Radionuclide-labeled estrogen receptor molecular targeting compound and application thereof
A radionuclide and estrogen receptor technology, applied in the field of estrogen receptor molecular targeting compounds, can solve the problems of unsatisfactory uptake of radiotracers, non-specific uptake, etc., achieve suitable circulation time in the body, and improve specificity Effects of ingestion and reduction of radiation damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
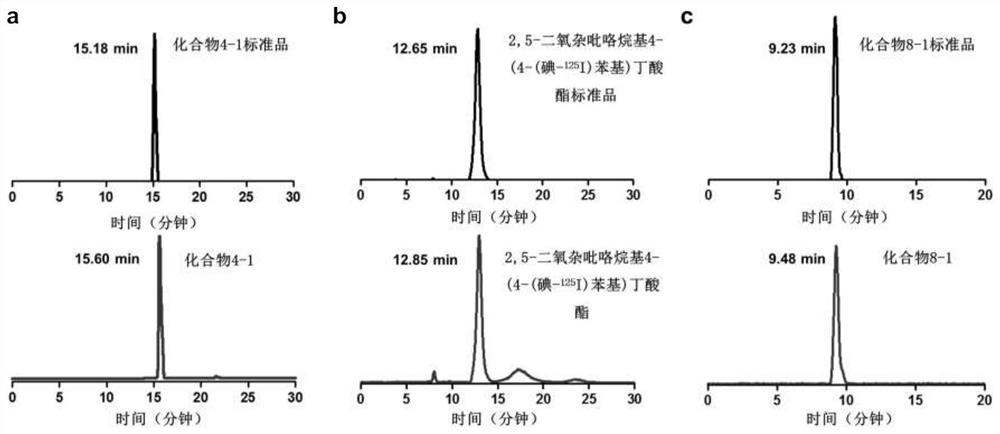
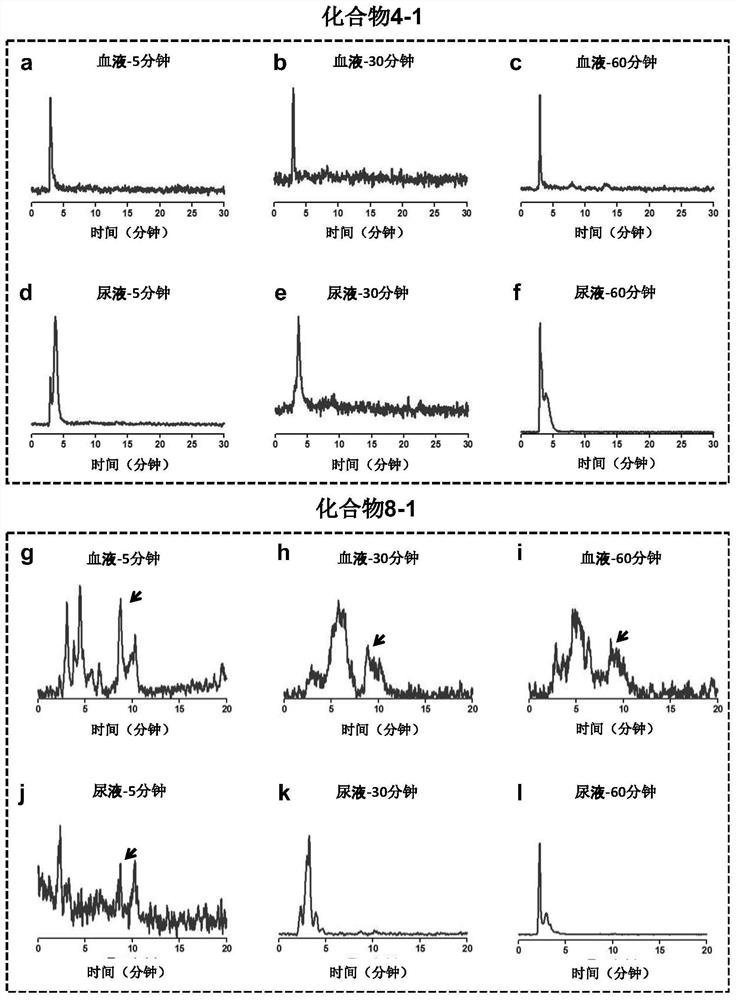
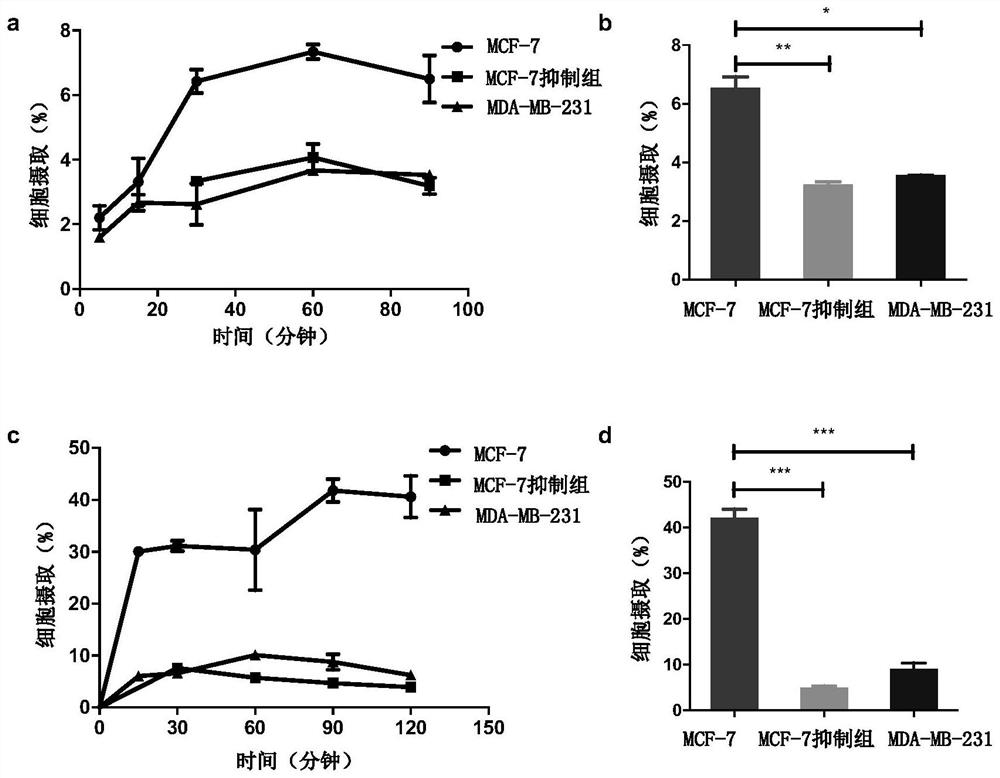
[0055] Synthesis of compound 4-1: when R in the above structural formula 1 to R 5 =H,R 6 = 131 I (radioactive iodine isotope), R 7 = 127 When I and n=0, it is compound 4-1. The radioactive iodine isotope is labeled by a "click" chemical reaction, and its synthetic route and preparation method are as follows:
[0056]
[0057] Specifically include the following steps:
[0058] 1) Synthesis of 3-azidopropylamine:
[0059] 3-Bromopropylamine hydrochloride (5 g, 38 mmol) was dissolved in 20 mL of deionized water at room temperature. At 0°C, aqueous sodium azide solution (7.5 g, 115 mmol, 20 mL) was added dropwise to the above solution. After the dropwise addition, the temperature was raised to 80°C, and the reaction was carried out overnight. The reaction system was cooled to 0°C, and 50 mL of ether was added. Weigh 4g of potassium hydroxide particles and slowly add it to the reaction flask, keeping the temperature at a low temperature. Extract with ether (50 mL×3), ...
Embodiment 2
[0065] Synthesis of compound 4-2: when R in the above structural formula 1 =H,R 2 =F,R 3 , R 4 =H,R 5 =OCH 3 , R 6 = 131 I (radioactive iodine isotope), R 7 = 127 When I and n=0, it is compound 4-2. The radioactive iodine isotope is labeled by a "click" chemical reaction, and its synthetic route and preparation method are as follows:
[0066]
[0067] 1) Synthesis of 4-fluoro-17α-alkynyl-11β-methoxyestradiol:
[0068] Under nitrogen atmosphere, N-fluoropyridinium triflate (1.83 g, 7.4 mmol) was added to 17α-alkynyl-11β-methoxy dissolved in 16 mL 1,1,2-trichloroethane estradiol (1.2 g, 3.7 mmol). After the reaction was complete, the solvent was removed by rotary evaporation and the residue was poured into water and extracted with dichloromethane. The crude product was purified by column chromatography (ethyl acetate / dichloromethane=1:9) to obtain 0.8 g of a pale yellow solid in 63% yield.
[0069] 2) Labeling of compound 4-2:
[0070] 4 mL of anhydrous acetonit...
Embodiment 3
[0072] Synthesis of compound 8-1: when R in the above formula 1 , R 2 , R 3 , R 4 , R 5 and R 6 =H, and R 7 = 125 I (radioactive iodine isotope), when n=3, is compound 8-1. The radioactive iodine isotope is labeled with phenylboronic acid precursor, and then connected to the estrogen structure. The synthetic route and preparation method are as follows:
[0073]
[0074] Specifically include the following steps:
[0075] 1) Synthesis of tert-butyl (2-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)carbamate:
[0076] 2-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethanamine (1 g, 4.6 mmol) and triethylamine (0.7 g, 6.9 mmol) were combined with Anhydrous 5mL of tetrahydrofuran was dissolved, and stirred at room temperature for 0.5h. Then di-tert-butyl dicarbonate (1.1g, 5mmol) was added, and the reaction was carried out at room temperature overnight. The reaction solvent was spin-dried and extracted with ethyl acetate (40mL×3). The organic layer was collected, The solvent was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com