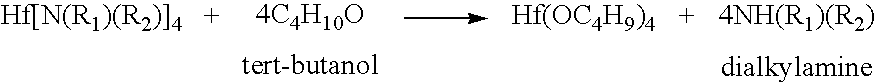Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Hexafluoro-acetylacetone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of 1,1,1,5,5,5-hexafluoro acetylacetone
InactiveCN102260151AReduce pollutionLow costOrganic compound preparationCarbonyl compound preparationTrifluoroacetic acidHexafluoro-acetylacetone
The invention provides a preparation method of 1,1,1,5,5,5-hexafluoro acetylacetone. The preparation method comprises the following steps: carrying out first-stage reaction on trifluoroacetyl acetate and trifluoroacetic anhydride under the action of a catalyst at 40-60 DEG C for 5-10 hours; then raising the temperature to 120-150 DEG C for second-stage reaction, and distilling out the resulting 1,1,1,5,5,5-hexafluoro acetylacetone while reacting to obtain a crude 1,1,1,5,5,5-hexafluoro acetylacetone product; and finally purifying the crude product to obtain a refined 1,1,1,5,5,5-hexafluoro acetylacetone product. In the preparation method provided by the invention, as inexpensive trifluoroacetic anhydride and trifluoroacetyl acetate are used as raw materials for the reaction, the cost of the product is dramatically reduced; and as no acid is generated in the reaction process, the environmental pollution is low.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
Heat-resistant filler for plastics and preparation method of heat-resistant filler
The invention discloses a heat-resistant filler for plastics. The filler is prepared from the following raw materials in parts by weight: 1-2 parts of magnesium stearate, 3-4 parts of zirconia ceramic microbeads, 0.1-0.2 part of sodium dodecyl benzene sulfonate, 2-3 parts of pure acrylic emulsion, 1-2 parts of hexafluoro-acetylacetone, 0.2-0.3 part of China wood oil, 0.2-0.3 part of carnauba wax, 2-3 parts of nanometer iron-ore slag, 200-220 parts of light calcium carbonate, 5-9 parts of aids and 20-30 parts of water. According to the filler, due to addition of the zirconia ceramic microbeads, the heat resistance of the plastic can be improved; due to addition of the nanometer iron-ore slag, the wear resistance and strength of the plastics can be improved; and moreover, calcium carbonate is subjected to surface modification by using pure acrylic emulsion, hexafluoro-acetylacetone, China wood oil and carnauba wax, so that calcium carbonate is high in lipophilicity, the compatibility of calcium carbonate with the plastics is increased, and the strength of the plastics can be improved.
Owner:青阳县吉祥塑胶有限公司
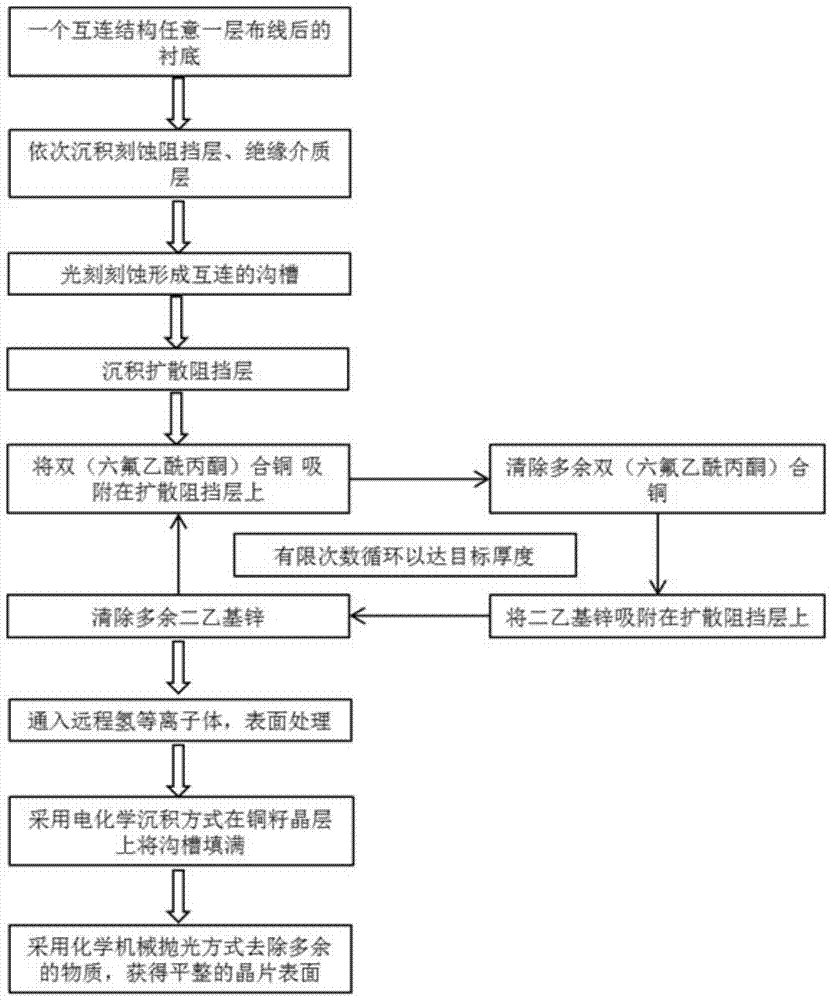
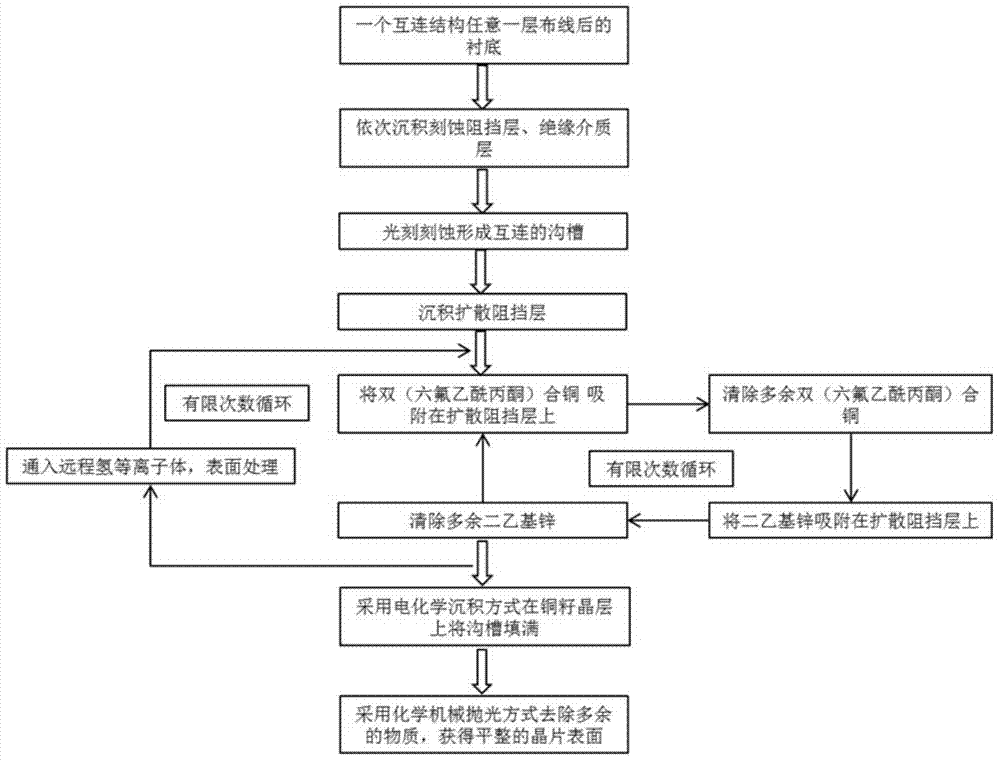
Method and application for preparing ultra-thin copper seed layer by processing surfaces of hydrogen plasmas
InactiveCN103681480AImprove performanceImprove reliabilitySemiconductor/solid-state device manufacturingHexafluoro-acetylacetonePhysical chemistry
The invention belongs to the technical field of semiconductor integrated circuits, specifically a method for preparing an ultra-thin copper seed layer by processing surfaces of remote hydrogen plasmas. The method disclosed by the invention comprises the following steps: absorbing bi(hexafluoro-acetylacetone) copper on a diffusion barrier layer to remove residual bi(hexafluoro-acetylacetone) copper; absorbing diethyl zinc on the diffusion barrier layer to remove residual diethyl zinc; repeating the above steps to achieve the target thickness of the ultra-thin copper seed layer; finally inputting the remote hydrogen plasmas to process the surface. With an ALD (Atomic Layer Deposition) method, the copper seed layer is grown, the thickness of the copper seed layer can be effectively controlled under lower technique temperature, and the copper seed layer has good groove filling performance; with remote hydrogen plasmas pulse, gas byproducts can be generated by reacting with impurities in a deposition film and are taken away through carrier gas, thus the quality of the deposition film is improved, the adhesion characteristic of electro-coppering and the copper seed layer is improved, and the reliability in the copper-connection application of the integrated circuits is kept.
Owner:FUDAN UNIV
Nitroxyl free radical metal complex with naphthalene ring structure and preparation method of complex
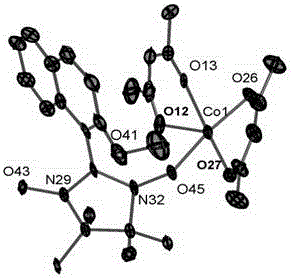
InactiveCN104177442ASimple processSynthesis temperature is lowCobalt organic compoundsOrganic/organic-metallic materials magnetismCubic crystal systemHexafluoro-acetylacetone
The invention discloses a nitroxyl free radical metal complex with a naphthalene ring structure. A chemical formula of the nitroxyl free radical metal complex is as follows: {Co(hfac)2(NIT-2-OMe-Naph)}n, wherein hfac is hexafluoro-acetylacetone, and NIT-2-OMe-Naph is a 2-(2-methoxy-1-naphthyl)-4,4,5,5-tetramethyl imidazoline-3-oxy-1-oxy free radical; a crystal is a cubic crystal system and a space group is P212121. The nitroxyl free radical metal complex has the advantages that the metal complex is a novel nitroxyl free radical ligand with greater electron density and steric hindrance; after the nitroxyl free radical containing a benzene ring is widely reported, the nitroxyl free radical with the benzene ring structure is expected to become a new concerned nitroxyl free radical main body, so that great possibility is provided for synthesizing various nitroxyl free radicals. A preparation method for the metal complex is simple in process, low in synthesis temperature, simple in crystal growth condition and high in synthesis yield which can be over 80%.
Owner:NANKAI UNIV
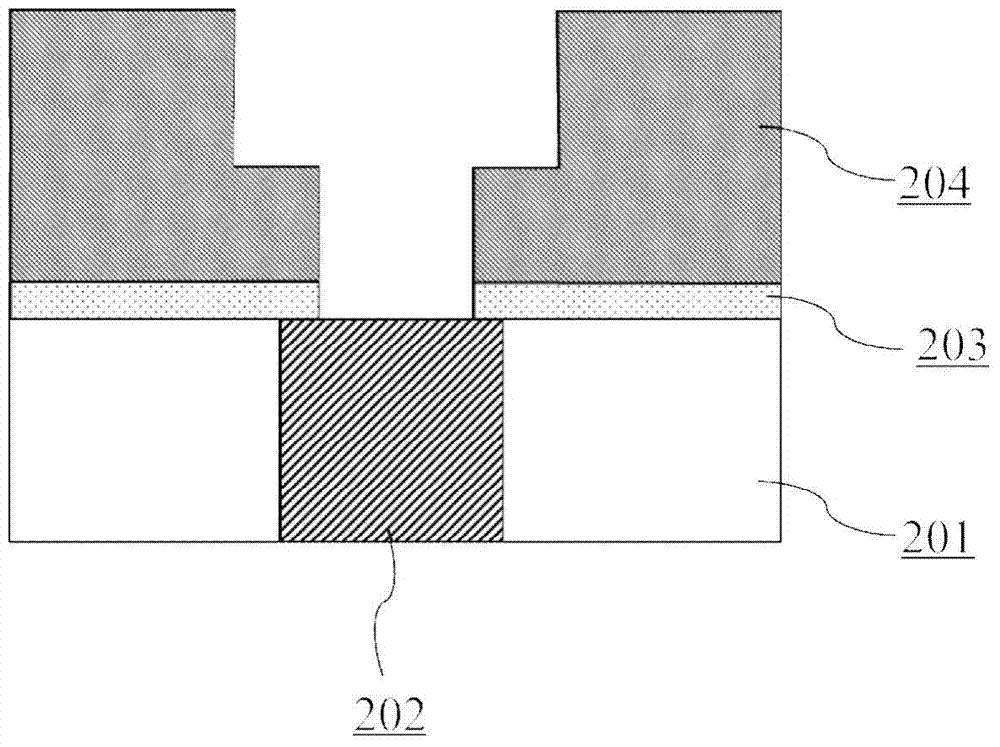
Processes for Producing Hafnium Complexes
InactiveUS20090005584A1High yieldEasily and safely removedOrganic compound preparationTitanium organic compoundsChemistryPropanol
Disclosed are first to sixth processes for respectively producing hafnium tetra-tertiary-butoxide, tetrakis(acetylacetonato)hafnium, tetrakis(1-methoxy-2-methyl-2-propanolato)hafnium, hafnium tetra-tertiary-amyloxide, tetrakis(3-methyl-3-pentoxy)hafnium, and tetrakis(hexafluoroacetylacetonato)hafnium. The first process includes the steps of (a) adding a compound A(OyXOnRf)m (e.g., CF3SO3H) to a crude hafnium amide Hf[N(R1)(R2)]4; (b) subjecting a product of the step (a) to a distillation under reduced pressure; (c) adding a lithium alkylamide Li(NR3R4) to a fraction obtained by the step (b); (d) subjecting a product of the step (c) to a distillation under reduced pressure; (e) adding tertiary butanol to a fraction obtained by the step (d); and (f) subjecting a product of the step (e) to a distillation under reduced pressure. The tertiary butanol of the step (e) is replaced with acetylacetone, 1-methoxy-2-methyl-2-propanol, tertiary amyl alcohol, 3-methyl-3-pentanol, and hexafluoroacetylacetone in the second to six processes, respectively.
Owner:CENT GLASS CO LTD
Rosin-containing geogrid
The invention discloses a rosin-containing geogrid. The rosin-containing geogrid consists of the following raw materials in parts by weight: 107-120 parts of high-density polypropylene, 8-10 parts of carboxyl graphene powder, 0.1-0.2 part of cadmium stearate, 6-10 parts of maleic anhydride grafted polypropylene, 2-3 parts of rosin, 1-2 parts of lanolin, 0.1-0.3 part of potassium bromoflouride, 1-1.6 parts of dispersing agent NNO, 0.1-0.4 part of antioxidant BHT, 2-3 parts of bamboo carbon powder, 0.2-0.4 part of hexafluoro-acetylacetone, 7-10 parts of blast furnace slag, 2-3 parts of rare earth auxiliary and 1-2 parts of sodium silicate. The rare earth auxiliary added into the rosin containing geogrid is formed through the full mixing of high-strength boron carbide, attapulgite and rare earth elements, the cooperation of a calcium acetate antiseptic and a polyvinylpyrrolidone stabilizer and the surface treatment action of a silane coupling agent, so that the formed auxiliary is high in not only surface strength, tenacity, weatherability and corrosion resistance, but also stability. The comprehensive quality of the finished product can be effectively improved by the auxiliary, and the adaptability to soil and the resistance to external pressure are improved.
Owner:ANHUI JIEAOMAKE SYNTHETIC MATERIAL TECH
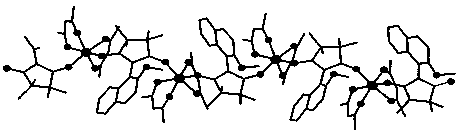
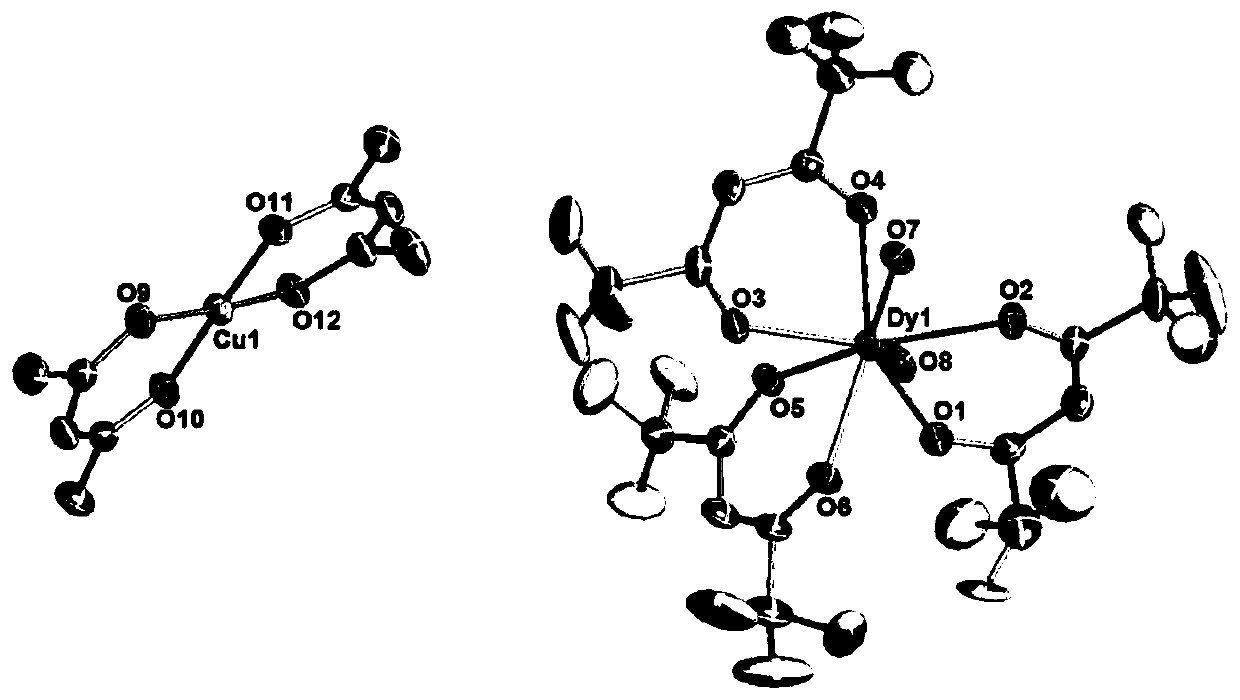
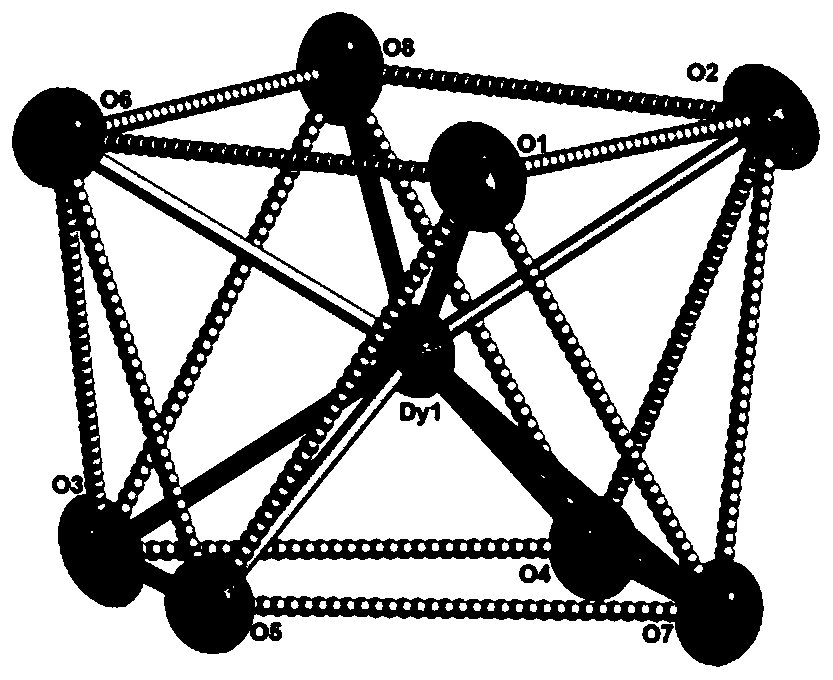
Dy (III)-Cu (II) eutectic monomolecular magnet and preparation method thereof
ActiveCN111116343ASimple filterSimple processOrganic chemistry methodsOrganic/organic-metallic materials magnetismMetallurgyQuantum chemical
The invention discloses a Dy (III)-Cu (II) eutectic monomolecular magnet and a preparation method thereof. The chemical formula of the Dy (III)-Cu (II) eutectic monomolecular magnet is [Dy(hfac)3(H2O)2]-[Cu(acac)2], wherein hfac is a hexafluoroacetylacetone anion, and acac is an acetylacetone anion. The preparation method comprises the following steps: adding a methanol solution dissolved with Dy(hfac)3(H2O)2 into a dichloromethane solution dissolved with Cu(acac)2, performing room-temperature stirring for 15-20 min, filtering to obtain a clear solution, naturally volatilizing the clear solution for 3 d to obtain dark green crystals, filtering, and washing and drying the dark green crystals to obtain the Dy (III)-Cu (II) eutectic monomolecular magnet. The Dy (III)-Cu (II) eutectic molecular material has the performance of a single-molecule magnet, and has a wide application prospect in the aspects of high-density information storage equipment, quantum chemical calculation, spintronicsand the like as a molecular-based magnetic material.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107020109AImprove adsorption capacityInhibition of segregationCatalyst carriersOther chemical processesChemical industryEthylenediamine
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical fields of environment protection and catalysts for chemical industry. The preparation method comprises the following steps of using diatomite, kyanite, talcum, trona, pulverized fuel ash and coal gangue as carriers, expanding pores of the carriers by lithium hypochlorite and bis(acetylacetone)beryllium, and adding a surfactant (monoalkyl ether trimethyl ammonium chloride) for activating treatment under the action of ultrasonic wave; then, performing hydrothermal reaction on the carriers, composite mineralizing agents (borax and potassium sulfate), catalytic activity additive precursors (yttrium tri(hexafluoroacetylacetone) (III) dihydrate, tri(cyclopentadienyl) promethium, tris(4,4,4-trifluoro-1-(2-thienyl)-1,3-butanediono)europium (III), and thulium(III) trifluoromethanesulfonate), and catalytic activity center precursors (pyruvic acid isonicotinyl hydrazone vanadium, cobalt gluconate, catechol ethylenediamine tungsten complex and gold potassium chloride) in a hydrothermal reaction kettle under the action of an emulsifier (oleamide propyl trimethyl methylsulfate ammonia), drying to remove water, and firing in a muffle furnace, so as to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Method for preparing highly effective green light rare earth compound film
InactiveCN1803972AEfficient transferUniform transferLuminescent compositionsFluorescenceHexafluoro-acetylacetone
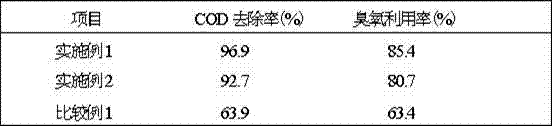
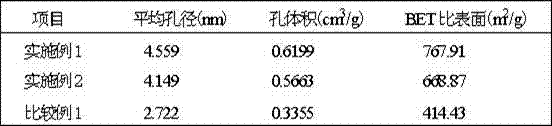
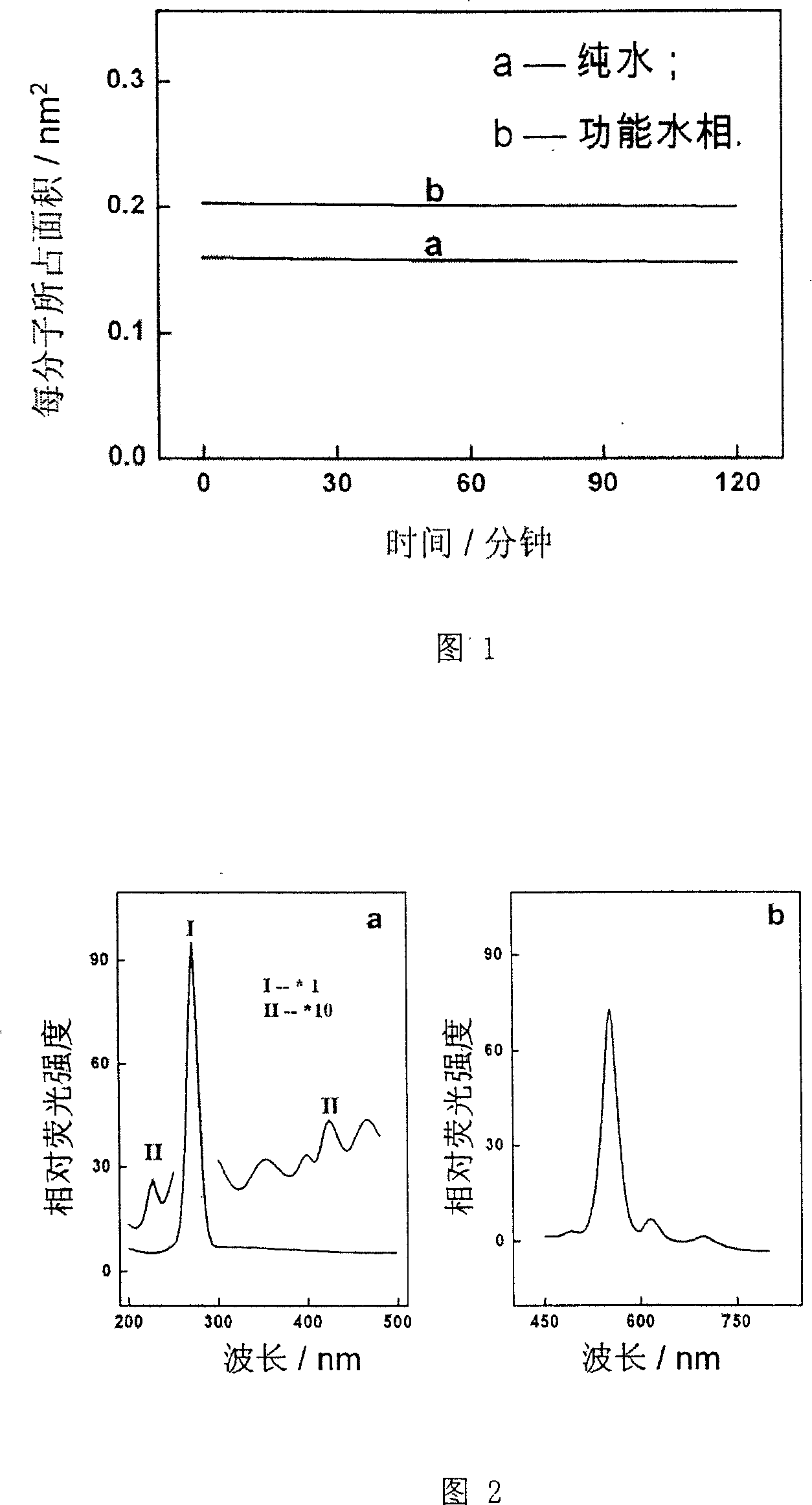
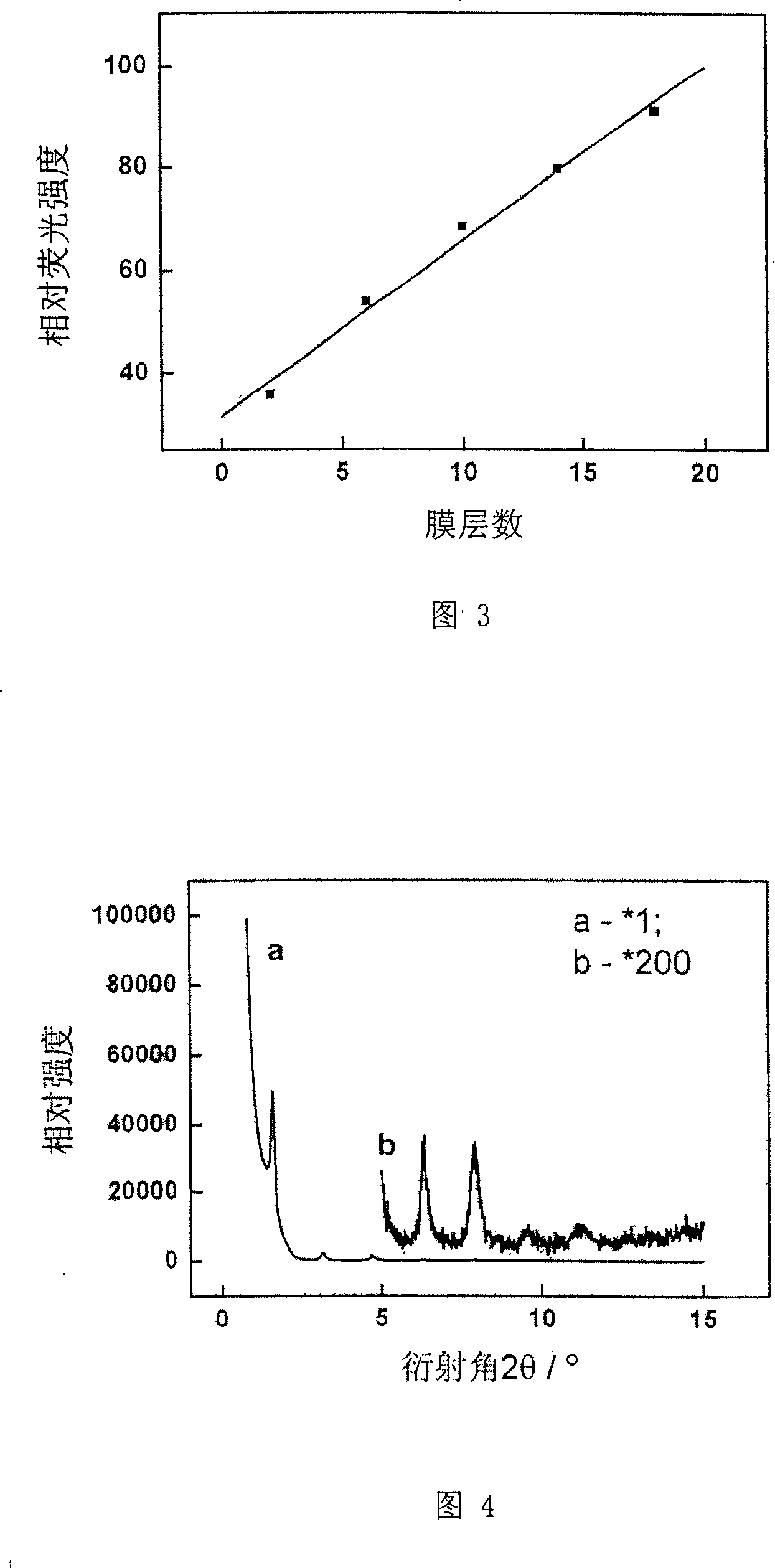
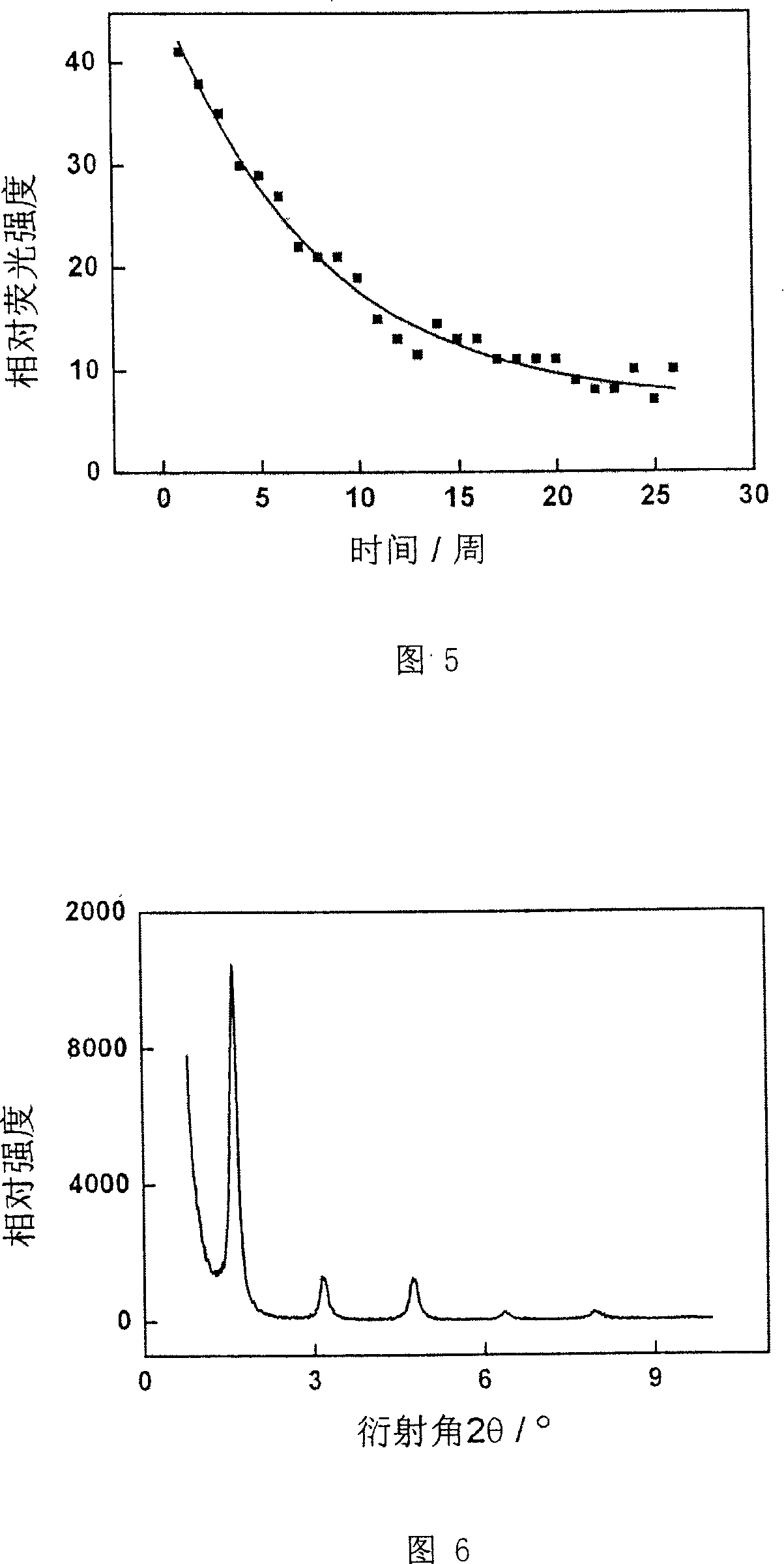
The disclosed preparation method for a high-efficient green-light rare-earth compound film comprises: (1) preparing any one rare-earth terbium (III) compound of Tb(acac)3Phen, Tb(TFA)3Phen, Tb(HFA)3Phen and Tb(TTA)3Phen (acac, TFA, HFA, TTA, and Phen for acetylacetone, trifluoro acetylacetone, hexafluoro acetylacetone, thenoyl trifluoroacetone, and 1, 10-ortho-phenanthroline, respectively); (2) preparing functional aqueous phase, mixing the terbium (III) compound with one fatty acid for ultrathin film preparation on gas-liquid two-dimensional surface and the transfer toward hydrophobic solid substrate. This product has characteristic fluorescence-emission peak on 535.0-557.5nm, keeps layer periodic structure after 26 weeks to detect out the fluorescent signal, and shows important application.
Owner:SHANDONG UNIV
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008299AImprove bindingInhibition of segregationCatalyst carriersWater contaminantsUltrasound - actionErionite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environmental-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking erionite, garnet, medical stones, wollastonite, activated carbon and carnallite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant dodecyl trimethyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle and a compound mineralizer borax and potassium sulfate and catalytic active auxiliary agent precursors isoproscandium oxide (III), a tri(hexafluoroacetyl acetone) yttrium (III) dihydrate, lanthanum stearate and terbium triacetate hydrate; and performing a hydrothermal reaction on catalytic active central compound precursors a titanocene ring substituted salicylic acid complex, vanadium isonicotinoyl hydrazone pyruvate, manganese lysine and dichlorodiamineplatinum under the action of an emulsifier myristyl tributyl ammonium chloride, drying a reaction product to remove water, and firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
A kind of cu/mgo catalyst that catalyzes the hydrogenolysis of glucose and its preparation method
ActiveCN104888778BSolve problems such as poor single selectivityImprove catalytic performanceOrganic compound preparationHydroxy compound preparationMagnesium Acetate TetrahydrateSolvent
Owner:DALIAN UNIV OF TECH +1
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107159246AEvenly dopedImprove adsorption capacityOther chemical processesCatalyst activation/preparationUltrasound - actionCerium
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmentally-friendly and chemical catalysts. The preparation method comprises the following steps: by taking erionite, garnet, medical stone, wollastonite, amazonite and kunzite as carriers, reaming the carriers with lithium hypochlorite and bis(acetylacetone) beryllium, then adding dodecyl trimethyl ammonium chloride serving as a surfactant for surface activation treatment under ultrasonic action, putting the carriers into a hydrothermal reaction kettle for hydrothermal reaction together with borax and potassium sulfate which serve as composite mineralizers, isopropyl scandium oxide (III), tri(hexafluoroacetylacetone) yttrium (III) dihydrate, tetra(2,2,6,6-tetramethyl-3,5-hepta-diketo acid) cerium (IV) and tri[N,N-bis(trimethylsilane)amine] erbium which serve as catalytic active auxiliary precursors and a dicyclopentadienyl titanium cyclo-replaced salicylic acid complex, pyruvate iso-nicotinazone vanadium, cobalt gluconate and dichlorodiamineplatinum which serve as catalytic active center component precursors under the action of bidodecyl dimethyl ammonium bromide serving as an emulsifier, drying a reaction product to remove water, and firing the reaction product in a muffle furnace, thus obtaining the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
A kind of nitrogen oxide free radical metal complex of naphthalene ring structure and preparation method thereof
InactiveCN104177442BSimple processSynthesis temperature is lowCobalt organic compoundsOrganic/organic-metallic materials magnetismCubic crystal systemHexafluoro-acetylacetone
The invention discloses a nitroxyl free radical metal complex with a naphthalene ring structure. A chemical formula of the nitroxyl free radical metal complex is as follows: {Co(hfac)2(NIT-2-OMe-Naph)}n, wherein hfac is hexafluoro-acetylacetone, and NIT-2-OMe-Naph is a 2-(2-methoxy-1-naphthyl)-4,4,5,5-tetramethyl imidazoline-3-oxy-1-oxy free radical; a crystal is a cubic crystal system and a space group is P212121. The nitroxyl free radical metal complex has the advantages that the metal complex is a novel nitroxyl free radical ligand with greater electron density and steric hindrance; after the nitroxyl free radical containing a benzene ring is widely reported, the nitroxyl free radical with the benzene ring structure is expected to become a new concerned nitroxyl free radical main body, so that great possibility is provided for synthesizing various nitroxyl free radicals. A preparation method for the metal complex is simple in process, low in synthesis temperature, simple in crystal growth condition and high in synthesis yield which can be over 80%.
Owner:NANKAI UNIV
Automobile power steering rubber hose material
InactiveCN103756160AIncrease elasticityEasy to useFlexible pipesPhosphoric Acid EstersButenedioic acid
The invention discloses an automobile power steering rubber hose material. The automobile power steering rubber hose material is prepared from following raw materials, by weight, 97 to 100 parts of EPDM 4770R, 3 to 4 parts of maleic acid di-n-butyl ester, 1 to 2 parts of hexafluoro- acetylacetone, 1 to 2 parts of ethyl naphthol, 0.9 to 1 part of bis(P,P-bis-ethylhexyl diphosphato)ethanediolato titanate, 5 to 7 parts of di-n-octyl phthalate, 2 to 3 parts of polyacrylamide, 7 to 10 parts of hydrotalcite, 3 to 4 parts of glass powder, 1 to 2 parts of 1,2-dimethylimidazole, 0.7 to 1.1 parts of sulphur, 0.7 to 1 part of promoter ZBX, and 17 to 25 parts of a composite filling material. The automobile power steering rubber hose material possesses excellent rebound resilience, and using effects; is long in service life; and is capable of realizing steering preferably.
Owner:WUHU JIACHENG ELECTRONICS TECH
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088422AStrong combinationStrong adsorptionCatalyst carriersOther chemical processesMuffle furnaceIridium
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, hydrotalcite, alunite, amazonite, and lithium limestone are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted by the subjoining of surfactant chlorination dodecyl dimethyl hydroxyethyl ammonium under the action of ultrasonic waves, then the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(acetylacetonate hexafluoride) yttrium (III) dihydrate, tricyclic cyclopentadienyl promethium, tri-(6, 6, 7, 7, 8, 8, 8-sevoflurane-2, 2-dimethyl-3, 5-octylene diketone) dysprosium (III), carbonate lutetium hydrate, catalytic active core precursor of pyruvic acid isonicotinoyl hydrazone vanadium, cobalt gluconate and hexa-nitroso rhodium tri-sodium and tetra-chloro dihydrate iridium under the action of emulsifier di-(alkyl) methyl bromide ammonium, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
Method for preparing ozone heterogeneous oxidation solid catalysts
InactiveCN107051493AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a method for preparing ozone heterogeneous oxidation solid catalysts, and belongs to the technical field of environmental protection and chemical catalysts. The method includes carrying out pore expansion and modification on carriers which are purification diatom, kyanite, nitratine, dolomite, amazonite and lithium hydroxyapatite porous materials by the aid of lithium hypochlorite and bis (acetylacetone) beryllium; adding chlorinated dodecyl dimethyl hydroxyethyl ammonium chloride into the carriers and carrying surface activation treatment on the carriers under the effects of ultrasonic waves; carrying out hydrothermal reaction on the carriers which are subjected to ultrasonic surface activation, borax, potassium sulfate, tri (hexafluoroacetylacetone) yttrium (III) dihydrate, samarium acetylacetone, tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium, holmium oxalate decahydrate rare earth metal organic compounds, vanadium pyruvic acid isonicotinyl hydrazone, nickel citrate, cupric glutamate and tetrachloro iridium dihydrate in hydrothermal reaction kettles under the effect of N-dimethyl dodecyl-N'-trimethyl-2-hydroxypropyl ammonium dichloride which is an emulsifier; drying reaction products to remove moisture; burning the reaction products in muffle furnaces at the certain temperatures to obtain the ozone heterogeneous oxidation solid catalysts. The chlorinated dodecyl dimethyl hydroxyethyl ammonium chloride is used as a surfactant. The borax and the potassium sulfate are used as composite mineralizers, the tri (hexafluoroacetylacetone) yttrium (III) dihydrate, the samarium acetylacetone, the tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium and the holmium oxalate decahydrate rare earth metal organic compounds are used as catalytic active auxiliary precursors, the vanadium pyruvic acid isonicotinyl hydrazone, the nickel citrate, the cupric glutamate and the tetrachloro iridium dihydrate are used as catalytic active central components, the vanadium pyruvic acid isonicotinyl hydrazone is a common transition metal organic compound, and the tetrachloro iridium dihydrate is a precious metal compound.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008407AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsPtru catalystUltrasound - action
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environmental-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking erionite, garnet, medical stones, wollastonite, coal ash and gangue as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant dodecyl trimethyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle and a compound mineralizer borax and potassium sulfate and catalytic active auxiliary agent precursors isoproscandium oxide (III), a tri(hexafluoroacetyl acetone) yttrium (III) dihydrate, tetra(2,2,6,6-tetramethyl-3,5-heptanedionato) cerium (IV) and thulium trifluoromethanesulfonate (III); and performing a hydrothermal reaction with catalytic active central compound precursors a titanocene ring substituted salicylic acid complex, vanadium isonicotinoyl hydrazone pyruvate, nickel citrate and potassium dithiocyanoargentate (I) under the action of an emulsifier dimyristyl dimethyl ammonium chloride, drying a reaction product to remove water, and firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIV
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088427AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, aluminum hydroxide, lapis lazuli stone, amazonite, and lithium limestone are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted after the subjoining of surfactant chlorination dodecyl dimethyl hydroxyethyl ammonium under the action of ultrasonic waves, then the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(hexafluoride acetylacetonate) yttrium (III) dihydrate, acetylacetonate samarium, tri-(4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-diacetyl) europium, hydrate tri-acetic acid terbium, catalytic active core precursor of pyruvic acid isonicotinoyl hydrazone vanadium, citric acid nickel, glutamic acid copper and di-chloride tetra ammine palladium under the action of emulsifier di-(octadecyl) lauryl methyl bromide ammonium, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107051456ACarbonization strengthens microporous structureImprove bindingCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment protection and chemical catalysts. The preparation method includes: using erionite, garnet, a diatom purifier, kyanite, fluorite and glauberite as the carriers, using lithium hypochlorite and bis(acetylacetone) beryllium to perform pore expansion on the carriers, adding surfactant chlorinated dodecyl trimethyl ammonium to activate the carriers under ultrasonic action, allowing the carriers to have hydrothermal reaction with compound mineralizer including borax and potassium sulfate, catalytic activity promoter precursors including scandium(III) isopropoxide, tri(hexafluoroacetylacetone) yttrium(III) dihydrate, 1, 1, 1-trifluoroacetylacetone neodymium and acetylacetone samarium, and catalytic activity central precursors including titanocene ring substituted salicylic acid complex, pyruvic acid isonicotinoyl hydrazone vanadium, zinc lactate and a precious metal compound K[Ag(SCN)2] in a hydrothermal reaction kettle under the effect of an emulsifier nonylphenol biquaternary ammonium salt, drying to remove moisture, and burning in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008301AImprove bindingInhibition of segregationOther chemical processesWater treatment compoundsMuffle furnaceLanthanum
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environmental-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking erionite, garnet, medical stones, wollastonite, gamma-aluminum oxide and barite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant dodecyl trimethyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle and a compound mineralizer borax and potassium sulfate and catalytic active auxiliary agent precursors isoproscandium oxide (III), a tri(hexafluoroacetyl acetone) yttrium (III) dihydrate, lanthanum stearate and tricyclopentadiene promethium; and performing a hydrothermal reaction on catalytic active central compound precursors a titanocene ring substituted salicylic acid complex, vanadium isonicotinoyl hydrazone pyruvate, manganese lysine and rhodium hexanitroso trisodium under the action of an emulsifier cetyl trimethylammonium chloride, and drying a reaction product to remove water, firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Heat radiating coating containing soybean protein powder
InactiveCN103756431AImprove cooling effectSafe and non-toxic side effectsProtein coatingsPotassium perfluorobutanesulfonateHexafluoro-acetylacetone
The invention discloses a heat radiating coating containing soybean protein powder. The coating is prepared from the following raw materials in parts by weight: 87-90 parts of high density polyethylene, 6-8 parts of polyvinylidene chloride, 1-2 parts of zinc oxide, 0.3-1 part of potassium perfluorobutanesulfonate, 1-2 parts of hexafluoro-acetylacetone, 1-2 parts of trihydroxyl polyether, 12-15 parts of titanium dioxide, 0.4-1 part of soybean protein powder, 2-3 parts of 2-butoxyethanol, 0.1-0.3 part of diisodecylphenyl phosphite and 8-14 parts of modified filler. The powder coating disclosed by the invention has a good heat radiating effect, good adhesive force of the coating and strong stability, and is safe and free from toxic and side effects.
Owner:WUHU BAOYI AMUSEMENT EQUIP
Method for preparing highly effective green light rare earth compound film
InactiveCN100352887CGood fluorescence stabilityHigh fluorescence stabilityLuminescent compositionsHexafluoro-acetylacetoneFluorescence
Owner:SHANDONG UNIV
Method for preparing ozone heterogeneous oxidation solid catalysts
InactiveCN107051481AStrong combinationStrong adsorptionCatalyst carriersOther chemical processesMuffle furnaceMoisture
The invention relates to a method for preparing ozone heterogeneous oxidation solid catalysts, and belongs to the technical field of environmental protection and chemical catalysts. The method includes carrying out pore expansion and modification on carriers which are purification diatom, kyanite, illite, ulexite, aluminum hydroxide and celestine porous materials by the aid of lithium hypochlorite and bis (acetylacetone) beryllium; adding monoalkyl ether trimethyl ammonium chloride into the carriers and carrying surface activation treatment on the carriers under the effects of ultrasonic waves; carrying out hydrothermal reaction on the carriers which are subjected to ultrasonic surface activation, borax, potassium sulfate, tri (hexafluoroacetylacetone) yttrium (III) dihydrate, promethium tricyclic pentadiene, samarium acetylacetone, terbium acetate hydrate rare earth metal organic compounds, vanadium pyruvic acid isonicotinyl hydrazone, cobalt gluconate, ammonium zirconium carbonate and palladium dichloro-tetrammine in hydrothermal reaction kettles under the effect of chlorinated methylacryloyl oxo-ethyl trimethylammonium which is an emulsifier; drying reaction products to remove moisture; burning the reaction products in muffle furnaces at the certain temperatures to obtain the ozone heterogeneous oxidation solid catalysts. The monoalkyl ether trimethyl ammonium chloride is used as a surfactant. The borax and the potassium sulfate are used as composite mineralizers, the tri (hexafluoroacetylacetone) yttrium (III) dihydrate, the promethium tricyclic pentadiene, the samarium acetylacetone and the terbium acetate hydrate rare earth metal organic compounds are used as catalytic active auxiliary precursors, the vanadium pyruvic acid isonicotinyl hydrazone, the cobalt gluconate, the ammonium zirconium carbonate and the palladium dichloro-tetrammine are used as catalytic active central components, the vanadium pyruvic acid isonicotinyl hydrazone is a common transition metal organic compound, and the palladium dichloro-tetrammine is a precious metal compounds.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidized solid catalyst
InactiveCN107020095AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionCerium
The invention relates to a preparation method of an ozone heterogeneous oxidized solid catalyst, and belongs to the technical field of environmental protection and chemical catalyst. The preparation method comprises the following steps: taking maifanite, wollastonite, potassium feldspar, szaibelyite, talcum and trona as carriers, performing lithium hypochlorite and bis(acetylacetonato) beryllium reaming, adding octadecyl trimethyl ammonium chloride to perform activating treatment under an ultrasonic effect; and then enabling the active carriers to perform hydrothermal reaction with the compound mineralizing agent borax and potassium sulfate, catalytic active promoter precursors tri(hexafluoro acetylacetone)yttrium (III) dihydrate compound, lanthanum stearate, tetrakis(2,2,6,6-tetramethyl-3,5-heptanedionato)cerium (IV), tri(3-trifluoroacetyl-D-camphor)praseodymium (III), catalytic active center precursors titanocene ring substituted salicylic complex, L-aspartic acid molybdenum, gold potassium tetrachloride and dichlorodiamminoplatinum under the effect of beta-hydroxyethyl dimethyl dodecyl ammonium sulfate, drying to remove the moisture, firing in a muffle furnace to obtain the ozone heterogeneous oxidized solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008469AImprove bindingImprove adsorption capacityCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environmental-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking erionite, garnet, diatom pure, kyanite, activated carbon and carnallite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant dodecyl trimethyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle and a compound mineralizer borax and potassium sulfate and catalytic active auxiliary agent precursors isoproscandium oxide (III), a tri(hexafluoroacetyl acetone) yttrium (III) dihydrate, tri(3-trifluoro acetyl-D-camphor) praseodymium (III) and tri(2,2,6,6-tetramethyl-3,5- heptanedionato) gadolinium; and performing a hydrothermal reaction with catalytic active central compound precursors a titanocene ring substituted salicylic acid complex, vanadium isonicotinoyl hydrazone pyruvate, nickel citrate and diammineplatinum (II) dichloride under the action of an emulsifier trioctyl methyl ammonium dichloride, drying a reaction product to remove water, and firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088420AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsEthylenediamineUltrasound - action
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, talc, crystalline alkali stone, amazonite, and lithium limestone are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted by the subjoining of surfactant mono alkyl ether trimethyl ammonium chloride under the action of ultrasonic waves, the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(acetylacetonate hexafluoride) yttrium (III) dihydrate, tricyclic cyclopentadienyl promethium, tri-(4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-diacetyl) europium, tri-[N, N-di(n-trimethylsilyl) amine] erbium, catalytic active core precursor of pyruvic acid isonicotinoyl hydrazine vanadium, and glucose acid cobalt, catechol ethylenediamine tungsten complex and ammonia-platinum dichloride under the action of emulsifier oleamide n-propyl dimethyl hydroxyethyl ammonium chloride, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088419AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, fluorite, glauberite, nitratine and dolomite are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted by the subjoining of surfactant mono alkyl ether trimethyl ammonium chloride under the action of ultrasonic waves, then the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(acetylacetonate hexafluoride) yttrium (III) dihydrate, tricyclic cyclopentadienyl promethium, tri-(2, 2, 6, 6-tetramethyl-3, 5-pimelic ketonic acid) gadolinium, tri-[N, N-di-(trimethylsilyl) amine] erbium, catalytic active core precursor of pyruvic acid isonicotinoyl hydrazone vanadium, cobalt gluconate, and di-thiocyanate radical combined silver (I) potassium acid, dichloro-diamine platinum under the action of emulsifier stearamide propyl dimethylamine lactate, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
Method of preparation of solid catalyst by ozone heterogeneous oxidation
InactiveCN107088421AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a method of preparation of solid catalyst by ozone heterogeneous oxidation, belongs to the technical field of environmental protection and chemical engineering catalysts. Diatom pure, kyanite, talc, ceramic alkali stone, fluorite and glauberite are adopted as carriers after subjecting to bore-reaming by lithium hypochlorite and di-(acetylacetone) beryllium, activating treatment is conducted by the subjoining of surfactant mono alkyl ether trimethyl ammonium chloride under the action of ultrasonic waves, then the carriers are subject to hydrothermal reaction in a hydrothermal reaction kettle with a composite mineralizer borax and potassium sulfate, precursors of catalytic coagent tri-(acetylacetonate hexafluoride) yttrium (III) dihydrate, tricyclic cyclopentadienyl promethium, acetyl acetone samarium, and trifluoromethane sulfonic acid thulium (III), catalytic active core precursor of pyruvic acid isonicotinoyl hydrazine vanadium, glucose acid cobalt, pyrocatechol quadrol tungsten complex and di-thiocyanate radical combined silver (I) acid potassium under the action of emulsifying agent octadecyl di-ethoxy ammonium methyl sulfate, after stoving, the moisture content is removed, and a solid catalyst by ozone heterogeneous oxidation is obtained by firing in a muffle furnace.
Owner:SICHUAN NORMAL UNIVERSITY
Stable chlorobenzene composite plastic container
InactiveCN104861391AIncrease resistanceImprove high temperature resistancePolystyrenePolyvinyl chloride
The invention discloses a stable chlorobenzene composite plastic container which comprises, by weight, 3-4 parts of tailings, 3-5 parts of furnace dust, 2-3 parts of polyurethane, 0.4-1 part of silane coupling agents KH560, 0.5-1 part of hexafluoro-acetylacetone, 3-4 parts of dibutyl maleate, 0.6-1 part of 2-mercapto benzimidazole, 2-3 parts of boron oxide, 0.6-1 part of hexabromocyclododecane, 2-3 parts of calcium stearate, 0.3-1 part of potassium titanate, 2-3 parts of maleic rosin, 0.5-1 part of calcium ascorbate, 1-2 parts of magnesium fluoride, 71-80 parts of polyvinyl chloride, 10-18 parts of polystyrene and 10-16 parts of fluoride gel. The plastic container is made of main materials including polystyrene and polyvinyl chloride, heat preservation, heat insulation and shockproof effects are enhanced while strength is ensured, and articles stored in the container are more effectively protected.
Owner:ANHUI YIJIA COMMODITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107020107AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesChemical industryEthylenediamine
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical fields of environment protection and catalysts for chemical industry. The preparation method comprises the following steps of using diatomite, kyanite, talcum, trona, magnesia spinel and peridotite as carriers, expanding pores of the carriers by lithium hypochlorite and bis(acetylacetone)beryllium, and adding a surfactant (monoalkyl ether trimethyl ammonium chloride) for activating treatment under the action of ultrasonic wave; then, performing hydrothermal reaction on the carriers, composite mineralizing agents (borax and potassium sulfate), catalytic activity additive precursors (yttrium tri(hexafluoroacetylacetone) (III) dihydrate, tri(cyclopentadienyl) promethium, tris(4,4,4-trifluoro-1-(2-thienyl)-1,3-butanediono)europium (III), and terbium(III) acetate hydrate), and catalytic activity center precursors (pyruvic acid isonicotinyl hydrazone vanadium, cobalt gluconate, catechol ethylenediamine tungsten complex and tetraammine dichloropalladium (II)) in a hydrothermal reaction kettle under the action of an emulsifier (tetradecyl dimethyl(2-hydroxy)ethyl ammonia chloride), drying to remove water, and firing in a muffle furnace, so as to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com