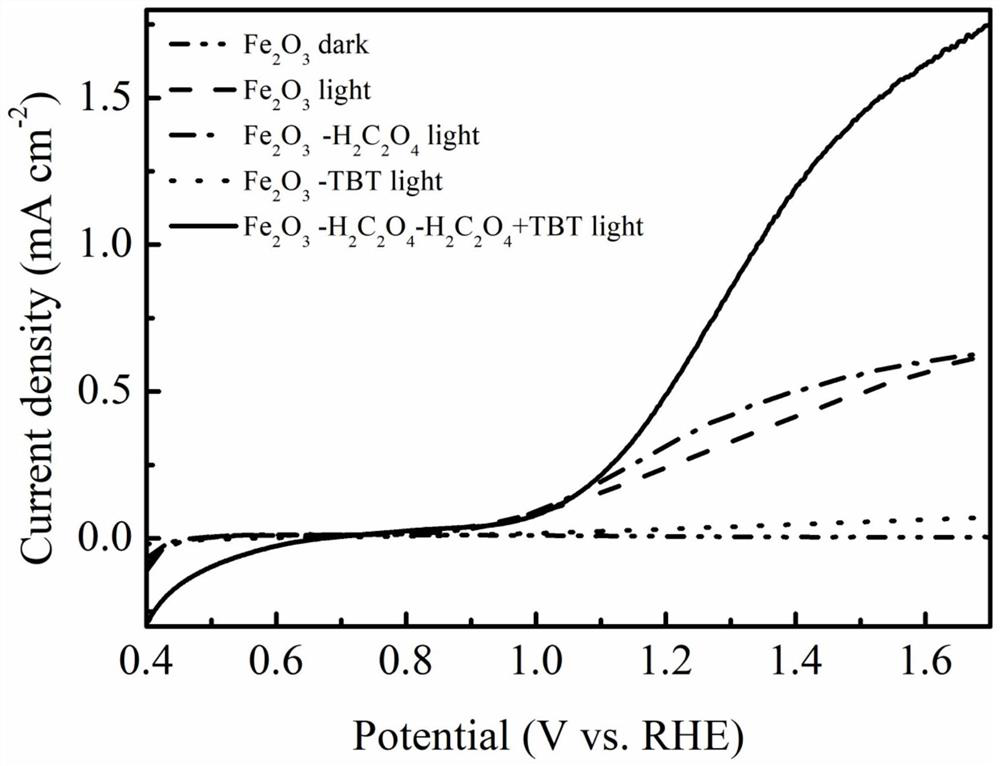
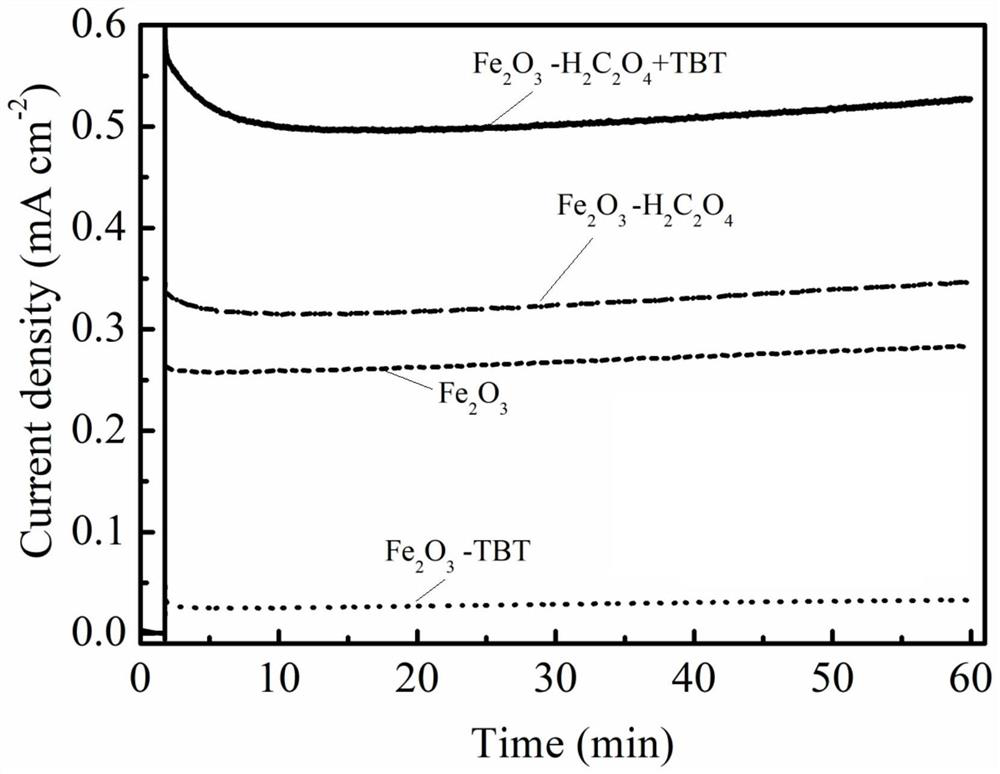
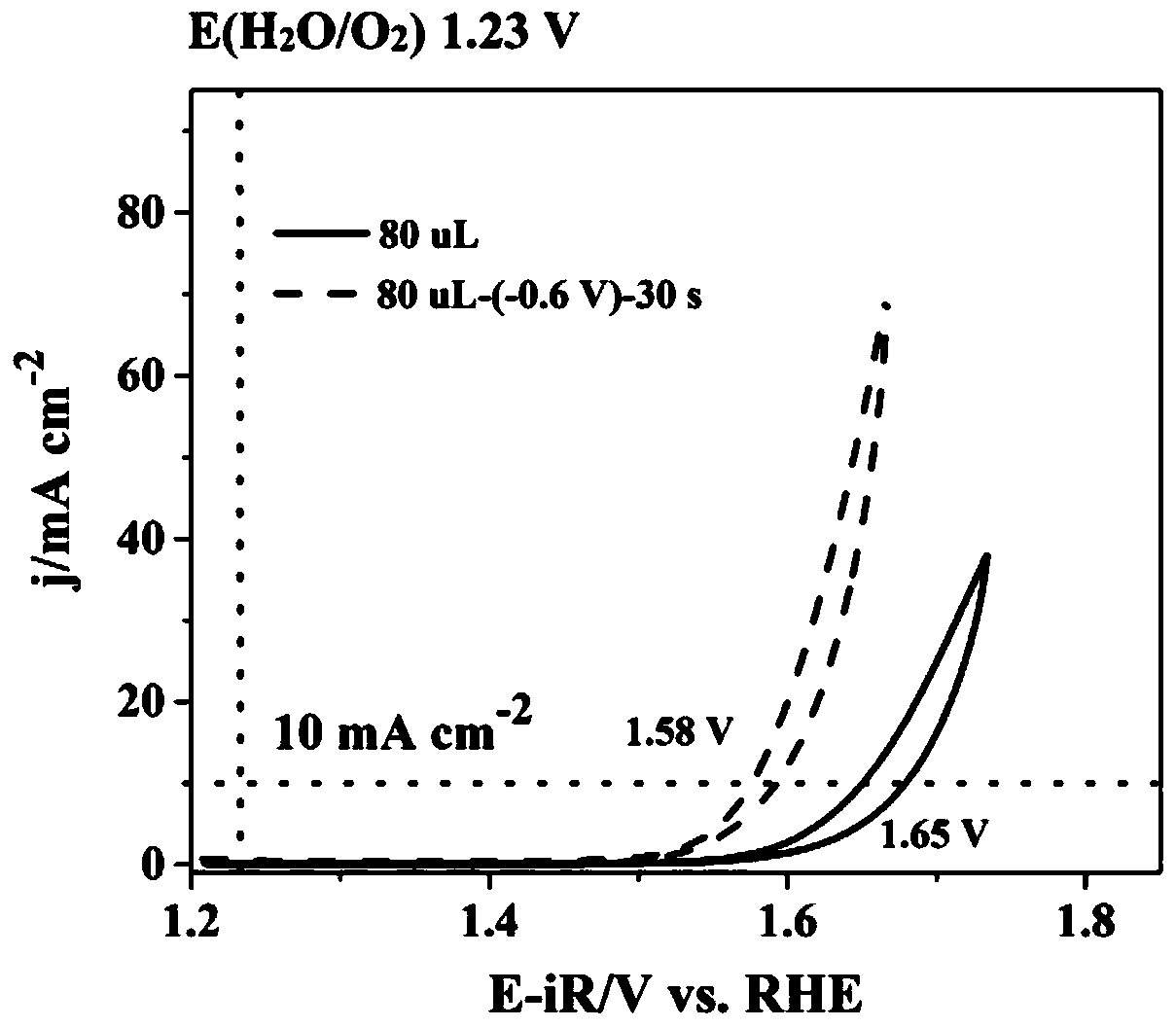
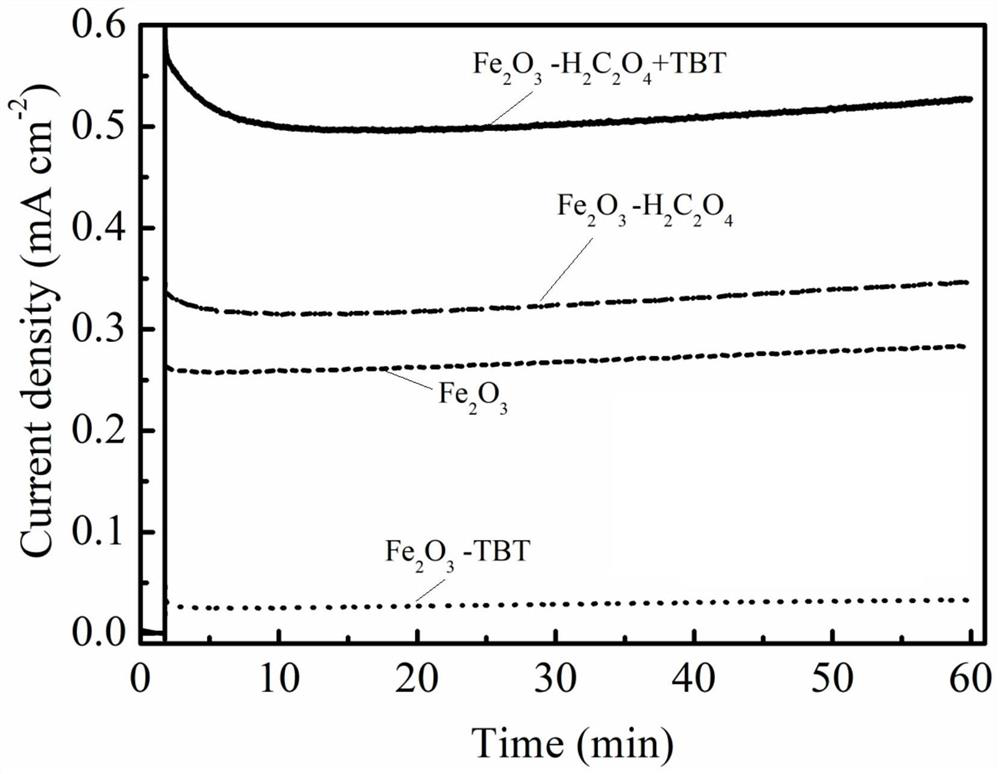
Method for preparing iron oxide photoanode under combined action of oxalic acid and tetrabutyl titanate
ActiveCN112038103AAchieve conversionImprove performanceLight-sensitive devicesPhotovoltaic energy generationOXALIC ACID DIHYDRATEChemical reaction
The invention relates to a method for preparing an iron oxide photoanode under the combined action of oxalic acid and tetrabutyl titanate. The method comprises the following steps: carrying out simpleelectrodeposition to obtain an an iron film, soaking the iron film in an oxalic acid (H2C2O4) ethanol solution while heating, carrying out ultrasonic soaking with tetrabutyl titanate (TBT), and carrying out annealing treatment to obtain the Fe2O3-H2C2O4+TBT photoanode. Due to oxalic acid and tetrabutyl titanate, the specific surface area of ferric oxide is increased, electron hole recombination is reduced, and the photoelectric property of ferric oxide is improved. Under AM1.5 standard test conditions, the photocurrent density can reach 0.59 mA cm<2> at 1.23 V relative to a reversible hydrogen electrode. Different from a reported method, the method of the invention has the advantages that raw materials are cheap and easy to obtain, the whole photoelectrode preparation process is fast, andlarge-batch preparation is easy. Different from a conventional method for controlling morphology, the method of the invention is completed by simple impregnation and chemical reaction.
Owner:CHANGZHOU UNIV
A kind of catalytic oxygen evolution electrode and its preparation method and application
The invention belongs to the technical field of electrolysis water catalyzation, and discloses a catalytic oxygen evolution electrode and a preparation method and application thereof. The catalytic oxygen evolution electrode is prepared in a manner that the voltage equivalent to -0.8 V--0.4 V of the voltage of a saturated Ag / AgCl reference electrode is applied in an electrolyte and the constant-pressure treatment time ranges from 30 s to 1800 s. According to the method that the catalytic oxygen evolution electrode can be used for reducing electrolysis water energy consumption, the catalytic activity of a noble metal catalyst (RuO2, IrO2), a metal catalyst (Co3O4) and a double perovskite material PrBa0.5Sr0.5Co1.5Fe0.5O5+alpha nanowire is improved; the treatment method is simple and practicable, and in the electrode preparation process, the shape and size of the electrode can be controlled; and the oxygen evolution performance of the catalytic oxygen evolution electrode prepared by themethod under the voltage ranging from 1 V to 2 V (relative to a reversible hydrogen electrode) is greatly improved, and application prospects are good.
Owner:SOUTH CHINA UNIV OF TECH
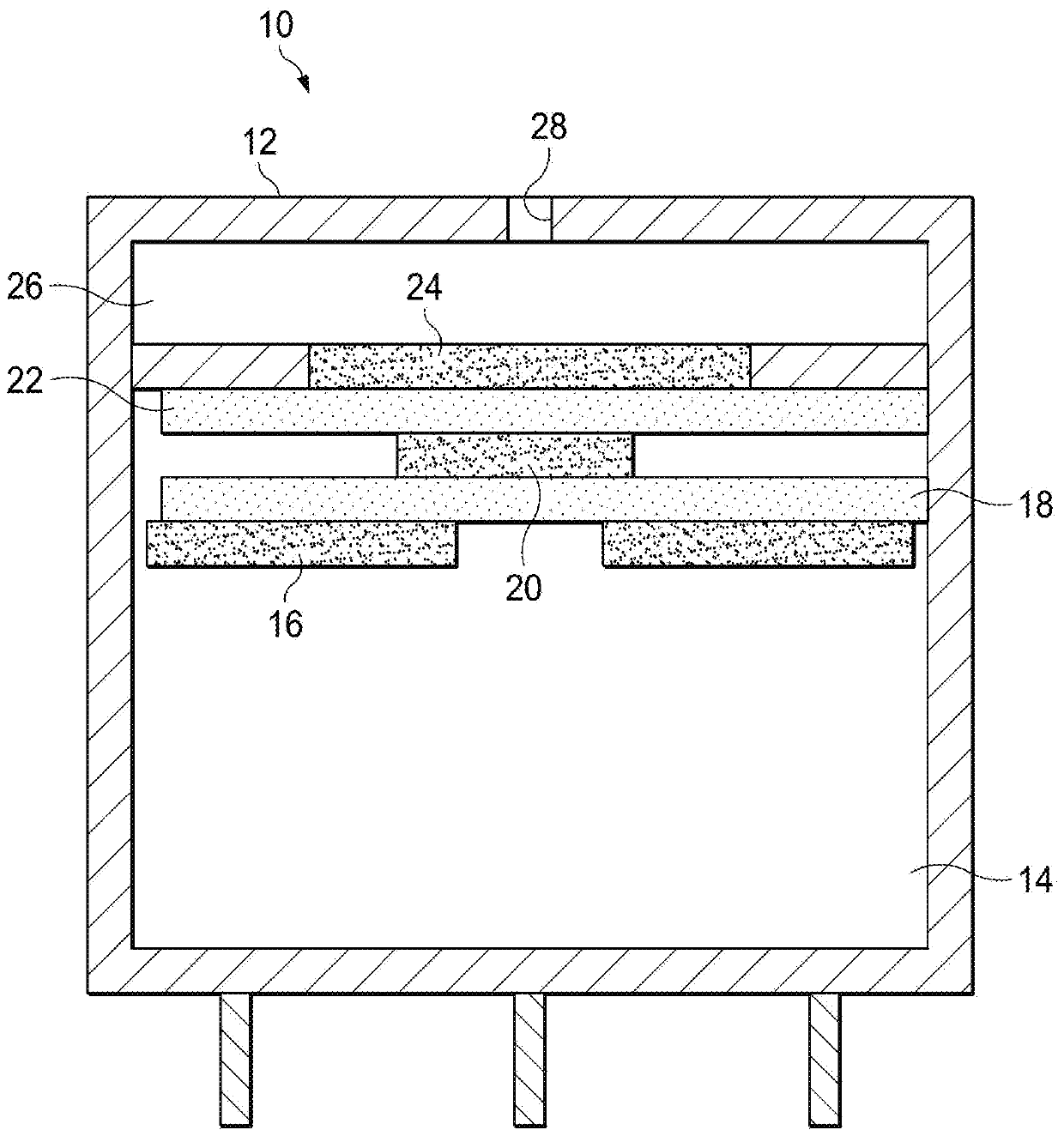
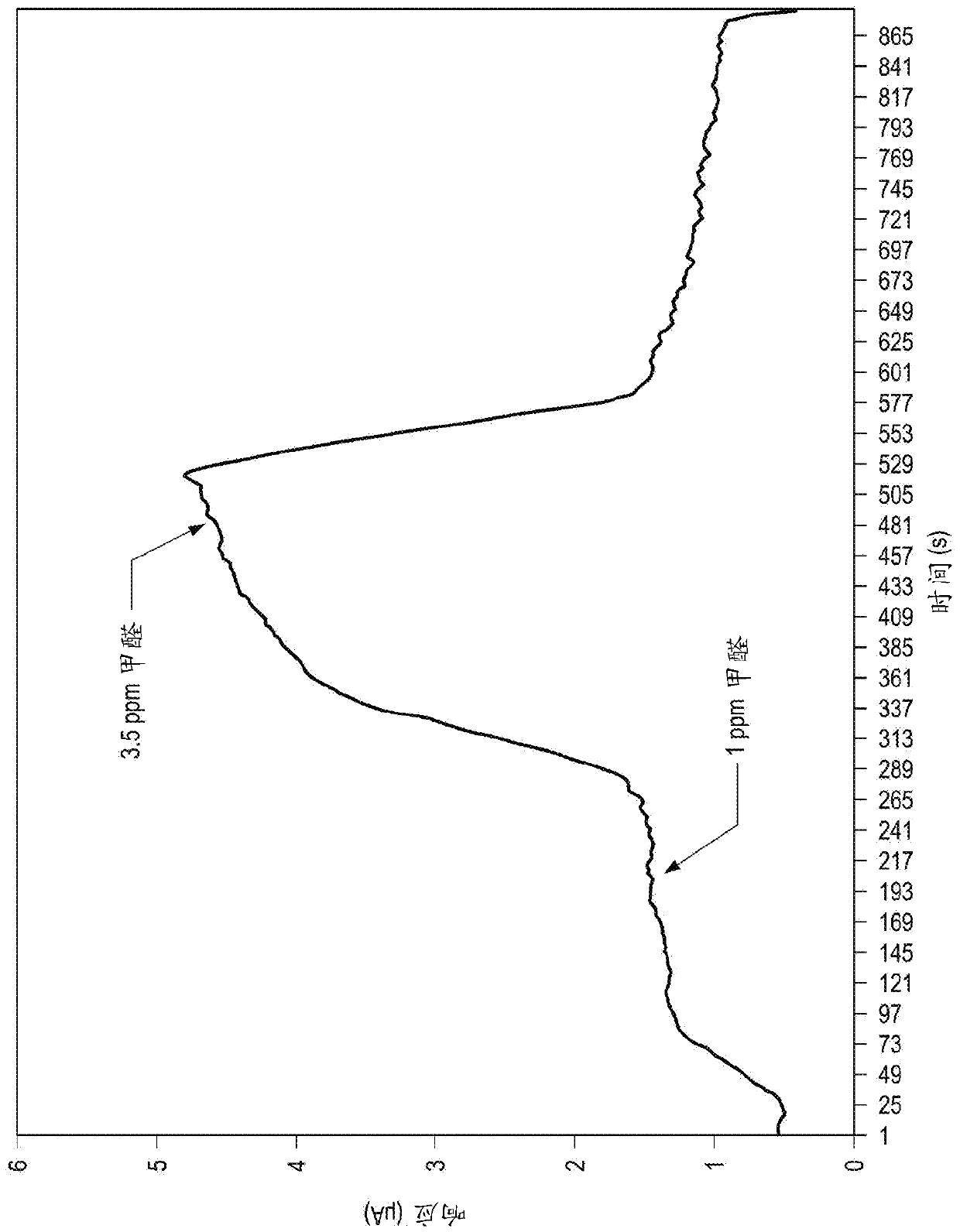
Improved electrochemical sensor and method for detecting formaldehyde by regulating voltage to reduce cross-sensitivity
An electrochemical formaldehyde sensor (10) and a method of detecting formaldehyde are disclosed. The electrochemical formaldehyde sensor (10) may comprise a housing (12); an electrolyte disposed within the housing (12); and a plurality of electrodes in contact with the electrolyte within the housing (12), wherein the plurality of electrodes comprise a working electrode (24) comprising iridium andan applied potential of between approximately 0.9 to 1 V relative to a reversible hydrogen electrode; and a counter electrode (16). The cross-sensitivity of the sensor (10) to alcohols is less than approximately 25% of the sensitivity to formaldehyde.
Owner:HONEYWELL INT INC
A kind of preparation method of iron oxide photoanode with oxalic acid and tetrabutyl titanate acting together
ActiveCN112038103BAchieve conversionImprove performanceLight-sensitive devicesPhotovoltaic energy generationOXALIC ACID DIHYDRATEElectron hole
The present invention relates to a kind of preparation method of the iron oxide photoanode that oxalic acid and tetrabutyl titanate act together, obtain iron film by simple electrodeposition, soak oxalic acid (H 2 C 2 o 4 ) ethanol solution, ultrasonically soak tetrabutyl titanate (TBT), and finally anneal to obtain Fe 2 o 3 -H 2 C 2 o 4 +TBT photoanode. Because oxalic acid and tetrabutyl titanate increase the specific surface area of iron oxide and reduce electron-hole recombination, its photoelectric performance has been improved. Under AM1.5 standard test conditions, the photocurrent density can reach 0.59mA cm at 1.23V relative to the reversible hydrogen electrode ‑2 . Different from the publicly reported methods, the raw materials of this method are cheap and easy to obtain, the whole photoelectrode preparation process is quick and easy to prepare in large quantities. And different from the previous method of controlling the morphology, it is done by simple impregnation and chemical reaction.
Owner:CHANGZHOU UNIV
ferroelectric composite cu 2 oVisible light photolysis of water to produce hydrogen photocathode and its preparation method
ActiveCN108385131BPrevent oxidationImprove stabilityVacuum evaporation coatingSputtering coatingHeterojunctionPhotocathode
The invention relates to a ferroelectric composite Cu2O visible light water photolysis hydrogen production photocathode and a preparing method thereof. The photocathode is sequentially composed of a BiFeO3 ferroelectric film layer, a gold nano-rod granular layer, a Cu2O film layer and a silicon wafer substrate from top to bottom, and heterojunctions are formed between the Cu2O film layer and the BiFeO3 ferroelectric film layer. According to the ferroelectric composite Cu2O visible light water photolysis hydrogen production photocathode and the preparing method of the photocathode, adverse effects of energy band barriers between the Cu2O film layer and the BiFeO3 ferroelectric film layer are eliminated through gold nano-rod granules by means of the LSPR effect, absorption of visible light with the wave length ranging from 650 nm-750 nm is strengthened, the Cu2O film layer is protected by the BiFeO3 ferroelectric film layer, residual polarization electric fields are used for eliminatingupwarp barriers on photoelectrode / electrolyte interfaces, the water photolysis efficiency and the hydrogen production efficiency are improved, the light current density reaches 91 microampere / cm<2>, and the threshold voltage relative to a reversible hydrogen electrode reaches 1.01 V.
Owner:SUZHOU TAIHU ELECTRIC ADVANCED MATERIAL CO LTD
Iron-nitrogen-carbon composite oxygen reduction catalyst as well as preparation method and application thereof
PendingCN114068962AIncrease current densityFuel and primary cellsCell electrodesCarbon compositesPtru catalyst
The invention provides a preparation method of an iron-nitrogen-carbon composite oxygen reduction catalyst, which comprises the following steps: mixing activated carbon, ferrous chloride and a nitrogen source to obtain a mixture; and carbonizing the mixture in a nitrogen atmosphere to obtain the iron-nitrogen-carbon composite catalyst. Activated carbon with a high specific surface area is used as a catalyst carrier, ferrous chloride and a nitrogen source are used as active components, and the high-performance iron-nitrogen-carbon composite catalyst is prepared through carbonization in a nitrogen atmosphere. The iron-nitrogen-carbon composite catalyst is prepared by carbonization at 700-1000 DEG C, and the current density of the catalyst obtained by carbonization at a preferable interval of 750-950 DEG C in a potential interval greater than 0.85 V (relative to a reversible hydrogen electrode) is higher than that of a commercial Pt / C (20wt%) catalyst; the voltage range of a zinc-oxygen-air battery assembled by the iron-nitrogen-carbon composite oxygen reduction catalyst prepared by the preparation method disclosed by the invention is 1-1.5 V.
Owner:LINGNAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com