Copper porphyrin complex for electro-catalysis oxygen evolution reaction and preparation method of copper porphyrin complex
A technology for oxygen evolution reaction and complexes, which is applied in the directions of organic chemistry methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as the application of unreported porphyrin-copper complexes, and achieves The effect of high yield, energy saving and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
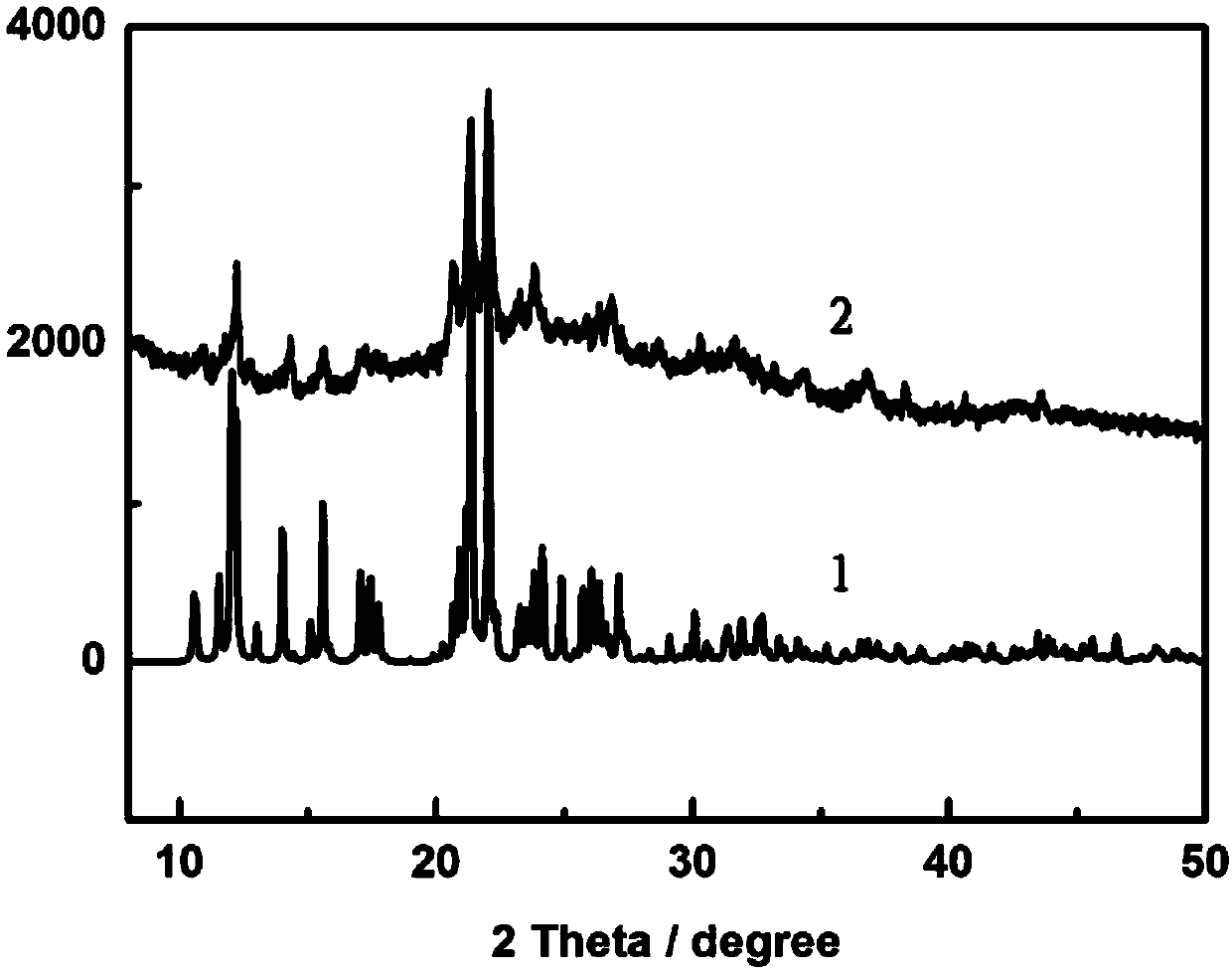
[0030] Embodiment 1: This embodiment relates to the preparation of porphyrin copper complex:
[0031] (1) The reactants 5,10,15,20-tetracyanophenylporphyrin (CNTCPP) and copper nitrate trihydrate are fed in a molar ratio of 1:5. Accurately weigh 0.02g, 0.028mmol of 5,10,15,20-tetracyanophenylporphyrin (CNTCPP) and 0.034g, 0.14mmol of copper nitrate trihydrate in the reaction vessel with an analytical balance; at room temperature, add 5mL of Water N, N' dimethylformamide solvent was stirred for 12 hours, the reaction was stopped, and the reaction solution was filtered with filter paper to obtain a blue-green filtrate;
[0032] (2) Add HBF to the filtrate 4 The pH value of the solution was adjusted to 6, and then the filtrate was transferred to a 25mL polytetrafluoroethylene-lined stainless steel reactor, and then placed in a constant temperature blower box at 85°C for 1000 minutes to obtain light blue crystals.
[0033]Elemental analysis theoretical value: C, 70.31; H, 4.15; ...
Embodiment 2
[0045] Embodiment 2: This embodiment involves complex electrocatalytic oxygen evolution reaction:
[0046] In order to study the oxygen evolution performance of the material, a three-electrode system was used to test on an electrochemical workstation (Gamry Reference600 Instruments, USA). Disperse 5.0 mg of the porphyrin copper complex prepared in Example 1 as a catalyst and 20.0 μL of Nafion (5 wt%) solution in 1 mL of ethanol-water mixed solution (volume ratio of ethanol: water is 1:1), and ultrasonically treat for 1 hour to obtain Uniform dispersion. Then 5.0 μL of this dispersion was loaded on a glassy carbon electrode with a diameter of 4 mm. Use 1.0M KOH as the electrolyte (bubble pure oxygen for 30 minutes to remove dissolved oxygen), a saturated calomel electrode as a reference electrode, a high-purity platinum electrode as a counter electrode, and a glassy carbon electrode loaded with a catalyst as a working electrode. the s -1 The scan rate was used to measure the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com