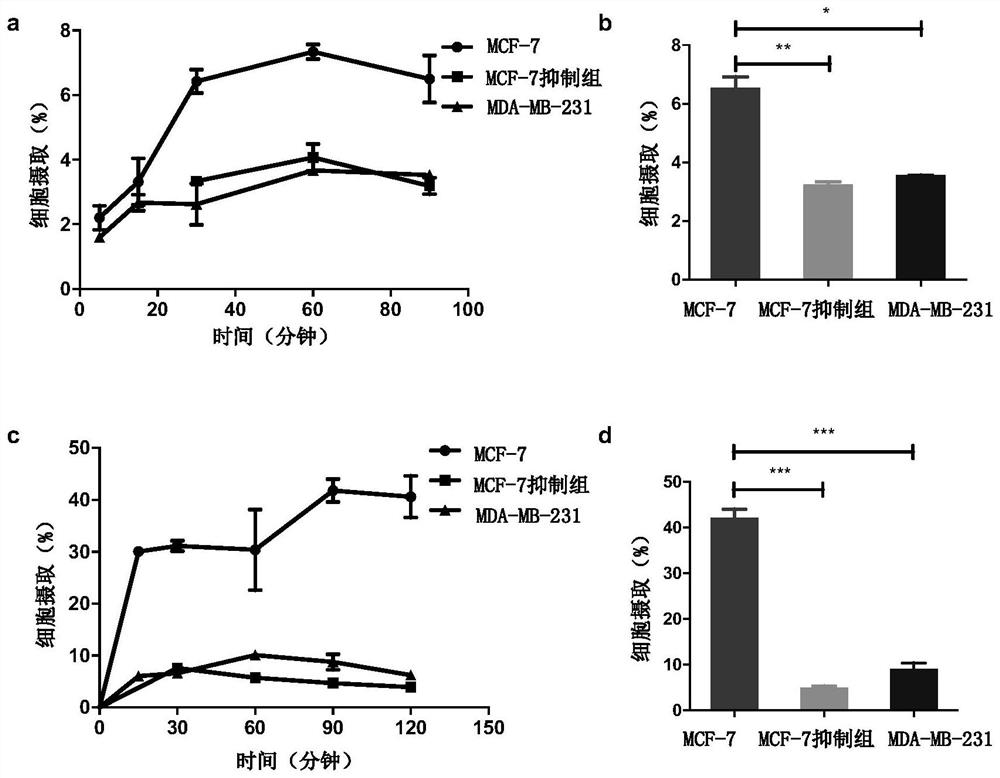
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "Prolong metabolic time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
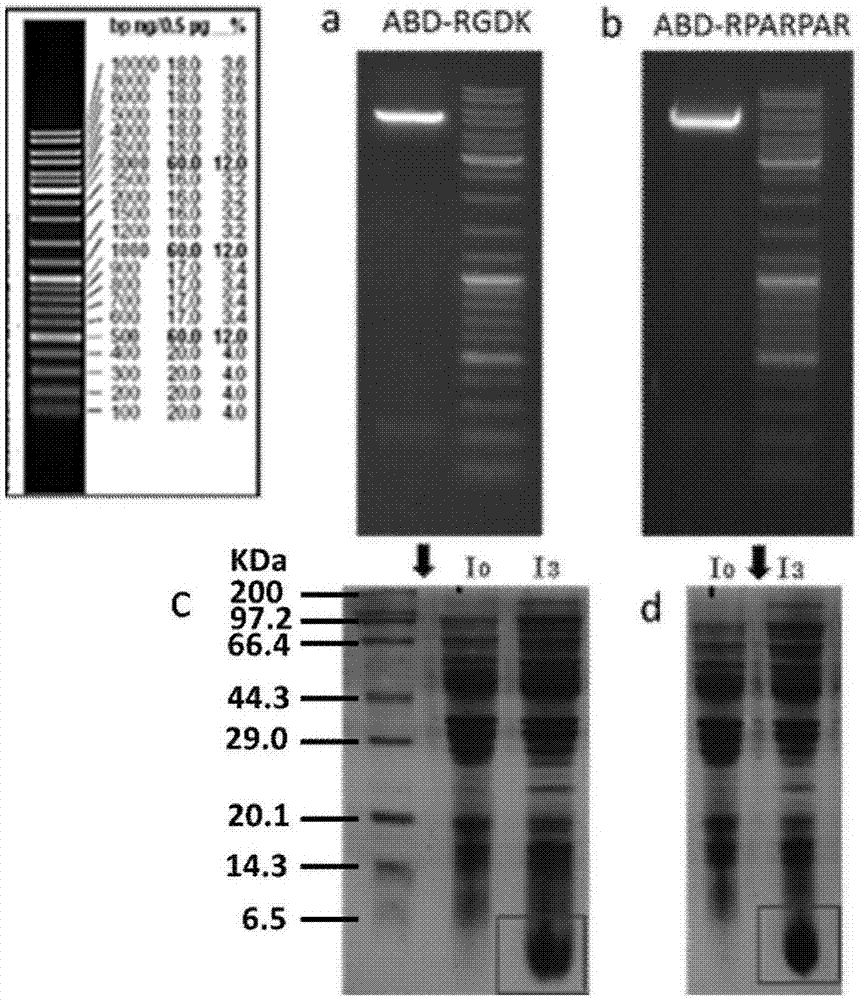
Tumor targeting polypeptide-medicine coupling derivative, and preparation method and application thereof
InactiveCN107952080AExtended half-lifeProlong the action timeOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetChemical Linkage
The invention belongs to the technical field of biological pharmacy, and relates to a tumor targeting polypeptide-medicine coupling derivative, and a preparation method and application thereof. The tumor targeting polypeptide-medicine coupling derivative comprises targeting polypeptides, long effect polypeptides and medicine molecules, wherein the targeting polypeptides are connected onto the endC of the long effect polypeptides through flexible connecting peptides; fusion peptides are used as carriers of the medicine molecules; the medicine carriers and the long effect polypeptides in the fusion peptides are connected through chemical bonds; and the long effect polypeptides are polypeptide or protein structural domains having the affine mutual effects with human serum albumin. The tumortargeting polypeptide-medicine coupling derivative can be combined with the human serum albumin; the remaining half-life period in the blood circulation system can be obviously prolonged; the long-term effectiveness in the body is realized; the targeting and seepage into the tumor tissues or cells can be realized; then, free effect molecules are released in tumor tissue micro acid environments orin cells in through an acid hydrolysis mechanism; and a better anti-tumor efficiency is achieved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
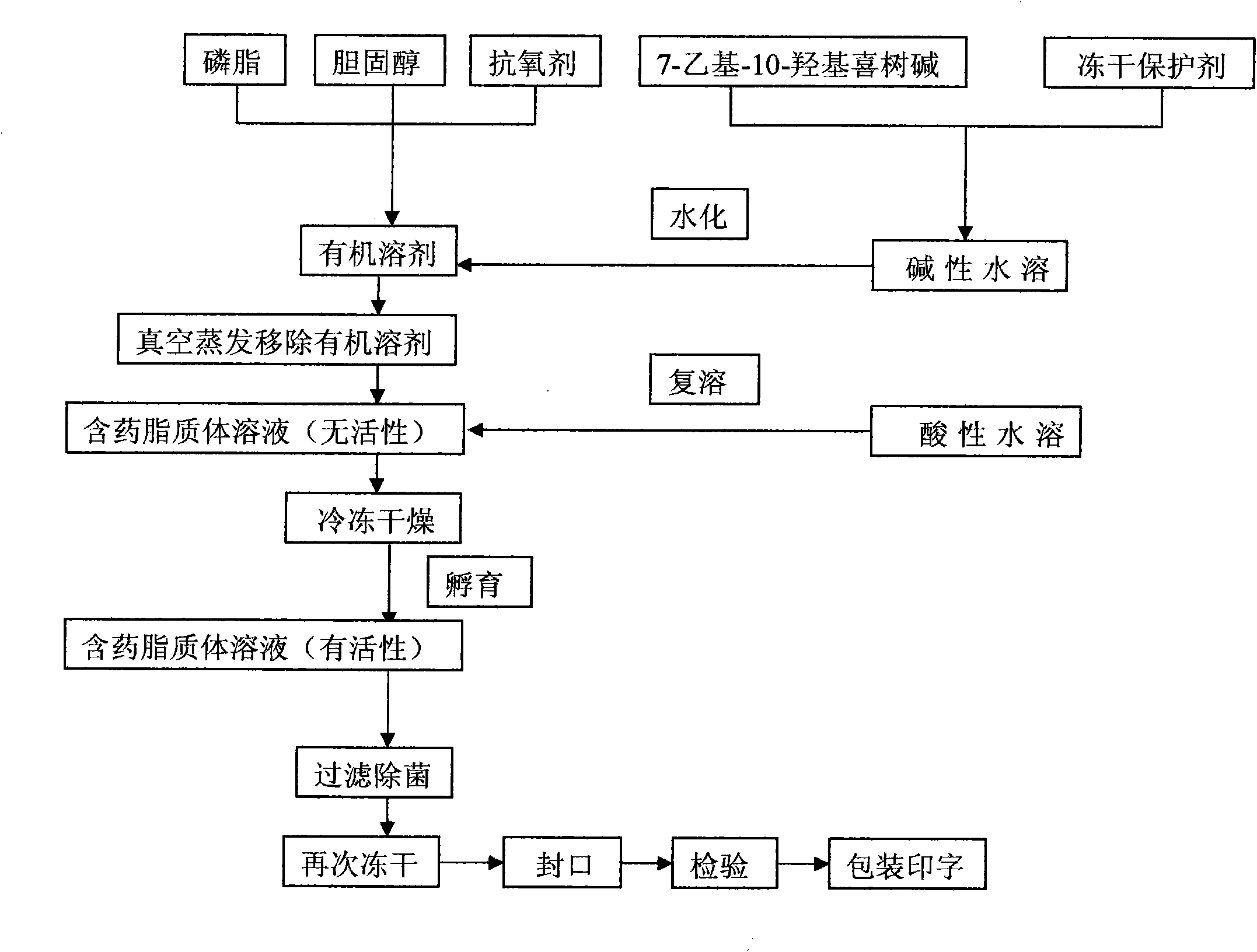
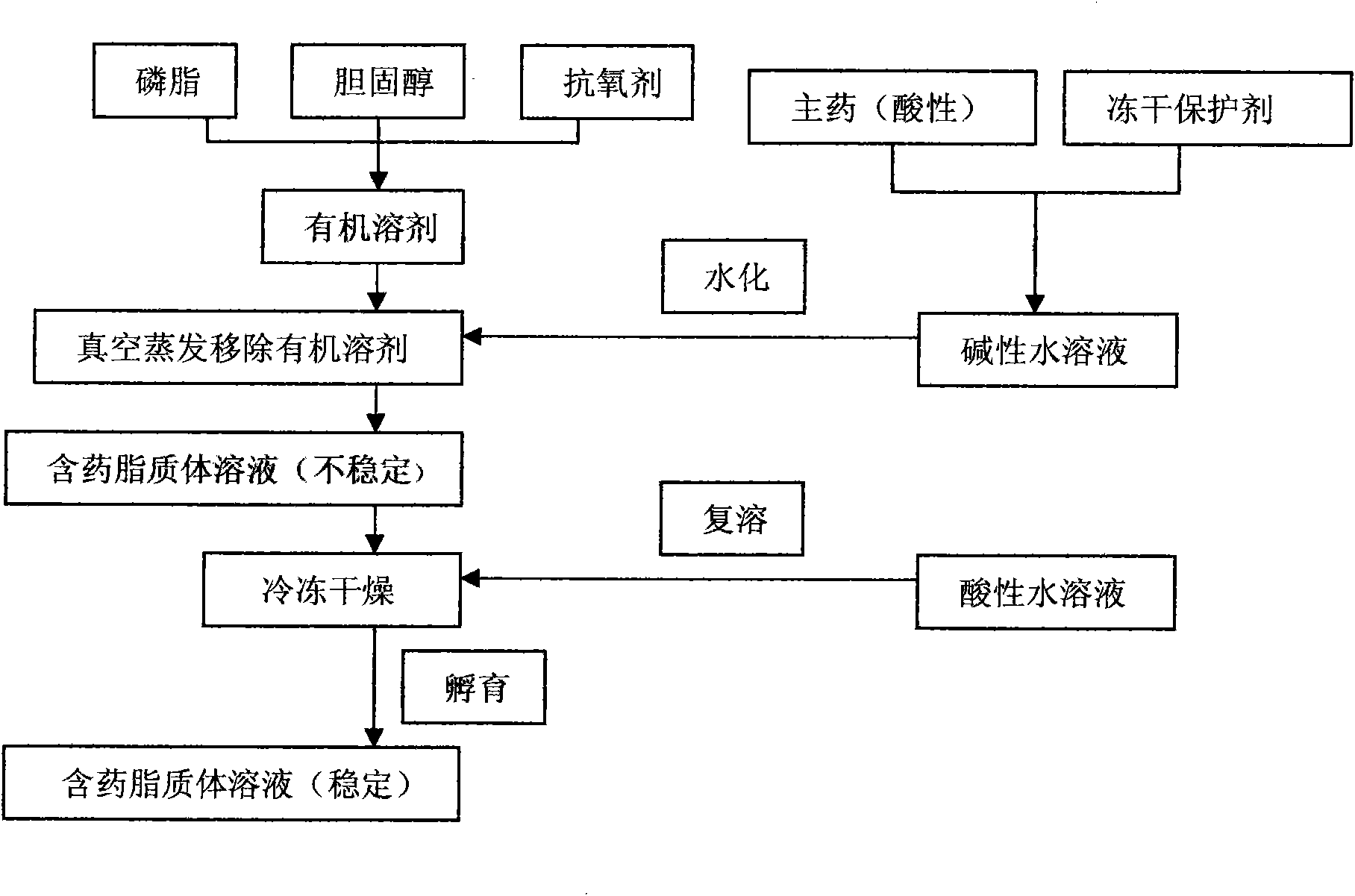
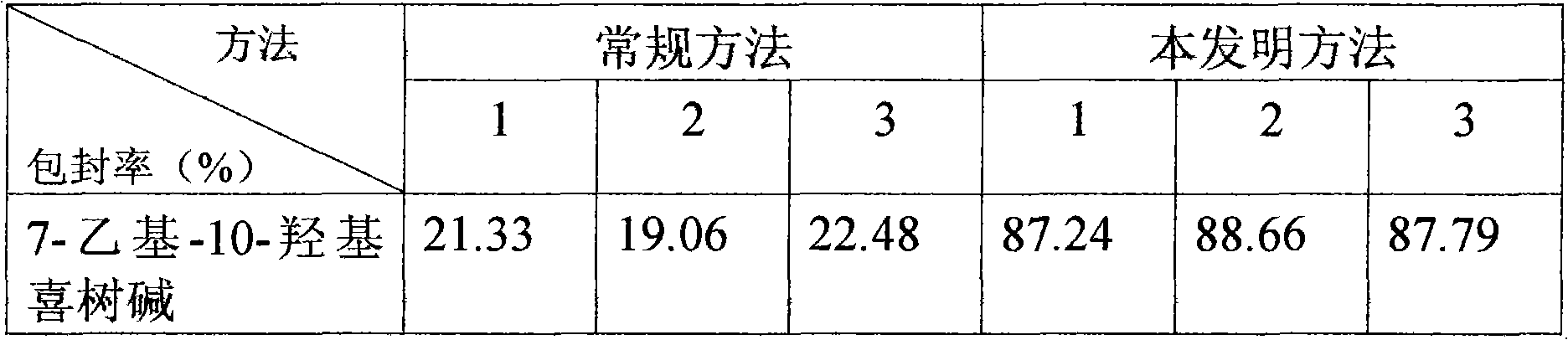
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
InactiveCN101874788AImprove stabilityImprove in vivo stabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the medical technical field, and discloses 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and a preparation method thereof. The 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection comprises the following components: 1-10g of 7-ethyl-10-hydroxycamptothecine, 30-60g of phospholipids, 10-40g of cholesterol, 2-8g of VE, 100-300g of a freeze drying protectant, 2000-8000ml of an organic solvent, 1000-4000ml of alkaline buffer salt solution and 1000-4000ml of acid buffer salt solution. The preparation method comprises the following steps: dissolving liposoluble components in the organic solvent and water-soluble components in the alkaline buffer salt; transferring the organic solvent, and then adding the alkaline buffer salt for hydration; and carrying freeze drying in vacuum, re-dissolving with the acid buffer salt, incubating, filtering, sterilizing, and carrying out freeze drying again to obtain the 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection for injection. The invention solves the problems of low solubility and fast in-vivo metabolism of the 7-ethyl-10-hydroxycamptothecine, thus lowering toxic reaction, eliminating side reaction, having higher target distribution characteristics, prolonging metabolism time and improving solubility and bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
Composition of liposome, and preparation method
InactiveCN1915222AInhibit transferRegulate immunityOrganic active ingredientsNervous disorderYolkDisease
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
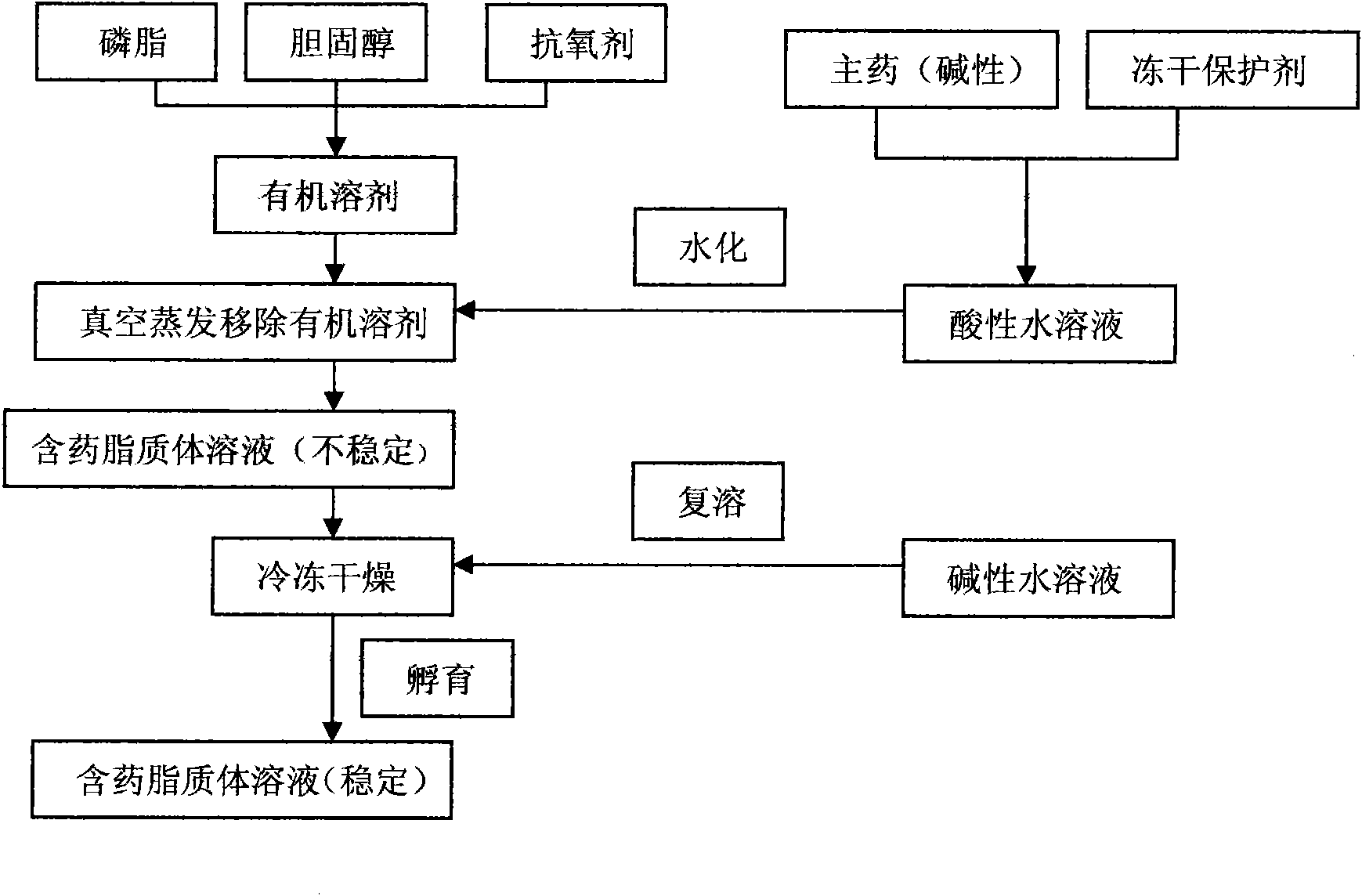
Preparation method for improving liposome entrapment efficiency
ActiveCN101874780AImprove stabilitySolve the problem of light and heat sensitivity and poor stabilityLiposomal deliverySolubilityOrganic solvent
The invention aims to provide a preparation method for improving liposome entrapment efficiency. The preparation method comprises the following steps: dissolving liposoluble components in an organic solvent, and dissolving water-soluble components in alkalescent or acidulous buffer salt; transferring the organic solvent, and adding buffer salt solution for hydration; and after vacuum freeze drying, redissolving the components in the alkalescent or acidulous buffer salt, and incubating to obtain a medicament-containing liposome. Acid compounds are easily dissolved in alkaline solution, and alkaline compounds are easily dissolved in acid solution; and in the method, the liposome is prepared by utilizing the characteristic of solubility change due to the acid-base difference, so that the entrapment efficiency of the compounds in the liposome is improved greatly, and a novel idea for preparing the liposome for indissoluble compounds is provided.
Owner:SHENYANG PHARMA UNIVERSITY +1
Berberine-baicalin compound and preparation method and application thereof
InactiveCN105541943ASimple processGood hypoglycemic effectOrganic active ingredientsSugar derivativesBerberineMedicine
The invention discloses a berberine-baicalin compound which has the structure shown as a general formula (I), and further discloses a preparation method of the compound and application of the compound in preparation of a drug for treating ulcerative enteritis. The compound is prepared by synthesizing berberine and baicalin. Compared with berberine and baicalin, the compound has the advantages that the toxicity of the multi-core molecular compound is significantly reduced, absorption is significantly improved, the bioavailability is improved, the metabolism time is prolonged, the long-acting effect can be achieved, and the medical efficacy of resisting the ulcerative enteritis and pathogenic bacteria is enhanced.
Owner:PI & PI BIOTECH
Preparation method of porous nano-hydroxyapatite sustained-release gel
ActiveCN110538346AUniform size distributionLarge size distributionOintment deliveryPharmaceutical non-active ingredientsPorosityApatite
The invention provides a preparation method of porous nano-hydroxyapatite sustained-release gel. Hydroxyapatite composite microspheres prepared by the method provided by the invention have uniform size distribution, a particle size distribution range of 10-100 mu m, and a central particle size of 20-60 mu m. Increase of an organic phase in a mixing process can effectively enhance the suspension stability of a suspension; the prepared microspheres have uniform size distribution and a larger particle size than the microspheres prepared by a traditional spray-drying method, and can be used as aninternal support material of injections; and the microspheres have a good spherical shape and high porosity, can enhance the injectability of a material, help to accelerate the metabolism time of drugs, maintain a stable and effective drug concentration in the body, and have good drug-release properties.
Owner:SHANGHAI MOYANG BIOTECHNOLOGY CO LTD
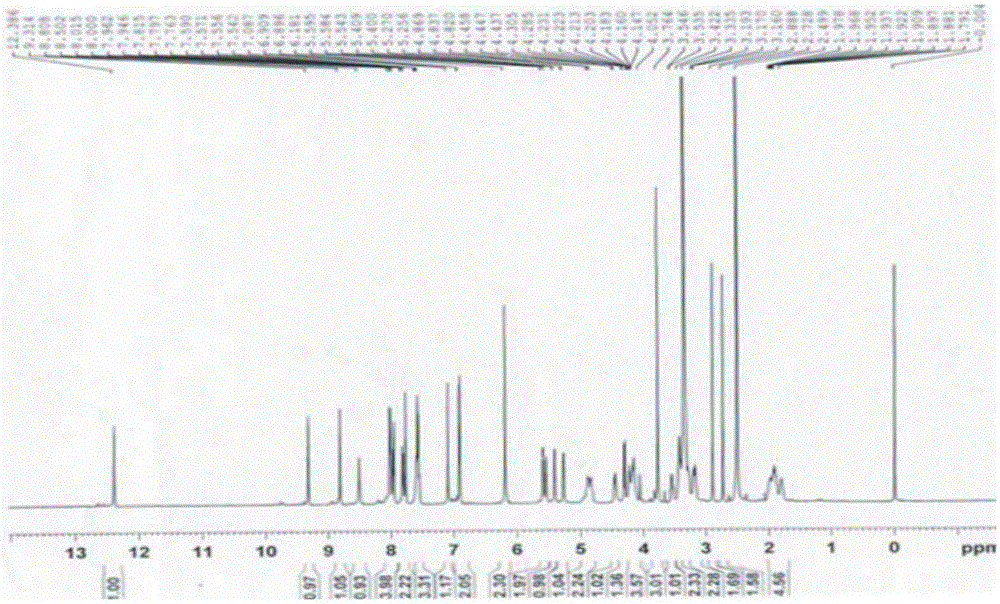
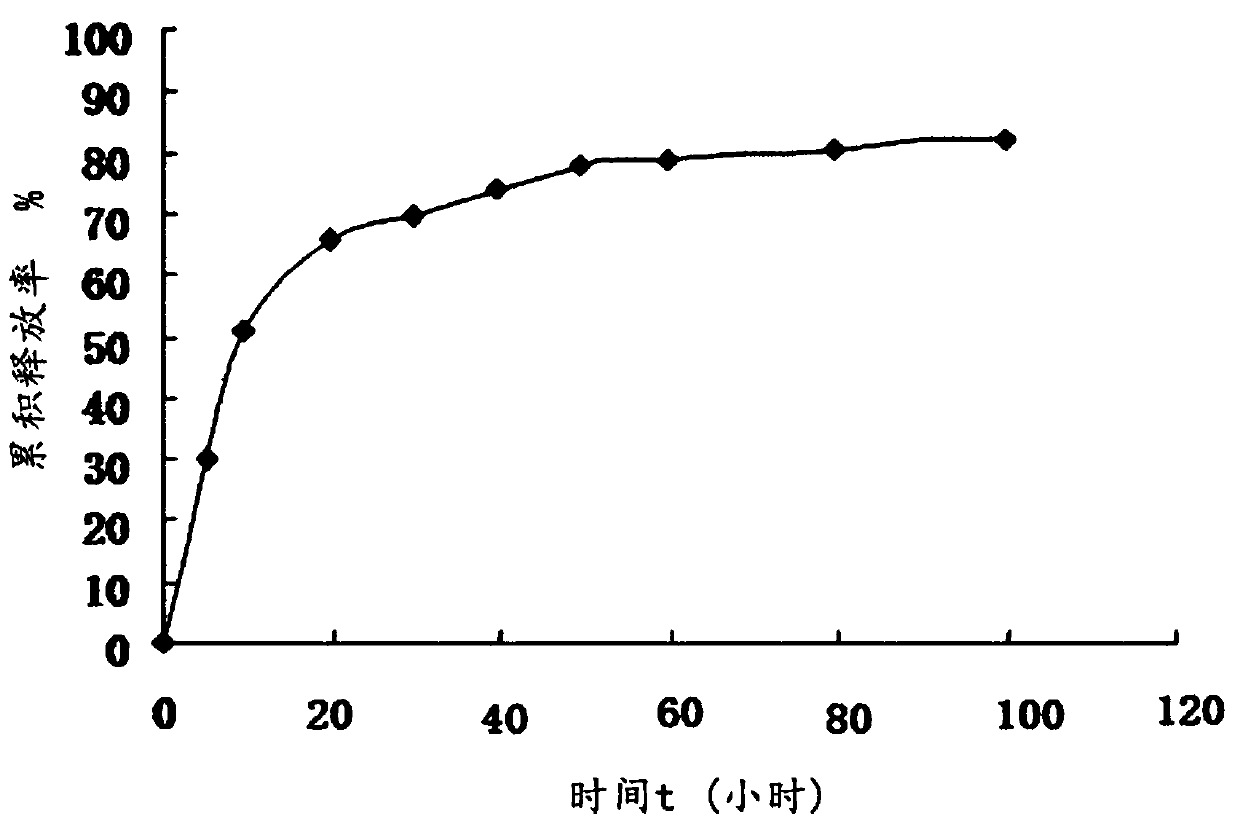
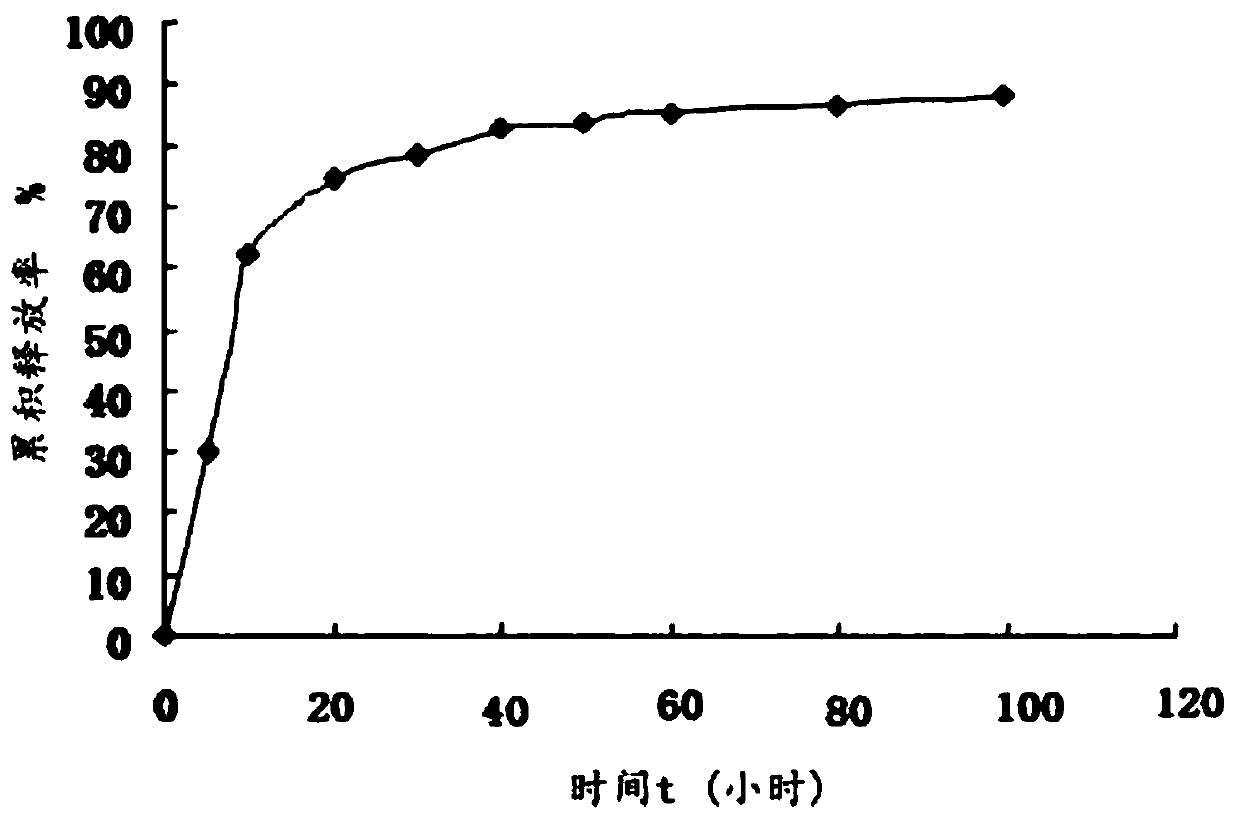
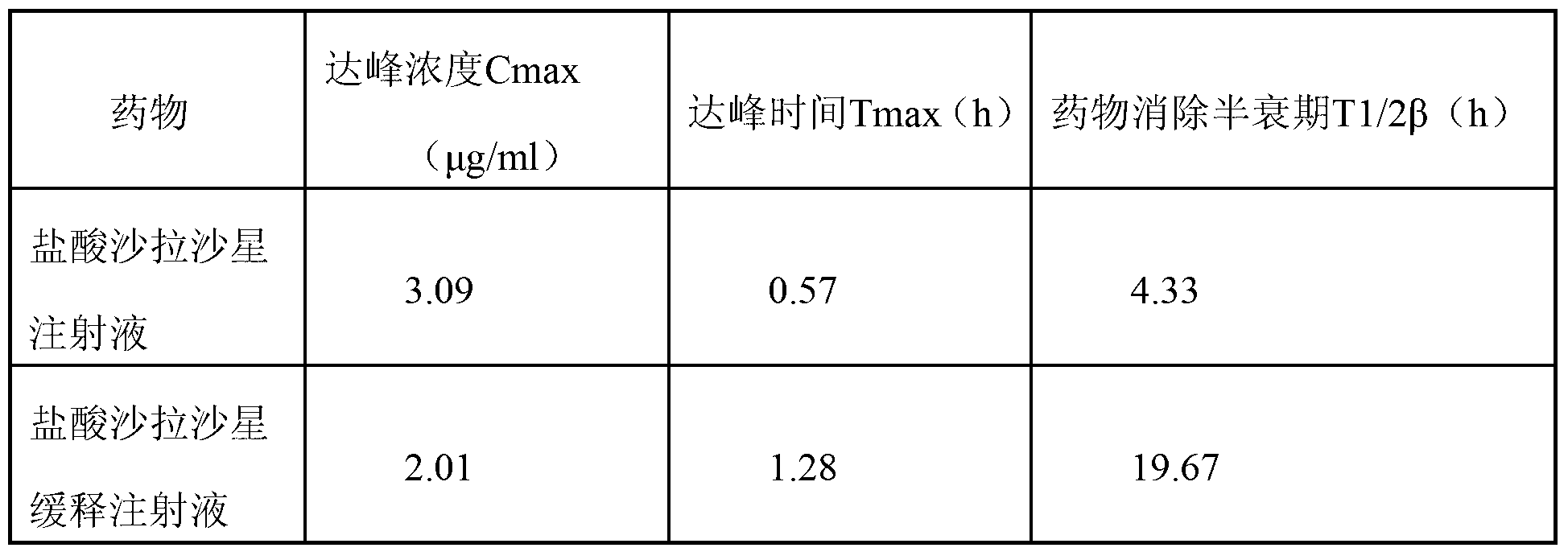
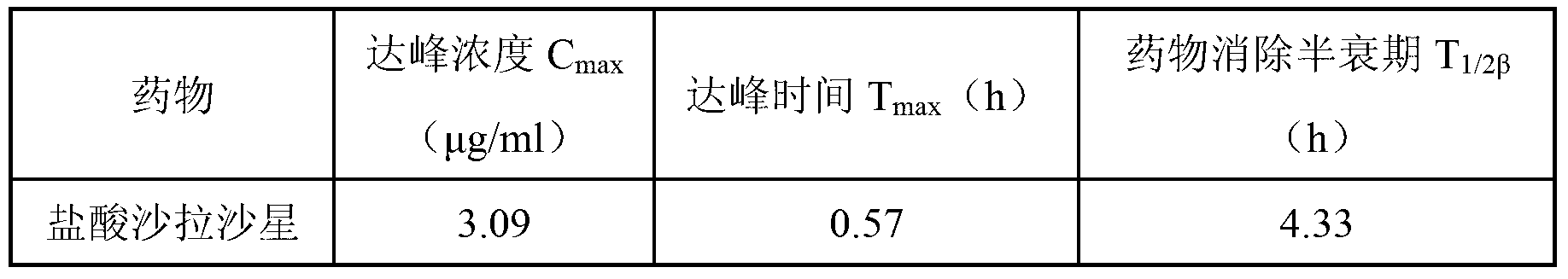
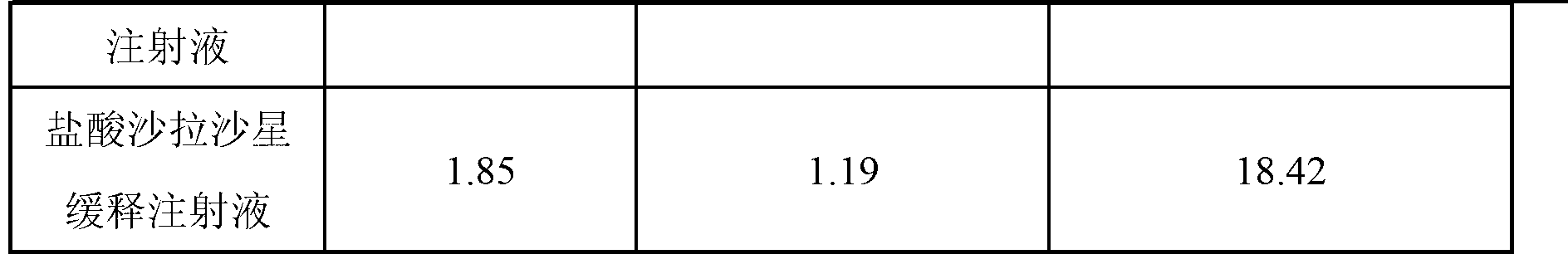
Sarafloxacin hydrochloride sustain-released injection and preparation method thereof
InactiveCN103315955AProlong metabolic timeImprove clinical treatment effectAntibacterial agentsOrganic active ingredientsMagnetic stirrerWater soluble
The invention relates to a sarafloxacin hydrochloride sustain-released injection and a preparation method thereof, belongs to a medicinal preparation field. The sarafloxacin hydrochloride sustain-released injection is composed of sarafloxacin hydrochloride, an emulsifier, a suspending agent, a stabilizing agent, an antioxidant, plant oil for injection, and injection water. The preparation method comprises the following steps of: heating sieved sarafloxacin hydrochloride raw material drug, the plant oil for injection, the emulsifier and the antioxidant in a constant temperature magnetic stirrer, to obtain a mixture A; heating the suspending agent, a water-soluble emulsifier, the stabilizing agent and the injection water in the constant temperature magnetic stirrer, to obtain a mixture B; continuously adding the mixture A into the mixture B, mixing and shearing to obtain colostrums; complementing margin of the colostrum with injection water, then homogenizing, loading, and disinfecting with cobalt 60 ray, to obtain the product. The sarafloxacin hydrochloride injection with obvious sustain-released effect on intramuscular injection of livestock and poultry, can reduce stress reaction, mitigates culture labor intensity, and raises economic benefits and social benefits for livestock and poultry culture.
Owner:SHANDONG LONGHAI BIOLOGICAL TECH +1
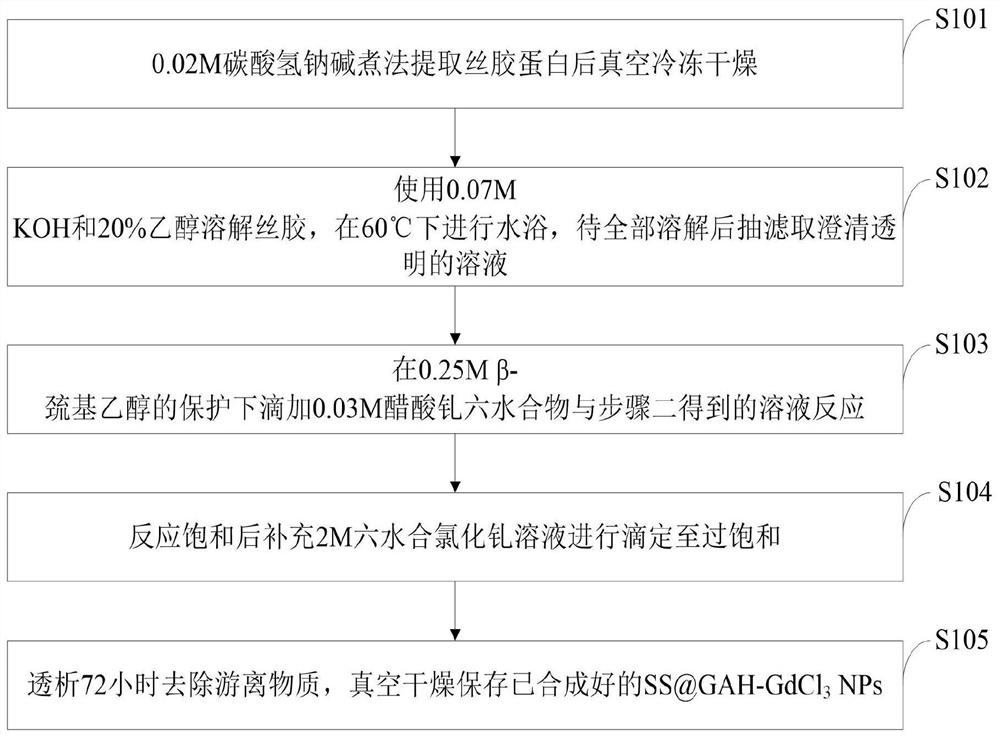
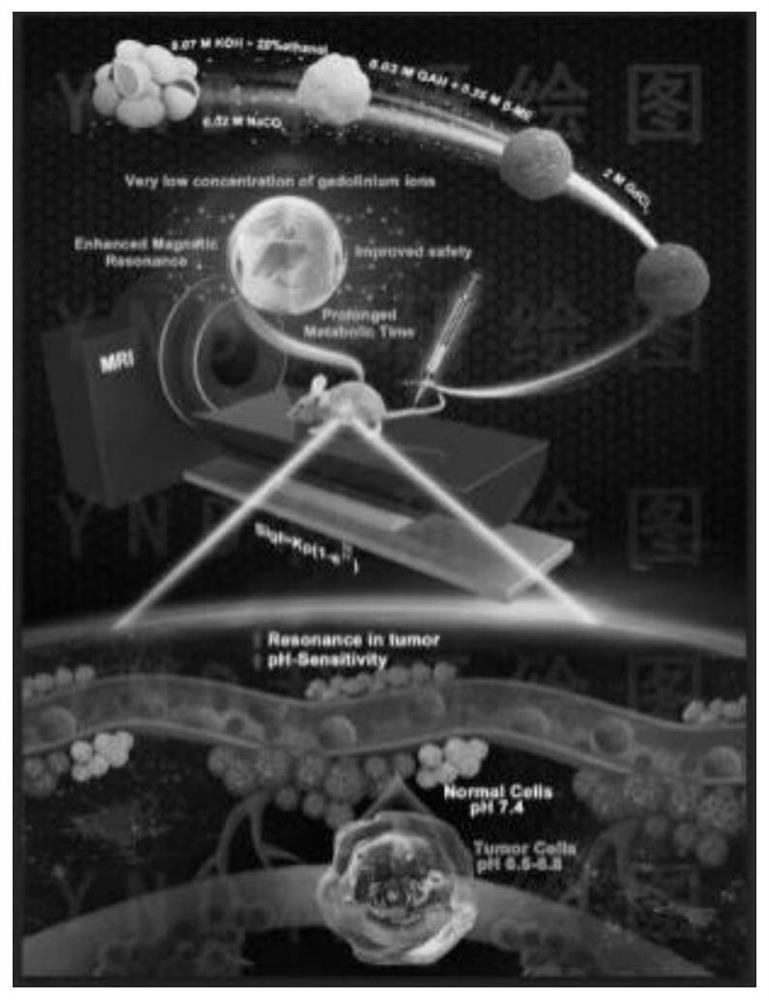
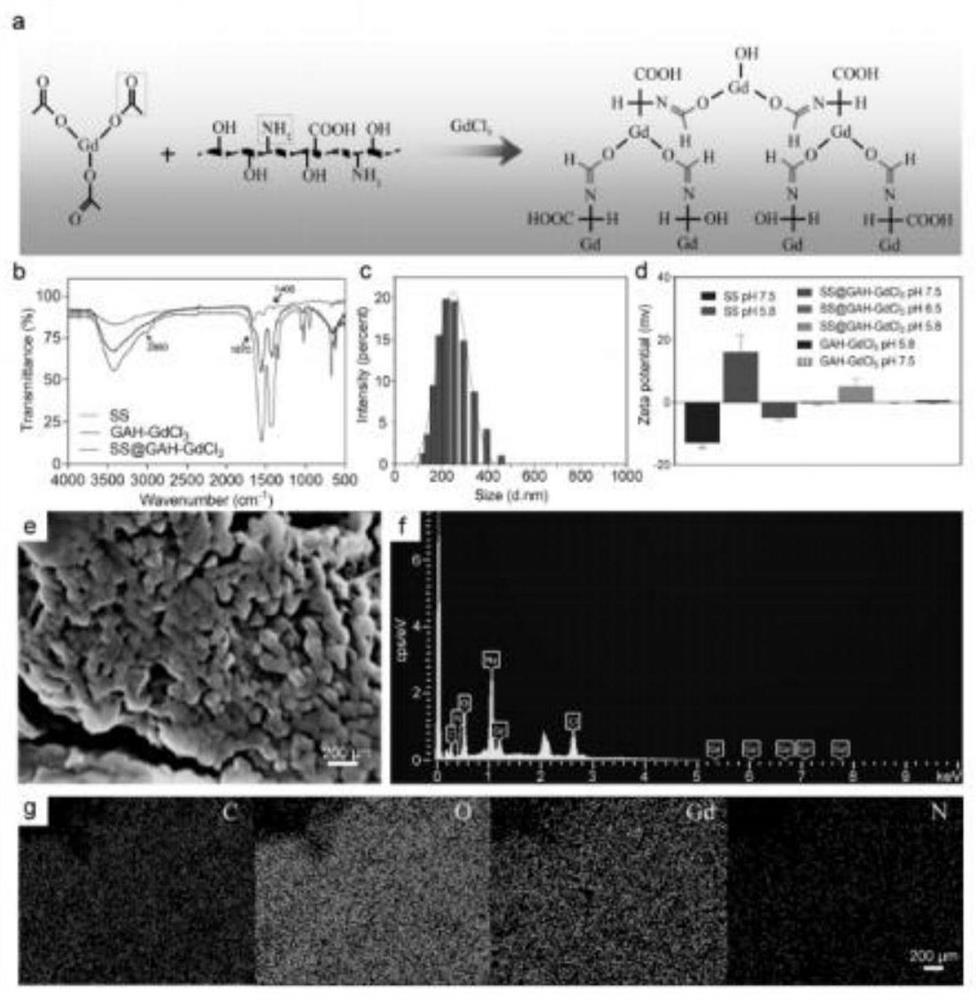
Preparation method of sericin-gadolinium pH responsive targeting tumor nuclear magnetic resonance contrast agent
ActiveCN111632155AImprove securityImprove imaging effectIn-vivo testing preparationsNuclear chemistryContrast medium
The invention belongs to the technical field of magnetic resonance imaging (MRI) contrast agents, and discloses a preparation method of a sericin-gadolinium pH responsive targeting tumor nuclear magnetic resonance contrast agent. The contrast agent is used for enhancing MRI research of tumor tissues, sericin, gadolinium acetate hexahydrate and gadolinium chloride hexahydrate are adopted as raw materials, and the nano contrast agent SS@GAH-GdCl3 is synthesized through a Schiff base reaction. Amino groups of the sericin and aldehyde groups of the gadolinium acetate hexahydrate are subjected to aone-step reaction by a two-step method to form Schiff base, and gadolinium ions are supplemented through electrostatic adsorption of the gadolinium chloride hexahydrate. The contrast agent prepared by the method can smoothly pass through normal tissues and blood vessels, and the surface potential of the contrast agent can be automatically reversed at a tumor part to enter tumor tissues, so that the uptake of tumor cells is increased, and the precise MRI contrast of solid tumors is realized; and the metabolism time of the nano contrast agent is remarkably prolonged by 30-60 min, and is far longer than the pharmacokinetic time of a commercial gadodiamide injection.
Owner:SOUTHWEST UNIV
Magnetic hydrogel device and preparation method and application thereof
ActiveCN113069544AStrong superparamagnetismStrong magnetismElectrotherapyEnergy modified materialsMagnetite NanoparticlesIonic Channels
The invention discloses a magnetic hydrogel device. According to the magnetic hydrogel device, hydrogel is used as a main body structure, and a plurality of magnetic nanoparticles are filled in the hydrogel. The invention further discloses a preparation method of the magnetic hydrogel device and application of the magnetic hydrogel device in nerve magnetic stimulation. Compared with a single magnetic nanoparticle, the magnetic hydrogel device has stronger superparamagnetism, is not easy to diffuse and metabolize in a living body (the metabolism time of a magnetic material in the living body is prolonged), the problem that the single magnetic nanoparticle is easy to diffuse and metabolize in the living body can be solved, the magnetic effect time of the magnetic nano-particles in the living body is further prolonged, damage caused by frequent injection of nano-drugs in nerve regulation and control is reduced, the magnetic nano-particles can exert the electromagnetic effect and the force effect at the same time, and then the ion channel activation capacity of nerve cells is cooperatively improved.
Owner:SOUTHEAST UNIV
Preparation method of porous nano-hydroxyapatite sustained release gel
InactiveCN109529110AUniform size distributionLarge size distributionOintment deliveryPharmaceutical non-active ingredientsPorosityApatite
The invention discloses a preparation method of porous nano-hydroxyapatite sustained release gel. Hydroxyapatite composite microspheres prepared by the preparation method disclosed by the invention are uniform in size distribution, and have a granularity distribution range of 10-100 microns as well as a central particle size of 20-60 microns. By adding organic phase in mixing process, suspension stability of a suspension can be effectively enhanced; so that, the prepared microspheres can be used as an internal support material used for injection for the microspheres are uniform in size distribution and larger in particle size compared with microspheres prepared by conventional spray-drying methods. Moreover, the microspheres are relatively good in shape and high in porosity; so that, the microspheres are capable of enhancing injectability of materials, promoting shortening of drug metabolism time, maintaining stable and effective drug concentration in vivo, as well as ensuring good drug release properties.
Owner:SHANGHAI MOYANG BIOTECHNOLOGY CO LTD
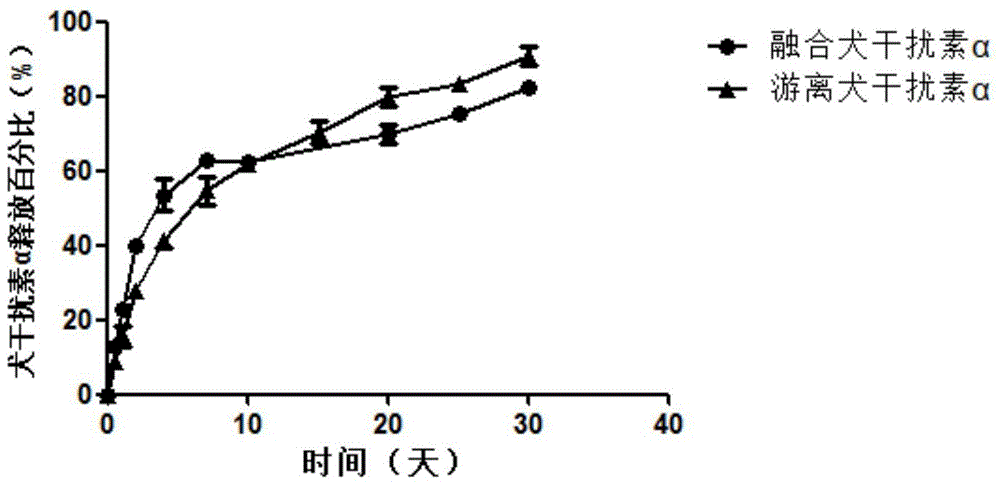
Canine interferon alpha particle compound as well as preparation method and application thereof
ActiveCN104587450ADegradableGood biocompatibilityPeptide/protein ingredientsAntiviralsSide effectAntiviral drug
The invention discloses a canine interferon alpha particle compound CaIFN alpha-NPa and a preparation method thereof. The compound comprises a gamma-polyglutamic acid-phenylalanine shell and canine interferon alpha wrapped in the shell and is formed by assembling the gamma-polyglutamic acid-phenylalanine shell and the canine interferon alpha through a self-polymerization process. The invention also discloses application of the canine interferon alpha particle compound in the preparation of canine antiviral drugs and an antiviral composition. The canine interferon alpha particle compound is high in wrapping rate, free of toxic and side effects and good in biocompatibility, can be used for retarding enzymatic degradation to realize the drug effect slow-release effect, is simple and easy to operate, low in cost and suitable for large-scale production, and has favorable application prospect.
Owner:EAST CHINA NORMAL UNIV
Composition of liposome, and preparation method
InactiveCN1915222BInhibit transferRegulate immunityOrganic active ingredientsNervous disorderDiseaseSterol
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
Difunctional nanoprobe for detecting mitochondrial cytochrome C as well as preparation method thereof
InactiveCN103550792AGood biocompatibilityGood dispersionIn-vivo testing preparationsBiocompatibility TestingEthyl group
The invention relates to a difunctional nanoprobe for detecting mitochondrial cytochrome C as well as a preparation method thereof. The preparation method comprises the following steps: taking a ligand of the mitochondrial cytochrome C; labeling fluorescein and aldehyde group on two ends of the ligand respectively; then adding magnetosome into the ligand again, and incubating overnight; then removing substances which do not react; then adding 1-(3-dimethyl amino propyl)-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide and methoxy-carboxylation-polyethylene glycol, and incubating overnight; and then removing substances which do not react, thereby obtaining the difunctional nanoprobe for detecting the mitochondrial cytochrome C. The difunctional nanoprobe and the preparation method thereof, which are provided by the invention, have good biocompatibility; the prepared probe for detecting mitochondrial cytochrome C has good dispersibility.
Owner:张薇薇
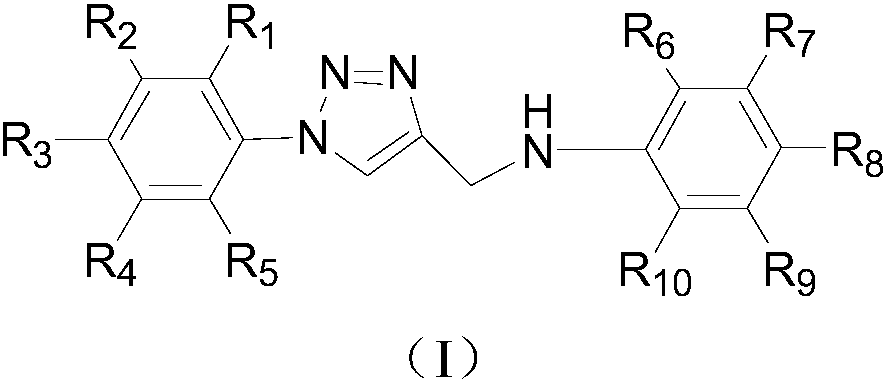
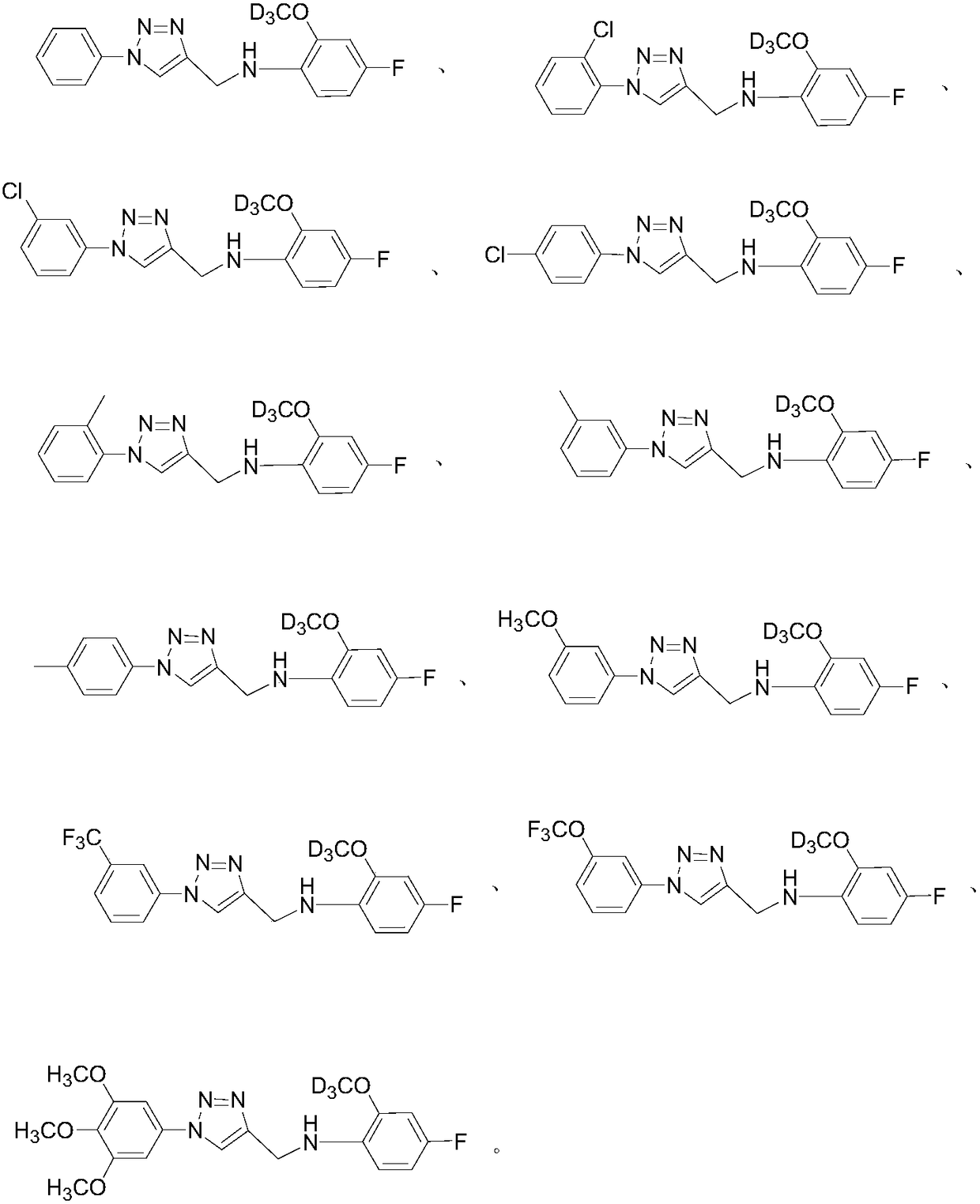
Deuterium labelled 1-substituted phenyl-4-substituted anilinomethyl-1,2,3-triazole derivatives as well as preparation method and application thereof
ActiveCN108358858AProlong metabolic timeImprove efficacyOrganic active ingredientsIsotope introduction to heterocyclic compoundsCancer cellDeuterium labelled
The invention relates to deuterium labelled 1-substituted phenyl-4-substituted anilinomethyl-1,2,3-triazole derivatives. The derivatives have the structure shown as general formula (I) in the description, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9 and R10 can be -F, -Cl, -Br, -I, -OCD3, -NO2, -SO3D, -D, deuterium labelled alkyl groups with 1-18 carbon atoms, deuterium labelled substituted phenyl,deuterium labelled substituted benzyl or deuterium labelled heterocyclic substituents. The invention further discloses a preparation method of the derivatives and an application of the derivatives topreparation of tumor treating or preventing drugs. Compared with the prior art, the deuterium labelled 1-substituted phenyl-4-substituted anilinomethyl-1,2,3-triazole derivatives have the effect of inhibiting cancer cells, and are convenient to synthesize.
Owner:SHANGHAI RES INST OF CHEM IND
Neural stem cells traced by fluorescence and SPECT/CT double-image functional microspheres and application
ActiveCN107384863AGood fluorescence propertiesLarge scattering interfacePowder deliverySenses disorderDiseaseFluorescence
The invention relates to neural stem cells traced by fluorescence and SPECT / CT double-image functional microspheres. The neural stem cells endocytose the fluorescence and SPECT / CT double-image functional microspheres. The fluorescence and SPECT / CT double-image functional microspheres comprise the following components, by mass, of 70%-99.9% of polymers, 0.05%-20% of cyclic metal iridium compounds and 0.05%-10% of I125 agents, wherein the polymers are degradable. The invention further discloses an application of the neural stem cells in preparation of tracers and / or therapeutic agents for a spiral neuronal injury or a neurodegenerative disease. According to the prepared neural stem cells traced by the fluorescence and SPECT / CT double-image functional microspheres, a noninvasive, dynamic, visual, simple and convenient method is provided for tracing tissue engineering seed cells implanted into a living body or a human body, and an observing and repairing technology aiming at the neurodegenerative disease is provided.
Owner:SOUTHEAST UNIV
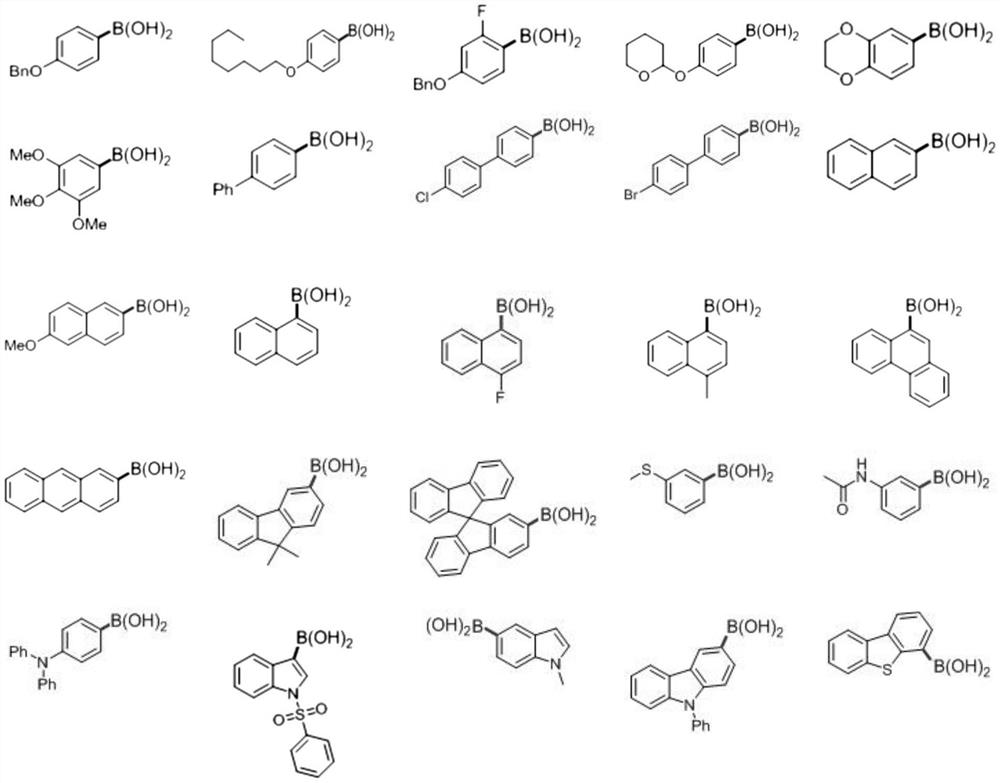
Preparation method of deuterated aromatic compound
PendingCN114105736AReduce generationProlong metabolic timeCarboxylic acid amides preparationAmino-hyroxy compound preparationBoronic acidOrganosolv
The invention relates to the technical field of fine chemicals, and provides a preparation method of a deuterated aromatic compound. The method comprises the following steps: mixing arylboronic acid, a photocatalyst, a Lewis base catalyst, a mercaptan catalyst, deuterated water and an organic solvent, and carrying out deboration deuteration reaction under a visible light irradiation condition to obtain the deuterated aromatic compound. According to the method, the deboration deuteration reaction of arylboronic acid is synergistically catalyzed by adopting visible light, Lewis alkali and mercaptan, the deuterated aromatic compound can be obtained in one step, the deuteration rate is high, the selectivity is good, and the product yield is high; by adopting the method disclosed by the invention, deuterated modification of complex arylboronic acid molecules and drugs can be realized. The result of the embodiment shows that the yield of the deuterated aromatic compound prepared by the preparation method provided by the invention can reach 90%.
Owner:TIANJIN JIKUN MEDICAL TECH CO LTD
Polynuclear compound and preparation method and application thereof
InactiveCN105622603AGood hypoglycemic effectSimple processOrganic active ingredientsOrganic chemistryOrganic chemistry
The invention discloses a polynuclear compound which is of the structure as indicated in the general formula (1).The invention further discloses a preparation method of the polynuclear compound and application of the polynuclear compound to preparation of medicine for treating diabetes.According to the methylated polynuclear compound, under the condition that medicine effects are maintained unchanged, stability of the polynuclear compound is improved.
Owner:PI & PI BIOTECH
Controllable degradation, filling-type complex bone implant of multivariant amino acid polymer-organic calcium/phosphorus salts, and prepration method thereof
ActiveUS20170340776A1Increase the degree of polymerizationHigh molecular weightTissue regenerationCoatingsAminocaproic acidBone implant
The present invention relates to the controllable degradation, filling-type complex bone implant of multivariant amino acid polymer-organic calcium / phosphorus salts, as well as to the preparative method thereof. The complex bone implant is consisted of multivariant amino acid polymers and medically acceptable organic calcium / phosphorus salts, while the content of organic calcium / phosphorus salts is 20-90% based on the total mass of composite material; the multivariant amino acid polymer is polymerized by ε-aminocaproic acid and at least two other amino acids, in which the molar content of ε-aminocaproic acid is at least 50% of the total molar quantity of amino acid polymers, while the amounts of other amino acids are at least 0.5% of the total molar quantity of amino acid polymers.
Owner:SICHUAN GUONA TECH
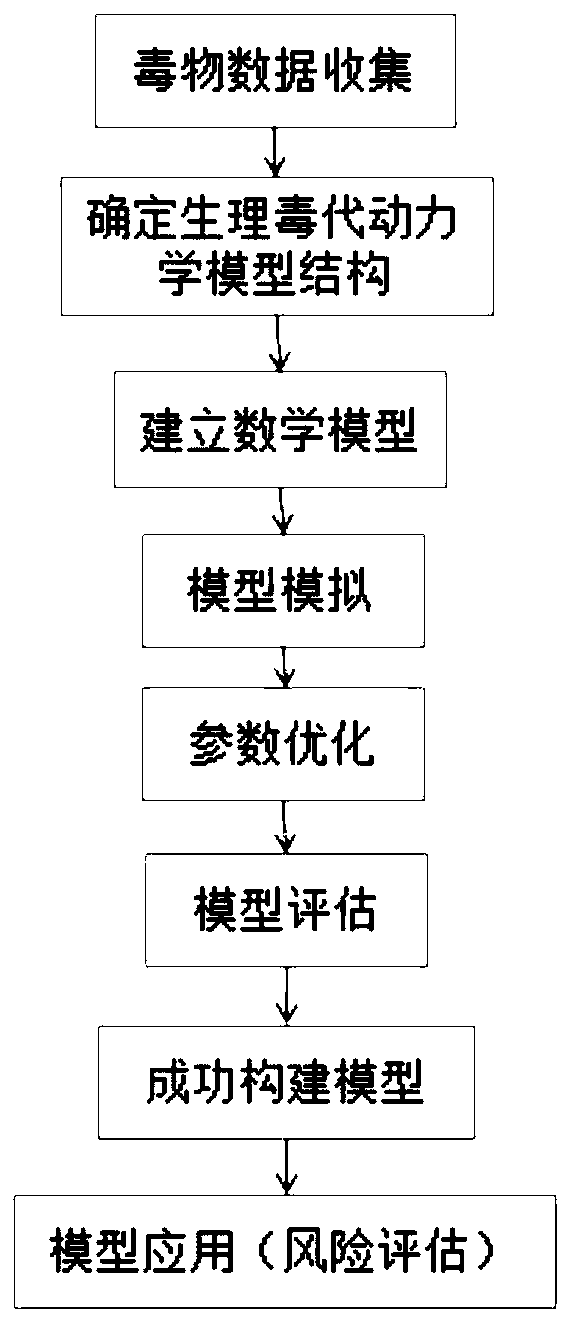
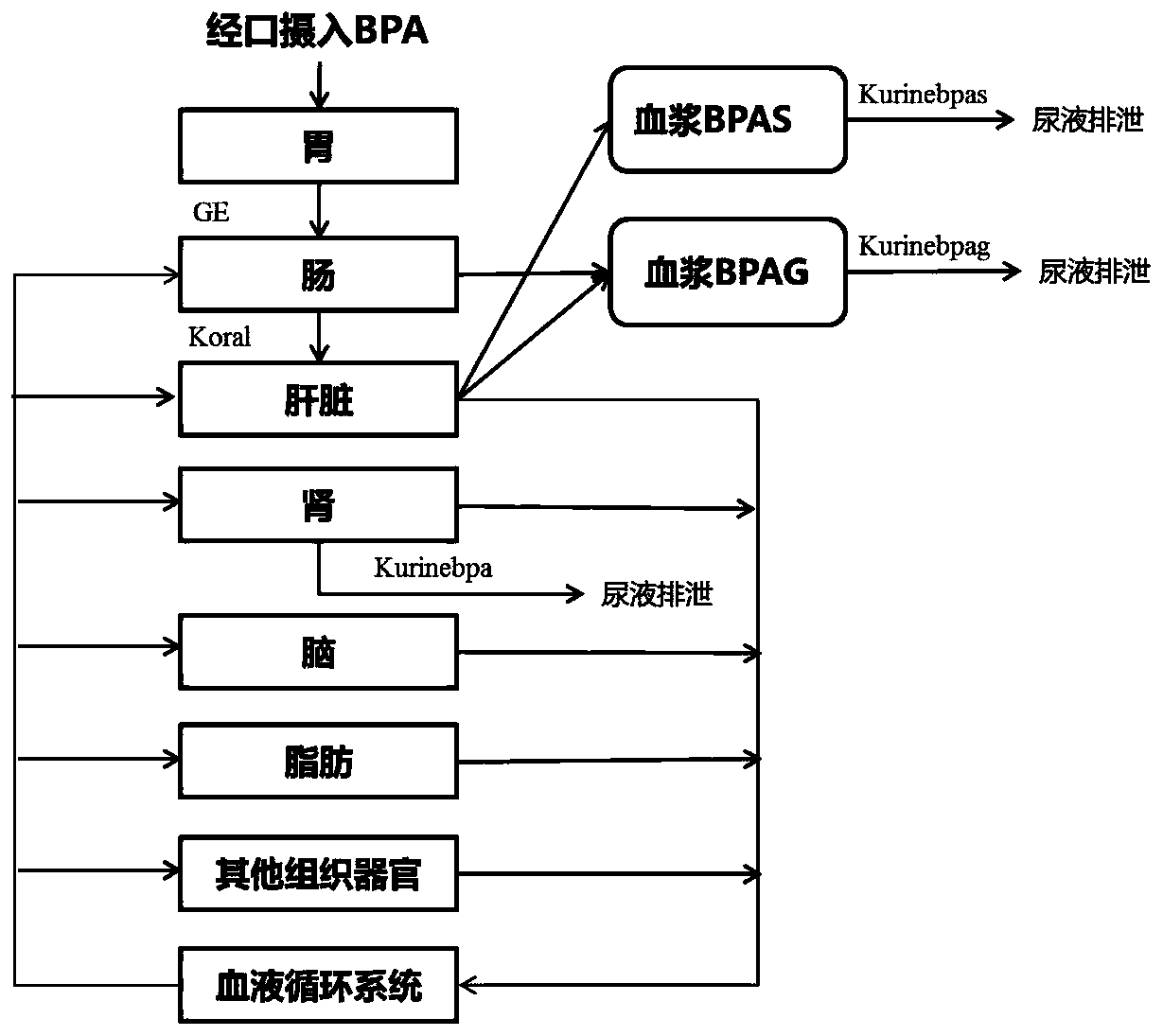
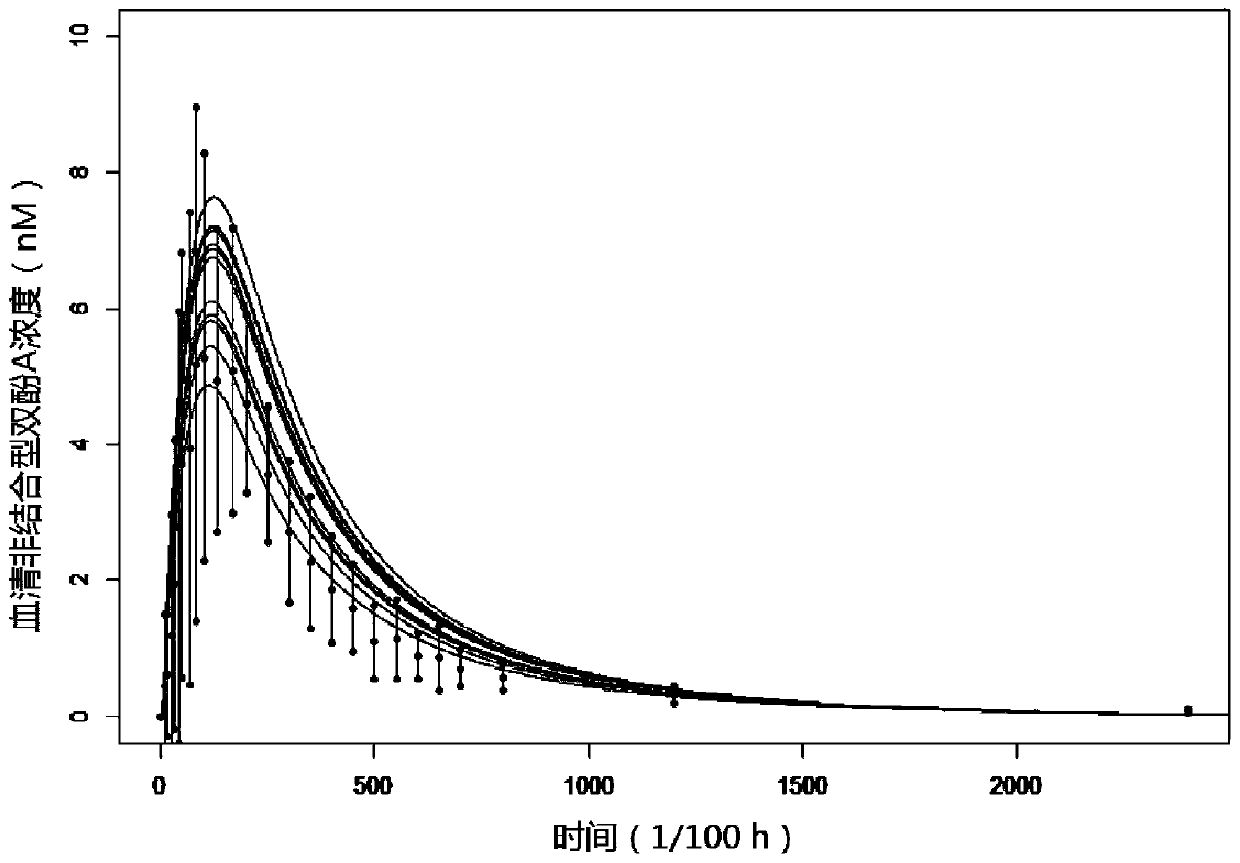
A physiological poison metabolism kinetic model for adults exposed to bisphenol A through mouths
InactiveCN111048143AExclude enterohepatic circulationComplete structureMedical simulationSystems biologyHuman bodyToxicant
The invention relates to the field of public health, particularly relates to chemical risk assessment, and especially relates to a physiological poison metabolism kinetic model for adults exposed to bisphenol A through mouths. The construction method of the model comprises the following steps: S1, determining a kinetic process of absorption, distribution, metabolism and excretion of the bisphenolA orally taken in a human body; S2, determining a physiological poison metabolism kinetic model structure of the adults exposed to bisphenol A through the mouths; S3, establishing a mathematical model, and compiling a differential equation; S4, acquiring human physiological parameters, bisphenol A biochemical parameters and bisphenol A toxicokinetic parameters; and S5, carrying out simulation andparameter optimization on the physiological poison metabolism kinetic model of the bisphenol A. The model and the method disclosed by the invention are helpful to establish bisphenol A internal and external exposure association of the human body, simulate the content level of bisphenol A in a target organ, and further clarify an effect mechanism and a dose-reaction relationship of the bisphenol Ain combination with an effect index of the bisphenol A, so that the health risk that people are exposed to bisphenol A through the mouths can be evaluated more accurately, and important scientific basic data are provided for making and correcting bisphenol A limit values.
Owner:FUDAN UNIV
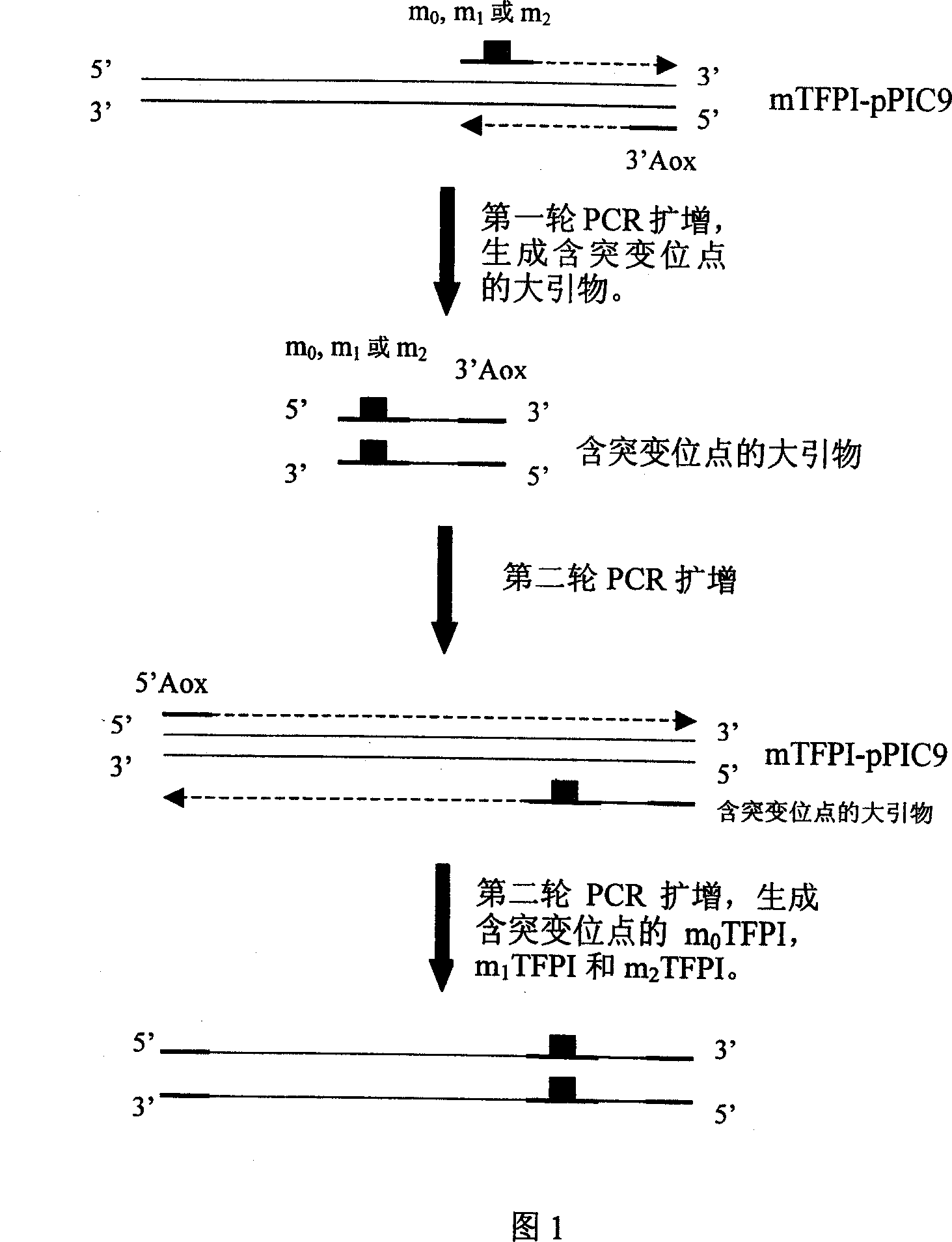
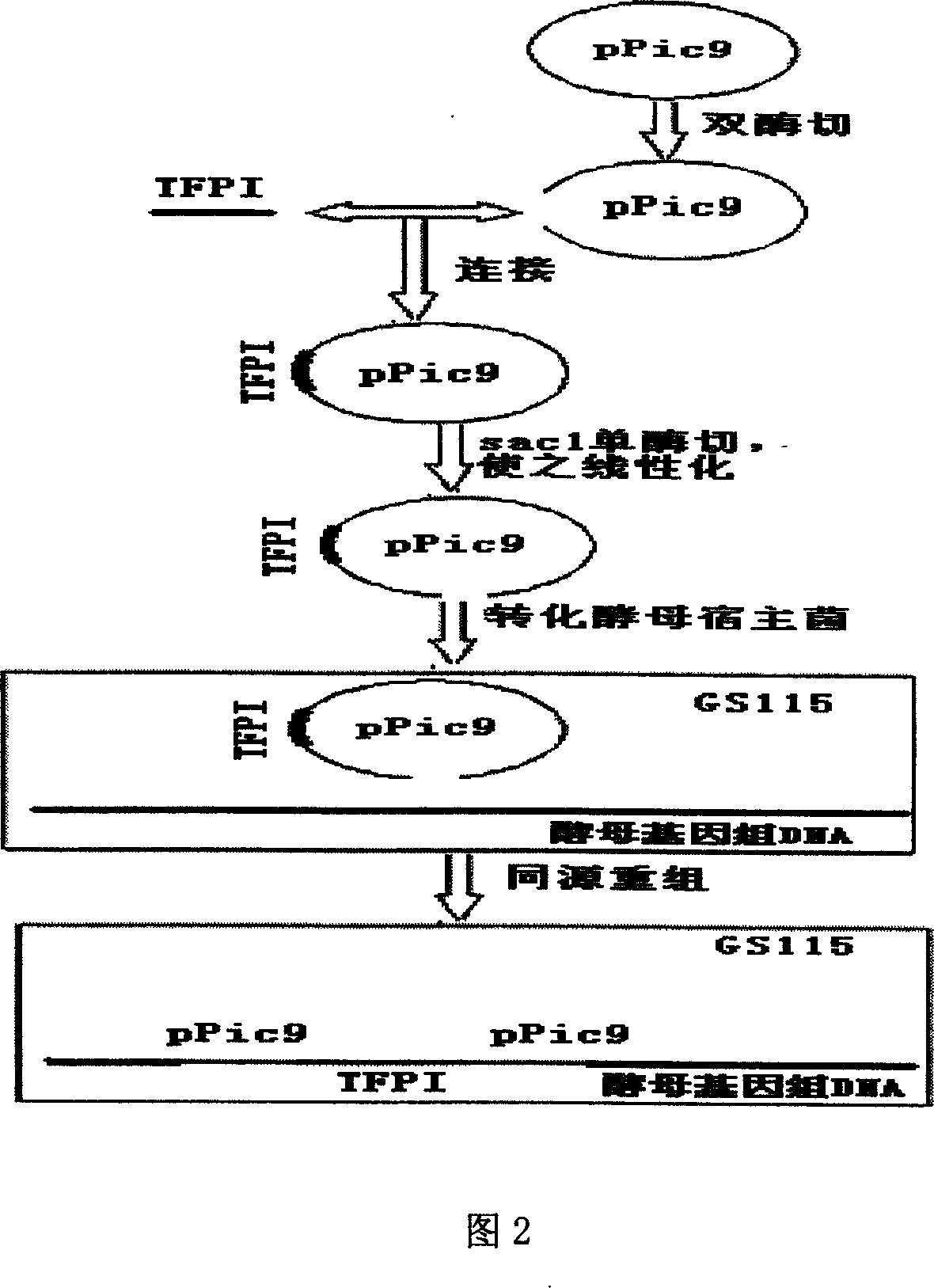
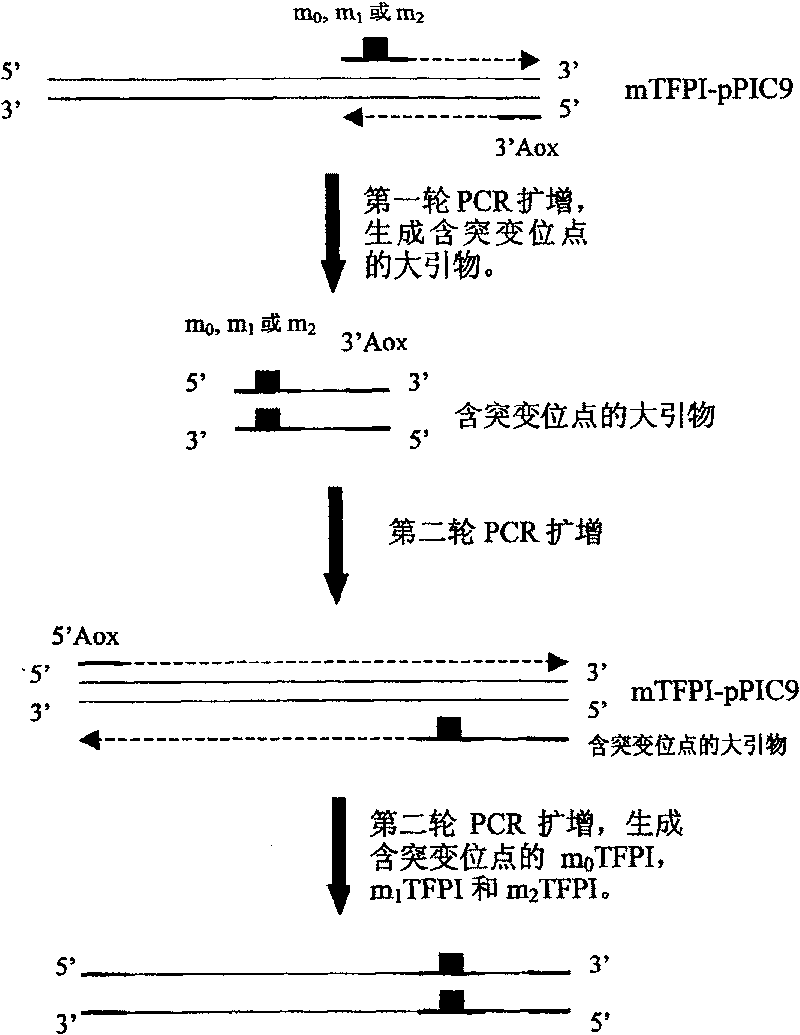
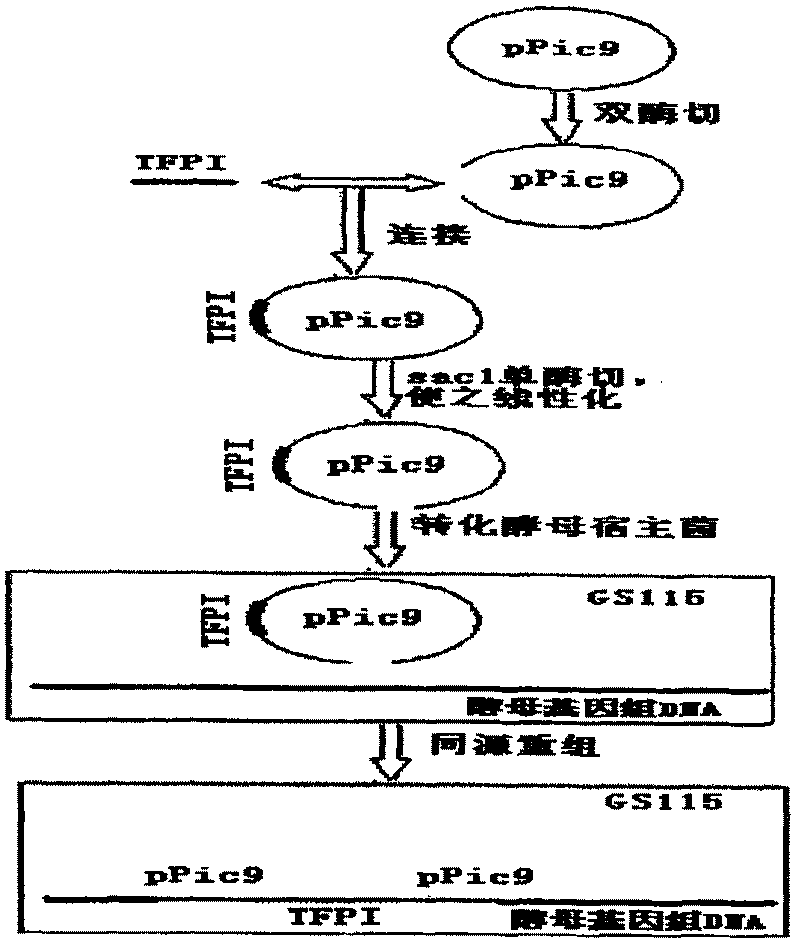
Human tissue factor pathway inhibitory factor mutation gene m2TFPI, recombination carrier and recombination microzyme including the same
InactiveCN1924016AProlong metabolic timeReduce the number of dosesFermentationAnimals/human peptidesNucleotideFactor ii
The invention discloses a mutation gene m2TFPI of human gene inhibitor factor with restructuring carrier and restructuring yeast, which possesses sequence as SEQ ID No.1, wherein the restructuring carrier connects m2TFPI DNA and pPIC9 carrier in the T4 connecting enzyme through digest segment of XhoI and EcoRI to form m2TFPI-pPIC9.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
A compound nano-preparation for treating T-cell acute lymphoblastic leukemia and its preparation method and application
ActiveCN107550887BGood curative effectReasonable formulaOrganic active ingredientsPharmaceutical non-active ingredientsPolyvinyl alcoholLymphoblastic Leukemia
The invention discloses a compound nano-preparation for treating T-cell acute lymphoblastic leukemia and its preparation method and application. Alcohol composition. The steps are: (1) dissolving polylactic acid-polyglycolic acid polymer, IRAK1 / 4 inhibitor and ABT-737 in acetonitrile solution to obtain a mixed solution; (2) emulsifying the mixed solution in polyvinyl alcohol solution to form The emulsion was emulsified on an ice bath using a micro-probe sonicator to obtain an emulsion; (3) the emulsion was stirred at room temperature to obtain inhibitors and compound nanoparticles; (4) the compound nanoparticles were ultracentrifuged in an ultracentrifuge and used Washed with distilled water, freeze-dried and stored to obtain inhibitors and nanoparticles. It also relates to the application of compound nano preparations. The formula is reasonable, the use is convenient, and the metabolism time of the drug is prolonged; the absorption of the drug in tumor cells is increased, thereby further enhancing the synergistic effect of the drug.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Canine interferon α particle complex and its preparation method and application
ActiveCN104587450BDegradableGood biocompatibilityPeptide/protein ingredientsAntiviralsSide effectBiocompatibility Testing
The invention discloses a canine interferon alpha particle compound CaIFN alpha-NPa and a preparation method thereof. The compound comprises a gamma-polyglutamic acid-phenylalanine shell and canine interferon alpha wrapped in the shell and is formed by assembling the gamma-polyglutamic acid-phenylalanine shell and the canine interferon alpha through a self-polymerization process. The invention also discloses application of the canine interferon alpha particle compound in the preparation of canine antiviral drugs and an antiviral composition. The canine interferon alpha particle compound is high in wrapping rate, free of toxic and side effects and good in biocompatibility, can be used for retarding enzymatic degradation to realize the drug effect slow-release effect, is simple and easy to operate, low in cost and suitable for large-scale production, and has favorable application prospect.
Owner:EAST CHINA NORMAL UNIV
A kind of diflavin, its preparation method and application
ActiveCN105693713BImprove efficacyLow toxicityOrganic active ingredientsOrganic chemistryEnteritidesDrug efficiency
The invention discloses a berberrubine and baicalein compound with the structure shown in the general formula (I). The invention further discloses a preparing method of the compound, and the compound is formed by synthesizing berberrubine and baicalein. The invention furthermore discloses application of the compound to preparation of a drug for treating ulcerative enteritis. Compared with berberrubine and baicalein, toxicity of the polynuclear molecular compound is reduced substantially, absorption is improved substantially, bioavailability is improved, the metabolism time is prolonged, the long-acting effect can be achieved, and the pharmacological effects of resisting ulcerative enteritis and pathogenic bacteria are enhanced.
Owner:PI & PI BIOTECH
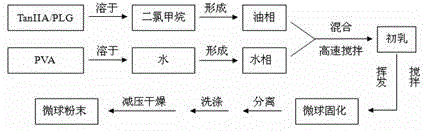
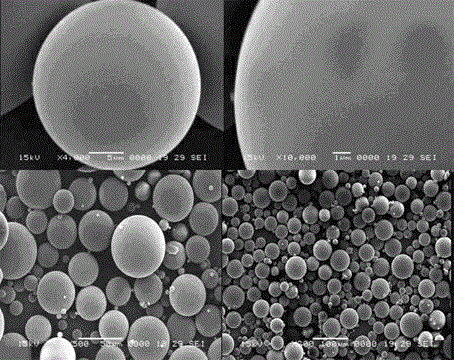
Tanshinone IIA-polyactic acid/hydroxyacetic acid microsphere and preparation method thereof
InactiveCN103083250BHigh specific activityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentPolyvinyl alcohol
The invention discloses a tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere and a preparation method thereof. The microsphere is prepared by drying oil-in-water type emulsion, wherein the oil phase is dichloromethane solution of a polyactic acid / hydroxyacetic acid copolymer and the water phase is the water solution of polyvinyl alcohol. The drug content of tanshinone IIA in the microsphere is 1-10% and the entrapment efficiency is 60-90%. The particle size range of the microsphere is 30-200mm. The tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere provided by the invention is suitable for interventional therapy of liver cancers, has a good liver tumor peripheral vascular thrombosis function, has an effective thrombosis time of 7-60 days, can be distributed in tumor tissues in a targeted manner, slowly release drugs, increase the local concentrations of the drugs, prolong the drug metabolism time, obviously inhibit animal liver tumor growth and prolong the animal lifetime and can inhibit expressions of a human hypoxia inducible factor 1alpha and a vascular endothelial growth factor and reduce the tumor tissue microvessel density after thrombosis.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M +1
Radionuclide-labeled estrogen receptor molecular targeting compound and application thereof
ActiveCN112409436AGood receptor targetingReduce non-target background uptakeRadioactive preparation carriersSteroidsEstrogen receptorBiochemistry
The invention discloses a radionuclide-labeled estrogen receptor molecular targeting compound and application thereof. The structural formula of the radionuclide-labeled estrogen receptor molecular targeting compound is described in the descriptions of the invention, contains an estrogen structure and has good estrogen receptor targeting in vitro and vivo; radioactive iodine isotopes (123I, 124I,125I or 131I) and 18F are used as radioactive signal groups to perform SPECT or PET development on estrogen receptors in organs or tissues of human or animals and treat positive tumors of the estrogenreceptors.
Owner:XIAMEN UNIV
Composite bone implant material and method of making thereof
ActiveUS10967099B2Provide certain mechanical strengthReduce regularityTissue regenerationCoatingsAminocapronsäureMedicine
The present invention relates to the controllable degradation, filling-type complex bone implant of multivariant amino acid polymer-organic calcium / phosphorus salts, as well as to the preparative method thereof. The complex bone implant is consisted of multivariant amino acid polymers and medically acceptable organic calcium / phosphorus salts, while the content of organic calcium / phosphorus salts is 20-90% based on the total mass of composite material; the multivariant amino acid polymer is polymerized by ε-aminocaproic acid and at least two other amino acids, in which the molar content of ε-aminocaproic acid is at least 50% of the total molar quantity of amino acid polymers, while the amounts of other amino acids are at least 0.5% of the total molar quantity of amino acid polymers.
Owner:SICHUAN GUONA TECH
A kind of high surface tension hydrogel glass body substitute and radiation preparation method thereof
ActiveCN106432810BIrradiation technology is non-toxicMild reaction conditionsTissue regenerationProsthesisHigh surfaceRetina
The invention discloses a high-surface-tension hydrogel vitreous substitute and a radiation preparation method thereof. The preparation method comprises the following steps: performing ultrasonic stirring on natural polysaccharides, supermolecules, a radiation sensitizer, a pH adjuster and a solvent which serve as main components to prepare a uniform dispersion system, feeding N2 for stewing and debubbling at negative pressure, performing vacuum encapsulation and quick circulating freezing-defreezing, and performing radiation crosslinking reaction on the dispersion system with the natural polysaccharides and the supermolecules, which serve as base materials, under ionizing radiation to prepare the high-surface-tension hydrogel vitreous substitute. The product is emulsification-free and dispersion-free, is higher in surface tension, can be self-degraded and absorbed slowly, is relatively long in metabolic time, can up press irregular surfaces and effectively close a retinal hole, is not liable to enter a retina, and has the characteristics of high light transparency, viscoelasticity, shock absorption property, uniformity, biological compatibility, safety and the like; preparation, plasticization, sterilization and the like can be synchronously completed, and a production process is simplified.
Owner:HUBEI UNIV OF SCI & TECH
Non-toxic cross-linked sodium hyaluronate gel articular cavity injection and preparation method thereof
PendingCN112675125AEasy to prepareMild reaction conditionsOrganic active ingredientsAerosol deliveryPtru catalystSolvent
The invention relates to the technical field of medical injections, in particular to a non-toxic cross-linked sodium hyaluronate gel articular cavity injection and a preparation method thereof. The product is gel formed by cross-linking sodium hyaluronate serving as a main body and amino acid containing two or more amino groups serving as a cross-linking agent under the action of a catalyst. The preparation method comprises the following steps of dissolving sodium hyaluronate in a certain amount of solvent I; then adding the cross-linking agent for dissolving; adding the catalyst, and carrying out a cross-linking reaction; dialyzing a product obtained by crosslinking through the solvent I to remove the cross-linking agent and the catalyst remained in the cross-linking reaction process; or carrying out precipitation collection on the product obtained by cross-linking by using a solvent II, drying, and dissolving the precipitation product in the solvent I until the required concentration is achieved; and filling the obtained product into a pre-filled syringe under a sterile condition, sterilizing the filled product, and carrying out light inspection to obtain the final product. The product is high in liquidity and long in degradation time, and reduces infection risks and pains of patients.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
Human tissue factor pathway inhibitory factor mutation gene m2TFPI, recombination carrier and recombination microzyme including the same
InactiveCN1924016BProlong metabolic timeReduce the number of dosesFermentationAnimals/human peptidesNucleotideFactor ii
The invention discloses a mutation gene m2TFPI of human gene inhibitor factor with restructuring carrier and restructuring yeast, which possesses sequence as SEQ ID No.1, wherein the restructuring carrier connects m2TFPI DNA and pPIC9 carrier in the T4 connecting enzyme through digest segment of XhoI and EcoRI to form m2TFPI-pPIC9.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Preparation method of gossypol acetate calcium alginate gel microspheres
ActiveCN107802602BHigh embedding rateGood swelling propertiesAldehyde active ingredientsPharmaceutical non-active ingredientsMicrosphereEthylic acid
The invention discloses a preparation method of gossypol acetate calcium alginate gel microspheres. Gossypol acetate is dissolved into N,N-dimethyl acetamide; epsilon-polylysine, diclofenac potassiumand potassium chloride are dissolved into water; the solution is uniformly mixed to obtain a stock solution; calcium chloride is dissolved into water; sodium alginate is dissolved into water to be prepared into a solution with the mass concentration being 1 to 2 percent; the stock solution is added into the sodium alginate solution; stirring is performed till the uniform mixing; a micro quantity injection pump is used for dripping the solution into the calcium chloride solution; gelatinization is performed for 20 to 40min to obtain microspheres. A buffer solution is used for flushing the microspheres to obtain the gossypol acetate calcium alginate gel microspheres. The gossypol acetate calcium alginate gel microspheres have the advantages of high embedding rate, good swelling property andgood medicine release property.
Owner:山东海诺知识产权运营管理有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com