A kind of diflavin, its preparation method and application
A technology of biflavin and baicalein, applied in the field of medicinal chemistry and pharmacotherapeutics, to achieve the effect of improving absorption, facilitating large-scale industrial production, and enhancing drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
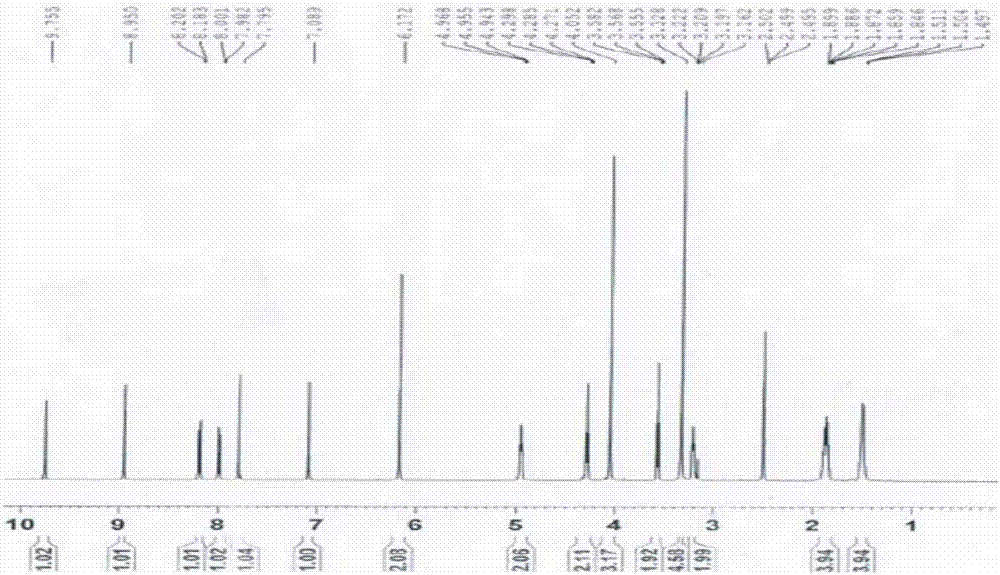
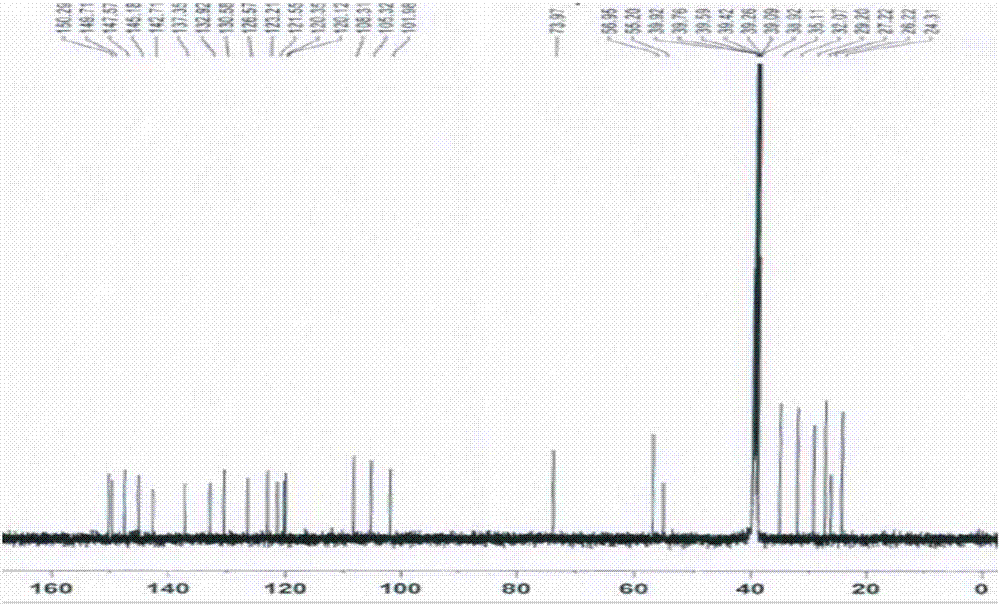
[0076] (1a) Synthesis of berberine derivatives: Weigh 1 part by weight of berberine, add 150 (150-200) parts by weight of acetonitrile, heat to boiling at 85°C, add X(CH 2 ) n Y 40 (20-40) parts by weight, reflux for 3 hours, concentrate the reaction solution to 50 (50-100) parts by weight, cool to obtain crystal A, filter, wash crystal A with an appropriate amount of acetonitrile, washing liquid and filtrate Combine and recover acetonitrile. The filter residue was dissolved with 30 parts by weight of methanol, cooled to obtain crystal B, filtered, crystal B was washed with methanol, and crystal A and crystal B were combined to obtain a berbererythrine derivative with a calculated yield of 56.34%. Among them, X(CH in this embodiment 2 ) n Y, X, and Y are all Br, n=4, and the gained berberine derivative is berberine-9-oxobutyl bromide, and its 1 H NMR picture as figure 1 as shown, 13 C NMR picture as figure 2 shown. in, figure 1 The attribution of H in is shown in Ta...
Embodiment 2
[0091] In addition to the amount of acetonitrile, X(CH 2 ) n Except that the amount of Y and the concentration of the reaction solution are different, the preparation method of the diflavin in this implementation is basically the same as that of Example 1. Among them, the X(CH of the present embodiment 2 ) n Y, X, Y are all Cl, n=5.
Embodiment 3
[0093] In addition to the amount of acetonitrile, X(CH 2 ) n Except that the amount of Y and the concentration of the reaction solution are different, the preparation method of the diflavin in this implementation is basically the same as that of Example 1. Among them, the X(CH of the present embodiment 2 ) n Y, X and Y are all S, n=6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com