Berberine-baicalin compound and preparation method and application thereof
A technology of diglucoside and baicalin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, and pharmaceutical formulations, to achieve the effects of enhancing drug efficacy, facilitating large-scale industrial production, and meeting various needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
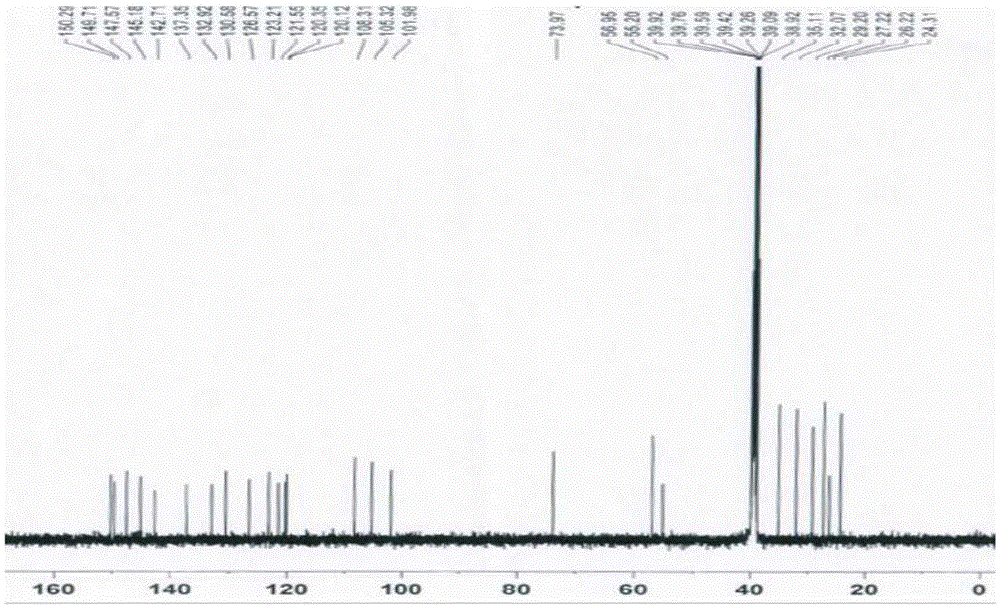
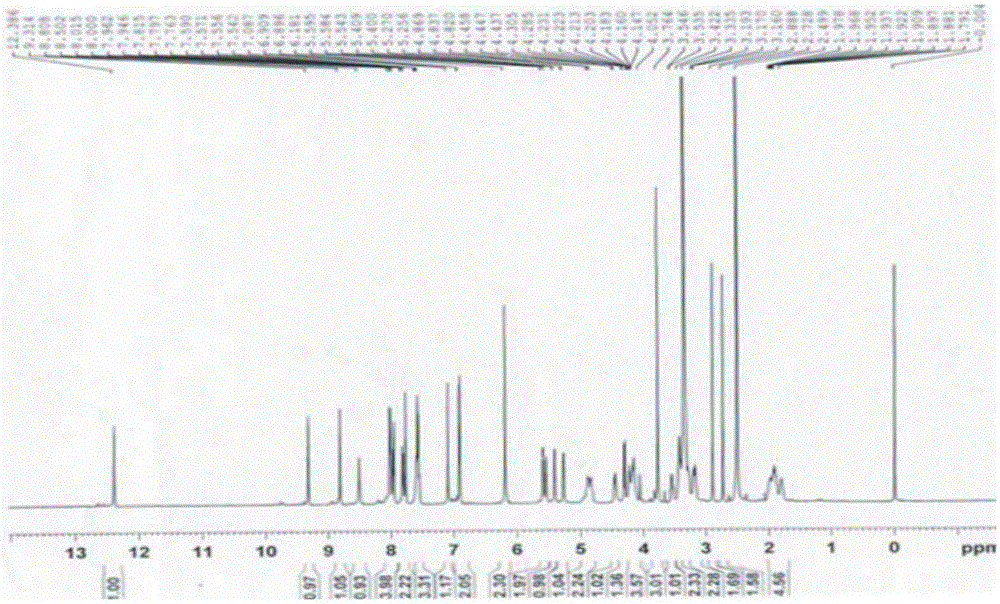
[0076] (1a) Synthesis of berberine derivatives: Weigh 1 part by weight of berberine, add 150 (150-200) parts by weight of acetonitrile, heat to boiling at 85°C, add X(CH 2 ) n Y40 (20-40) parts by weight, reflux for 3 hours, concentrate the reaction solution to 50 (50-100) parts by weight, cool and crystallize, filter, wash the crystals with an appropriate amount of acetonitrile, combine the washing liquid with the filtrate, and recover the acetonitrile . The filter residue was dissolved in 30 parts by weight of methanol, cooled to crystallize, filtered, washed with methanol, and combined with the above crystals to obtain a berberine derivative with a calculated yield of 56.34%. Among them, X(CH in this embodiment 2 ) n Y, X, and Y are all Br, n=4, and the gained berberine derivative is berberine-9-oxobutyl bromide, and its 1 HNMR picture as shown figure 1 as shown, 13 CNMR picture as figure 2 shown. in, figure 1 The attribution of H in is shown in Table 1, figure ...
Embodiment 2
[0091] In addition to the amount of acetonitrile, X(CH 2 ) n Except that the amount of Y and the concentration of the reaction solution are different, the preparation method of dixanthoside in this implementation is basically the same as that in Example 1. Among them, the X(CH of the present embodiment 2 ) n Y, X, Y are all Cl, n=5.
Embodiment 3
[0093] In addition to the amount of acetonitrile, X(CH 2 ) n Except that the amount of Y and the concentration of the reaction solution are different, the preparation method of dixanthoside in this implementation is basically the same as that in Example 1. Among them, the X(CH of the present embodiment 2 ) n Y, X and Y are all S, n=6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com