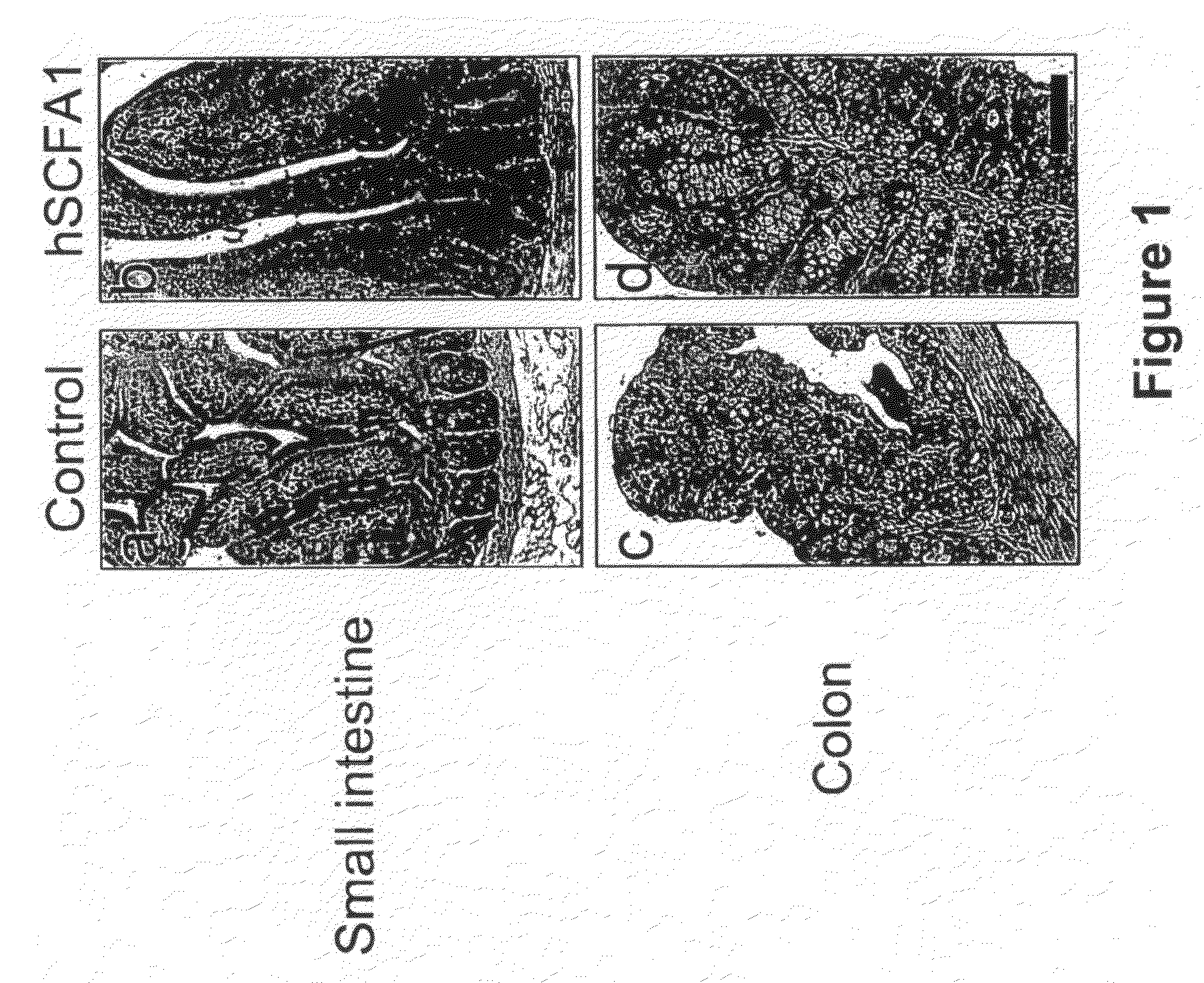
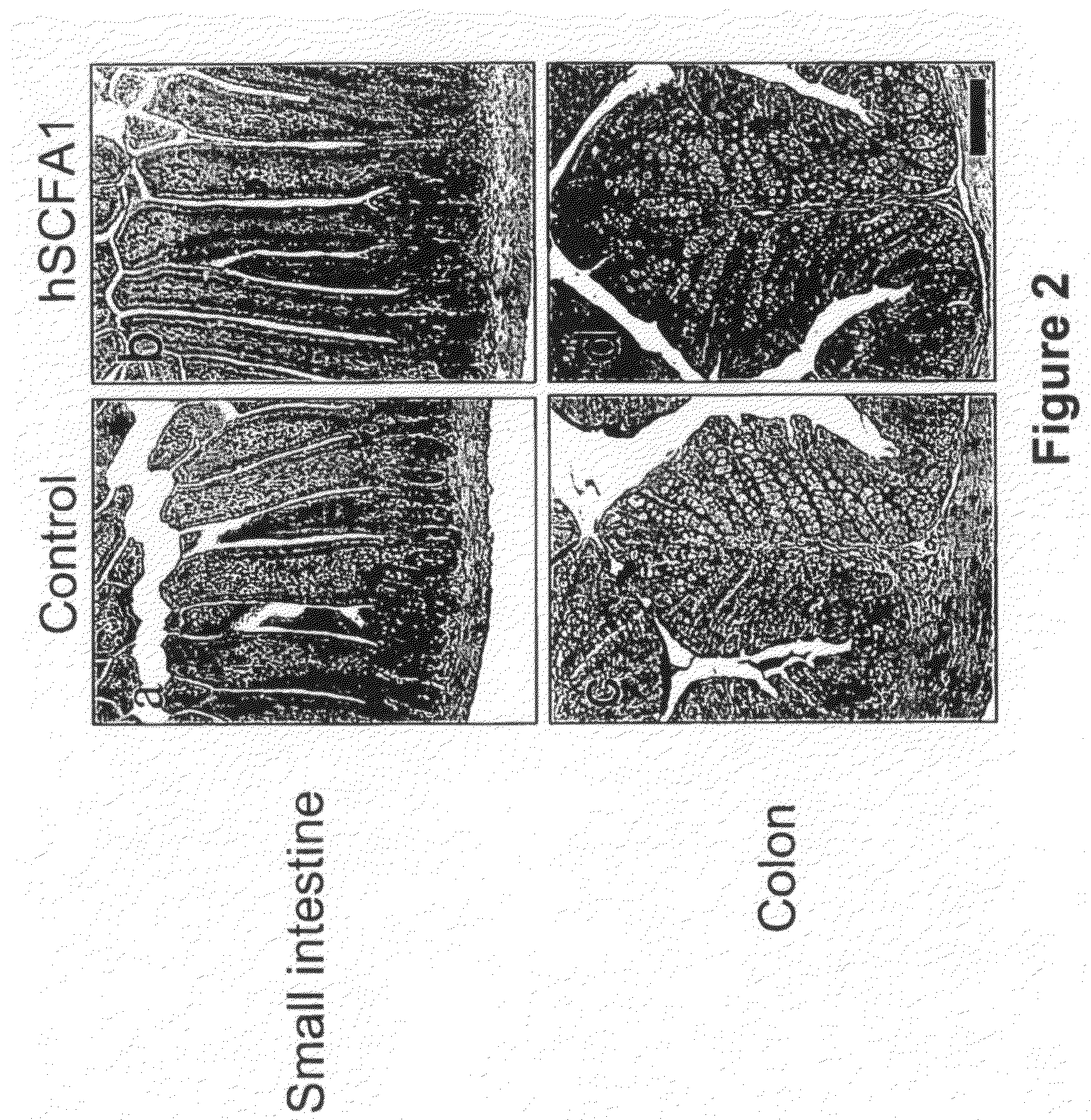
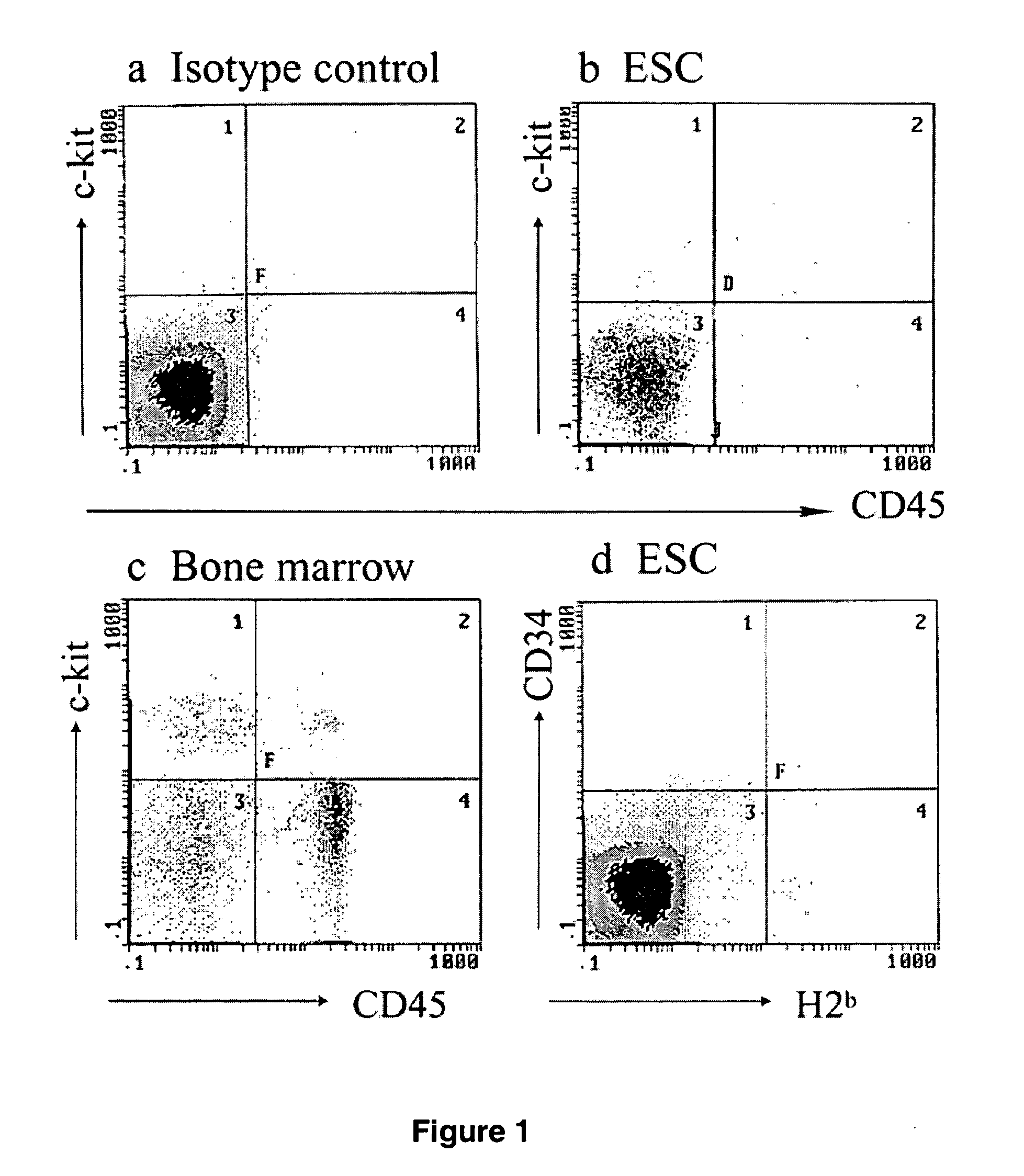
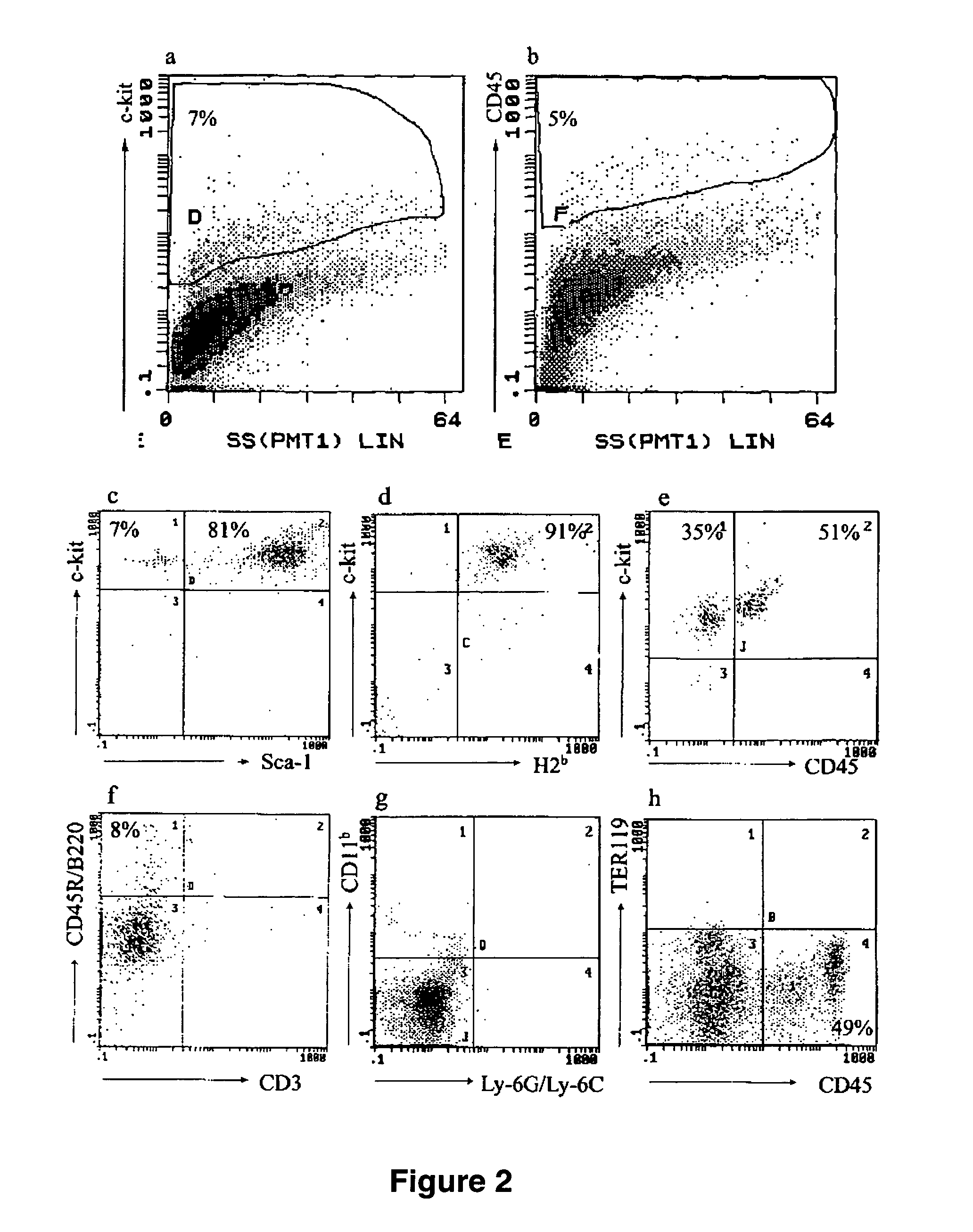
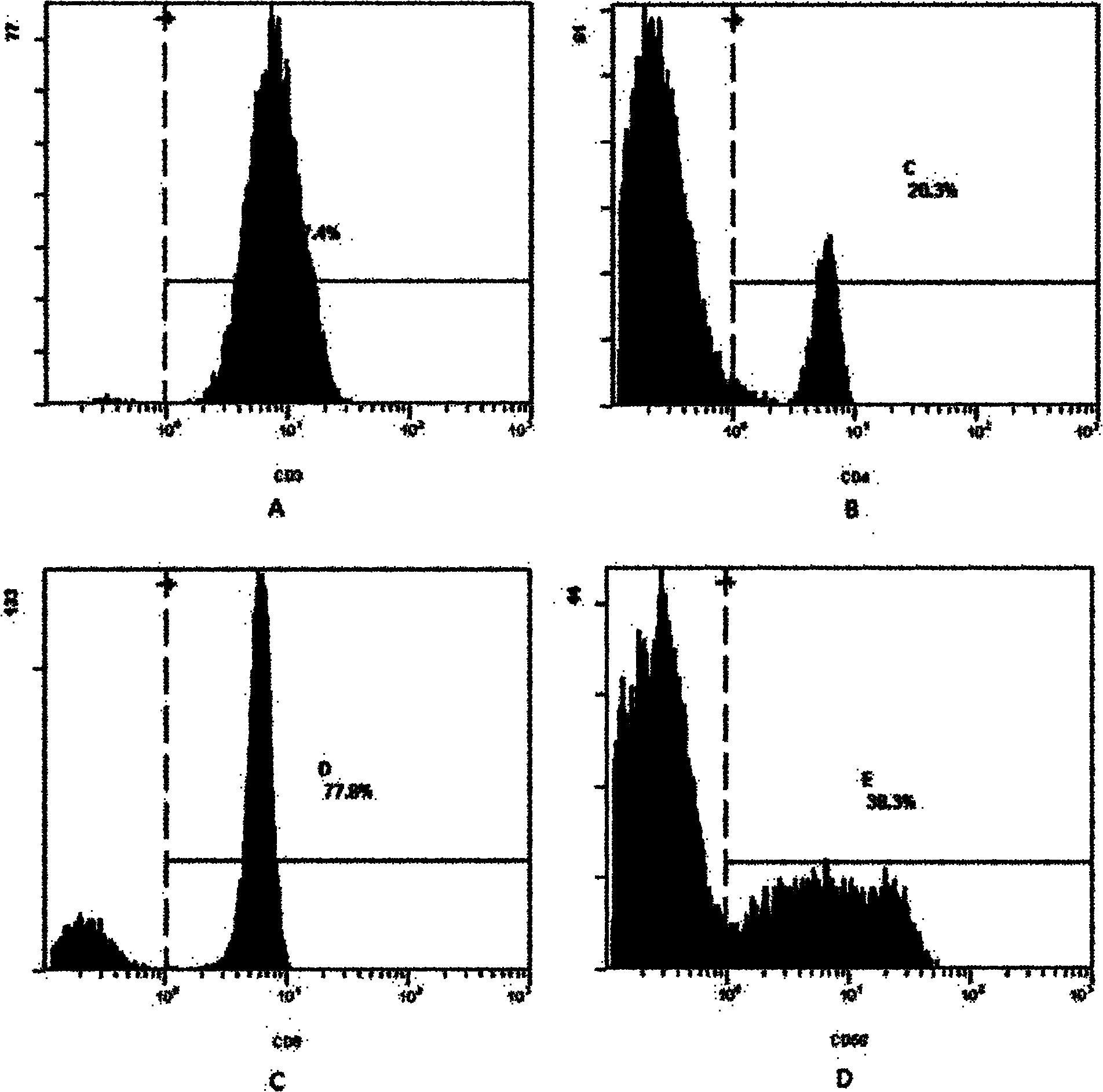
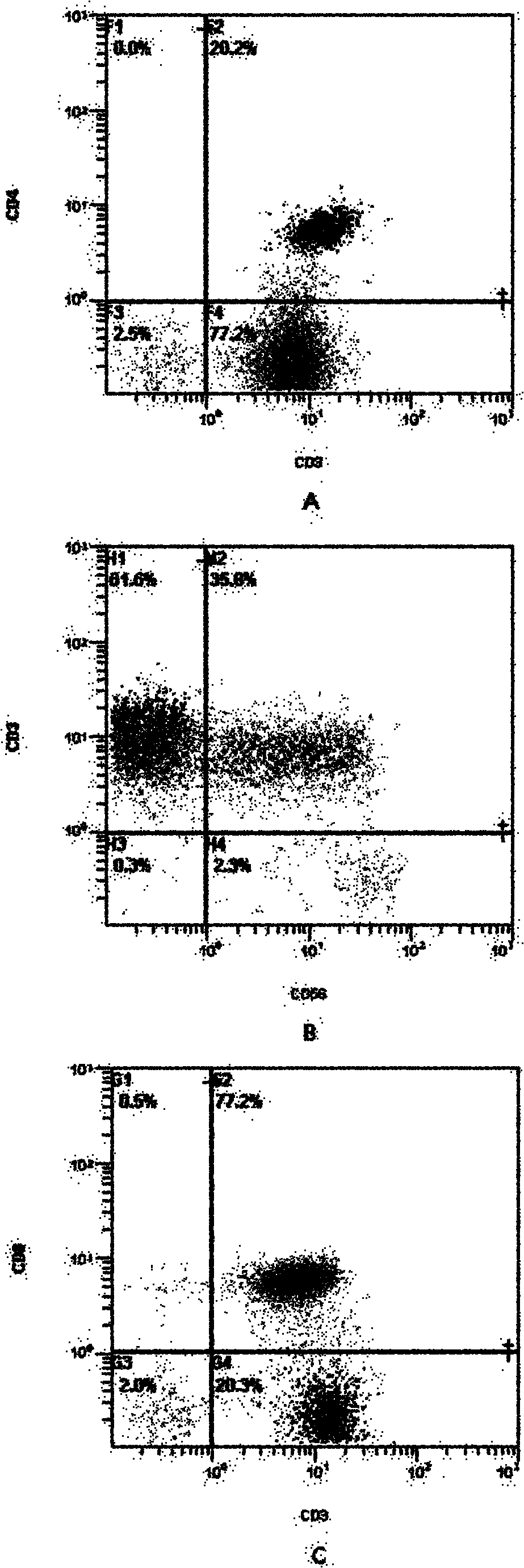
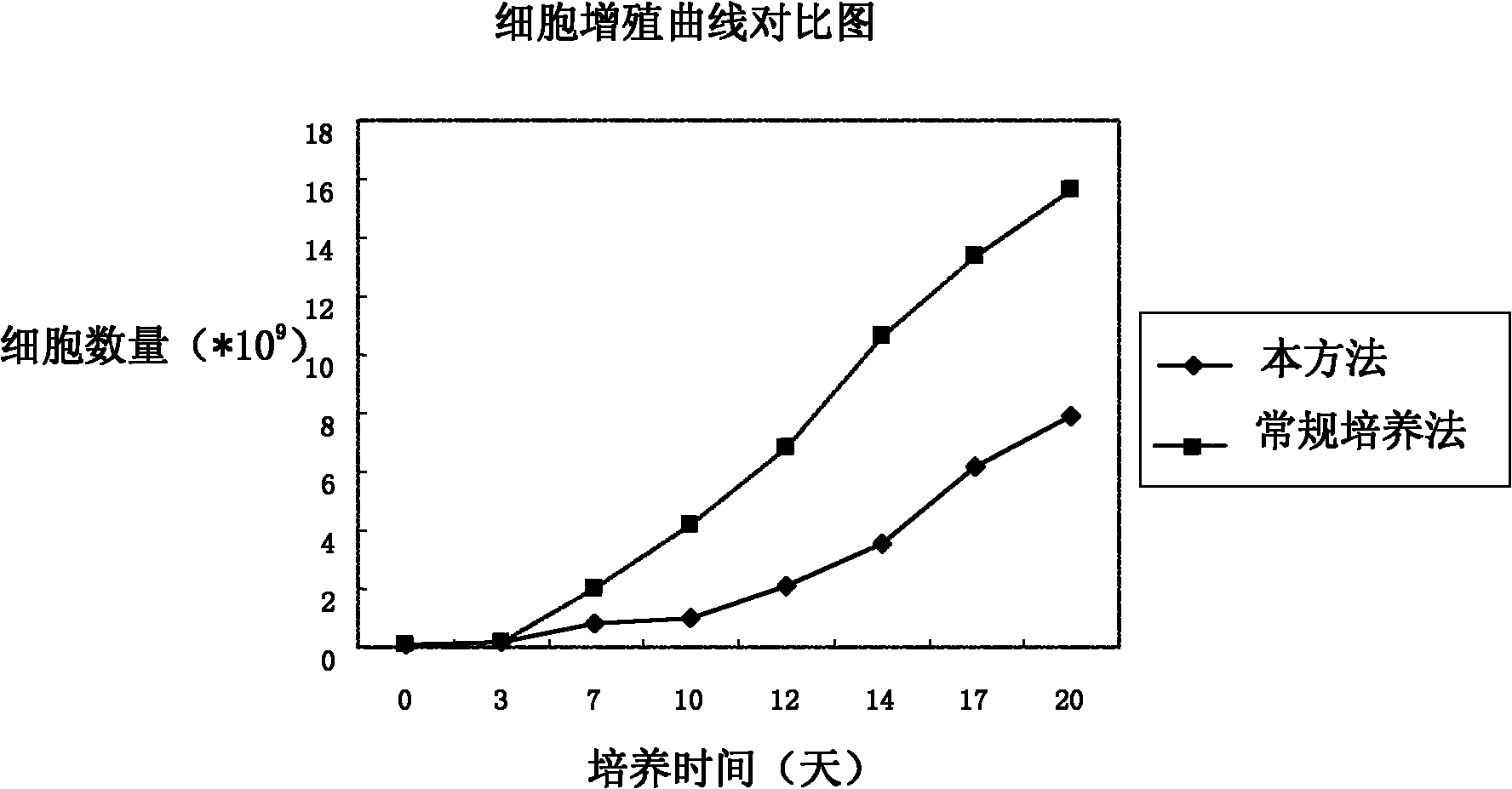
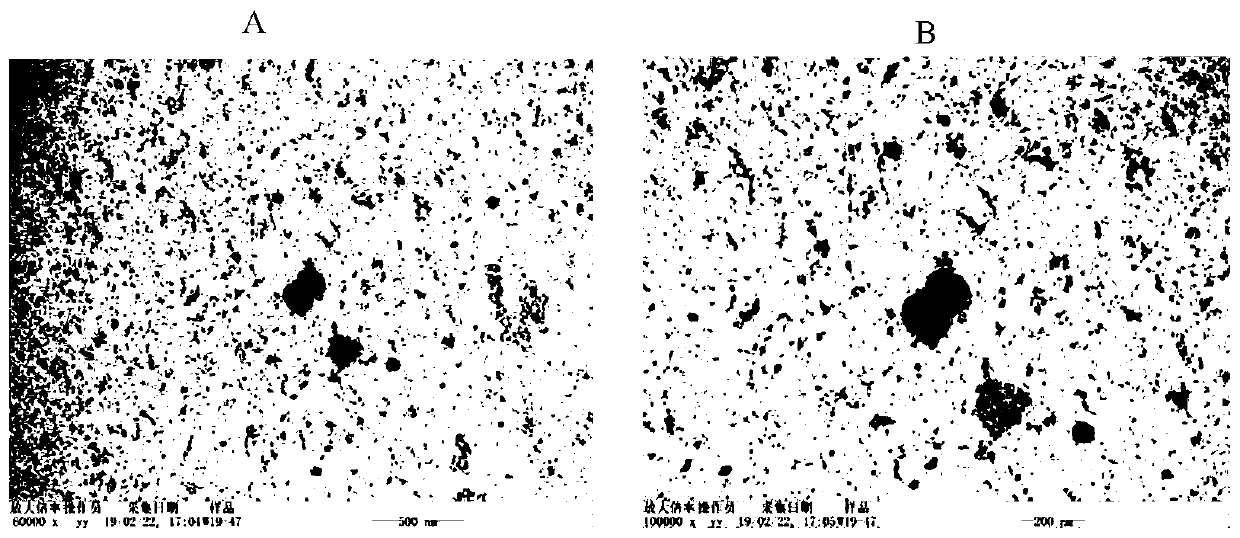
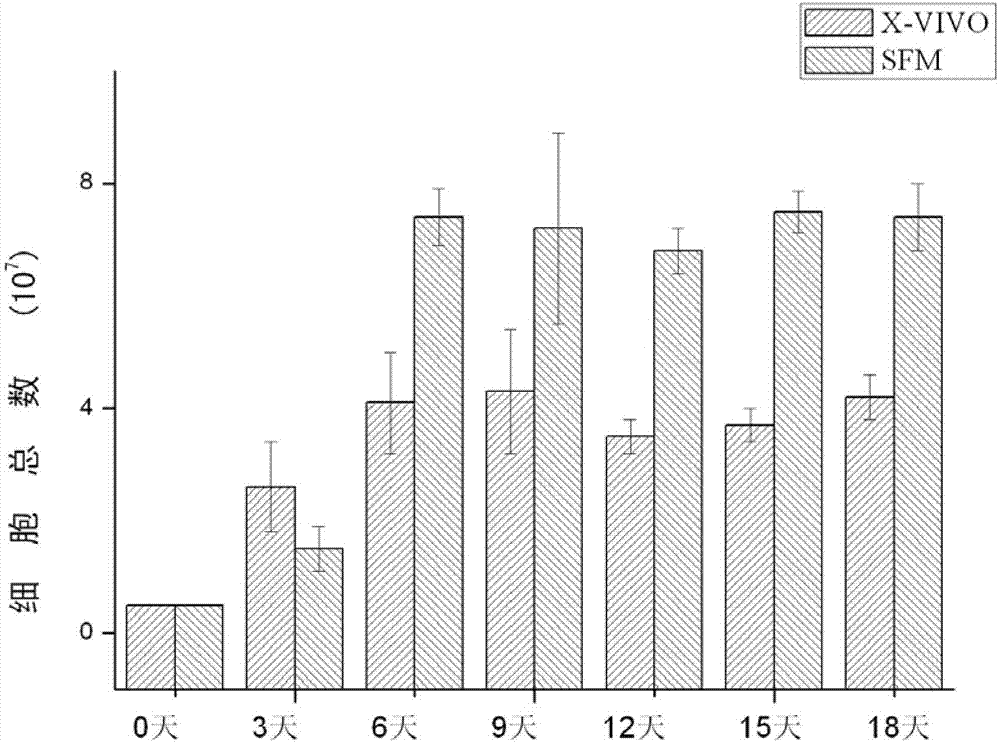
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
309 results about "Stem cell factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stem cell factor (also known as SCF, KIT-ligand, KL, or steel factor) is a cytokine that binds to the c-KIT receptor (CD117). SCF can exist both as a transmembrane protein and a soluble protein. This cytokine plays an important role in hematopoiesis (formation of blood cells), spermatogenesis, and melanogenesis.
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Encased stent
InactiveUS7311727B2Prevent restenosisImprove scalabilityStentsSurgeryCell Cycle InhibitionPolyethylene glycol
An encased stent that discourages restenosis by having a homogenous endothelial cell lining along the inner wall of the stent. The endothelial cell lining may be coated on the stent before the stent is placed in the artery, or the endothelial cell lining may be grown after placement by several factors that encourage such growth and discourage restenosis. The endothelial cells to coat the stent may be genetically modified to enhance the growth of the endothelial cells into a homogeneous lining. The stent has a continuous lining in the form of a multi-layer polymer coating, including a conducting biocorrosion inhibiting layer and a continuous film of polyurethane coupled by a coupling agent to polyethylene glycol. Various drugs and cell factors may be incorporated into the lining, such as anti-thrombin, anti-inflammatory and anti-coagulant drugs, cell cycle inhibitors, and vascular endothelial growth factors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
SCF antibody compositions and methods of using the same
InactiveUS7144731B2Peptide/protein ingredientsImmunoglobulins against growth factorsDiseaseAntiendomysial antibodies
Novel stem cell factors, oligonucleotides encoding the same, and methods of production, are disclosed. Pharmaceutical compositions and methods of treating disorders involving blood cells are also disclosed.
Owner:BIOVITRUM AB (PUBL)
Method for preparing stem cell secretion factor for beauty treatment and skin-protection
InactiveCN101461772AMaintain repair activityPromote proliferationCosmetic preparationsToilet preparationsDead cellDecomposition
The invention relates to a preparation method for stem cell secretion factors used for beauty culture and skin protection. The preparation method comprises the following steps: cell separation, stem cell amplification, the acquisition and concentration of a stem cell factor system, and the acquisition of the system containing the stem cell active factors through storage. The system comprises the active factors secreted by various types of currently verified stem cells. The obtained stem cell active factor system neither contains foreign culture solution components nor contains dead cell decomposition components; in addition, the stem cell active factor system can store and maintain the tissue and cell repairing activity for a long period, thereby truly turning the ideal of stem cell beauty culture into a reality, and improving the traditional beauty culture concepts at the biomedical depth. Simultaneously, the preparation method is simple and feasible and is suitable for mass production, thereby providing a shortcut for the stem cells to serve the beauty culture cause.
Owner:天津欧瑞生物科技有限公司
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS20070092967A1Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
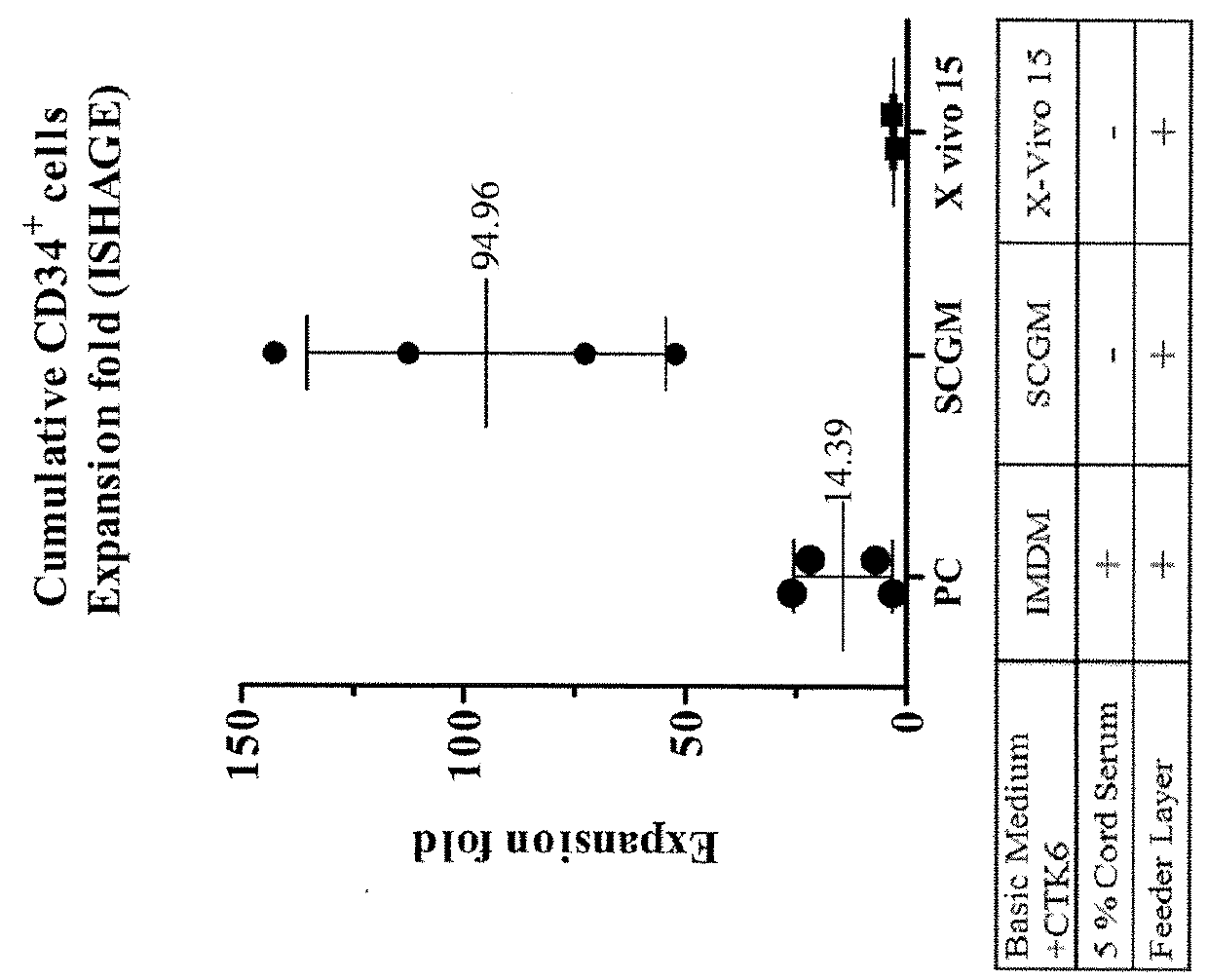
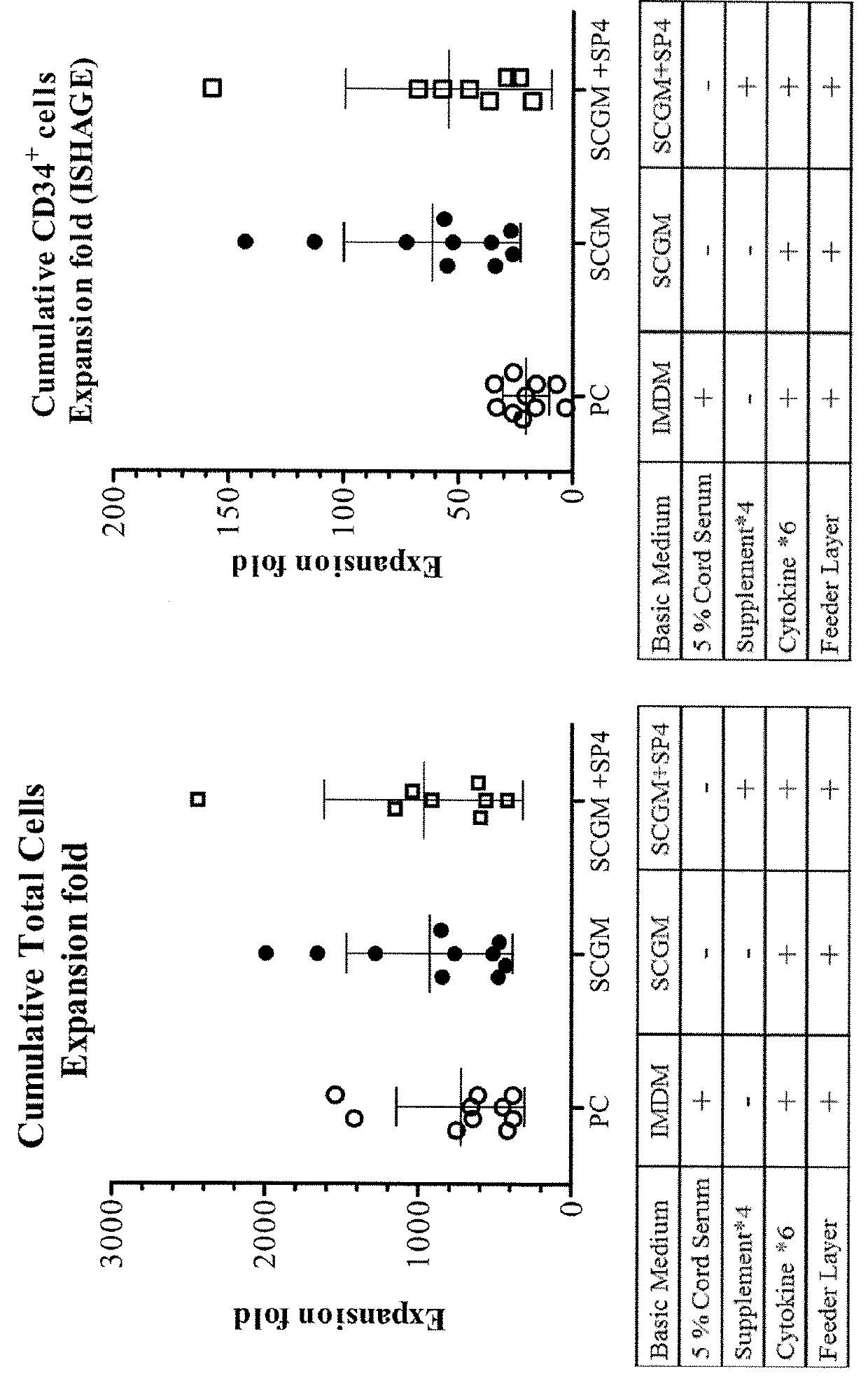
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
Cultured hematopoietic stem cells and method for expansion and analysis thereof
Hematopoietic stem cells and methods for ex vivo expansion of hematopoietic stem cells are provided. The methods comprise culturing the cells in a media containing an effective amount insulin-like growth factor (IGF), fibroblast growth factor (FGF), thrombopoietin (TPO), and stem cell factor (SCF), under conditions sufficient for expansion of said cells. Methods for identifying expanded hematopoeitc stem cells and kits for ex vivo expansion of hematopoietic stem cells are also provided.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Hydrogel bioscaffoldings and biomedical device coatings
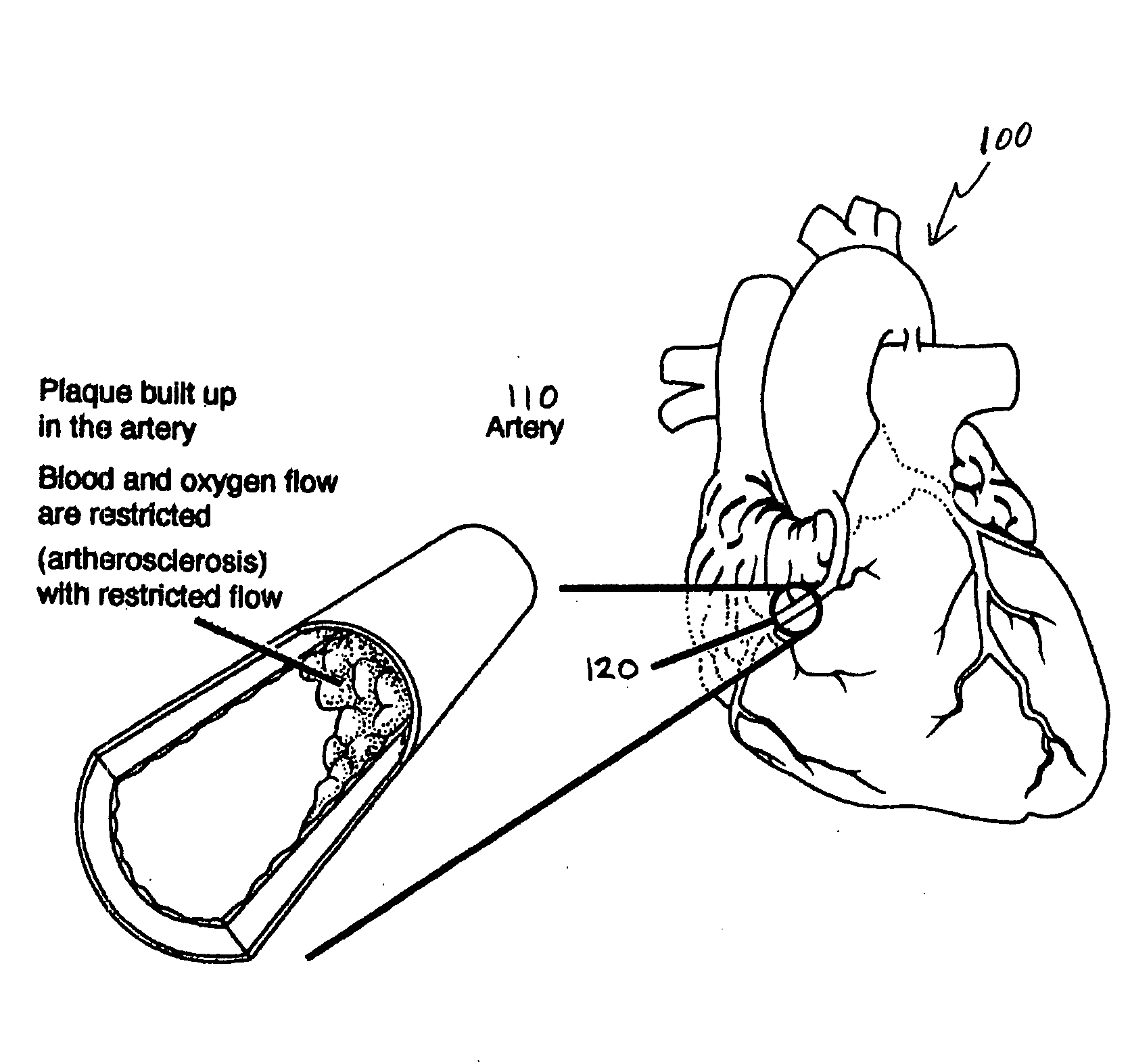
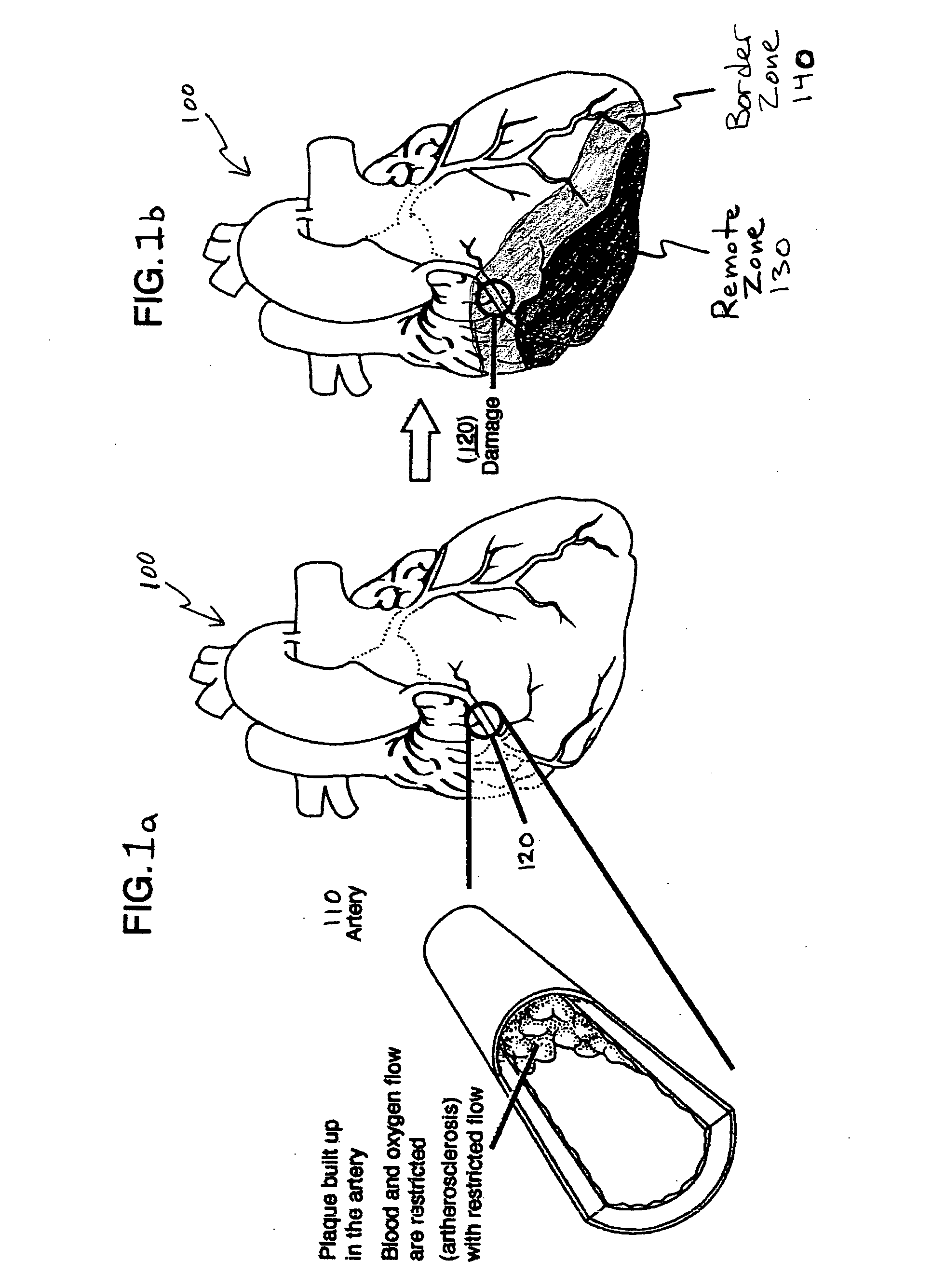
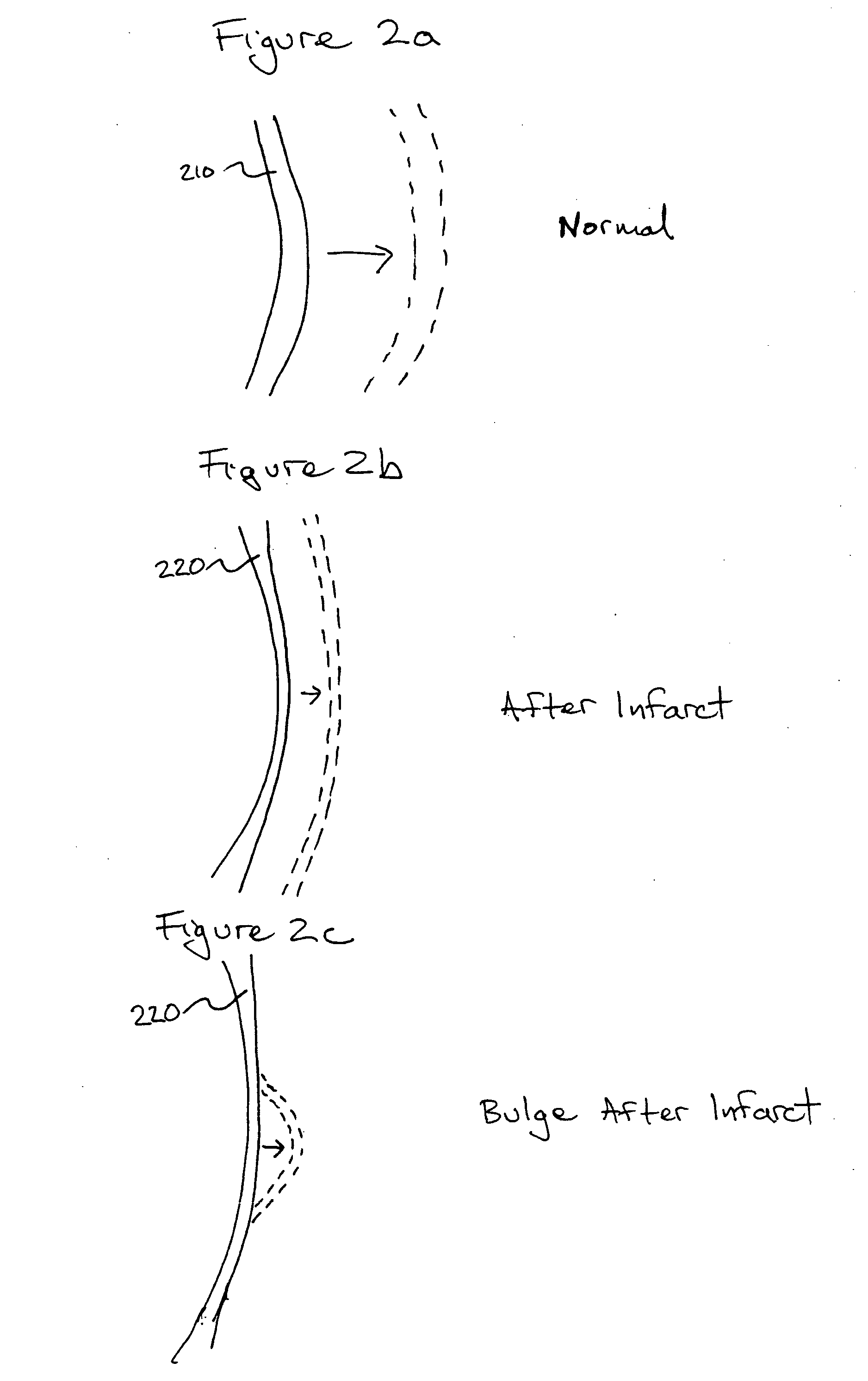
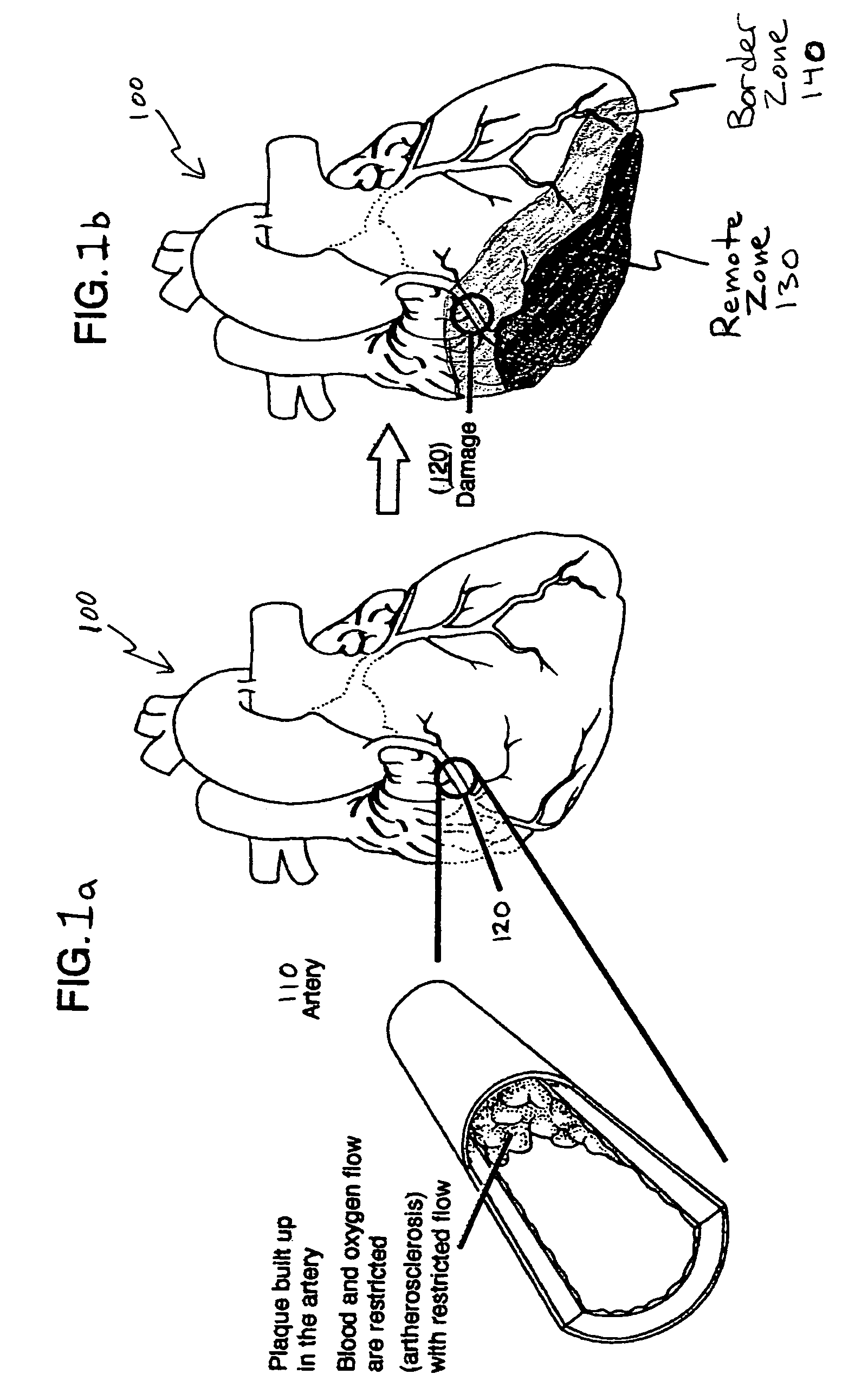
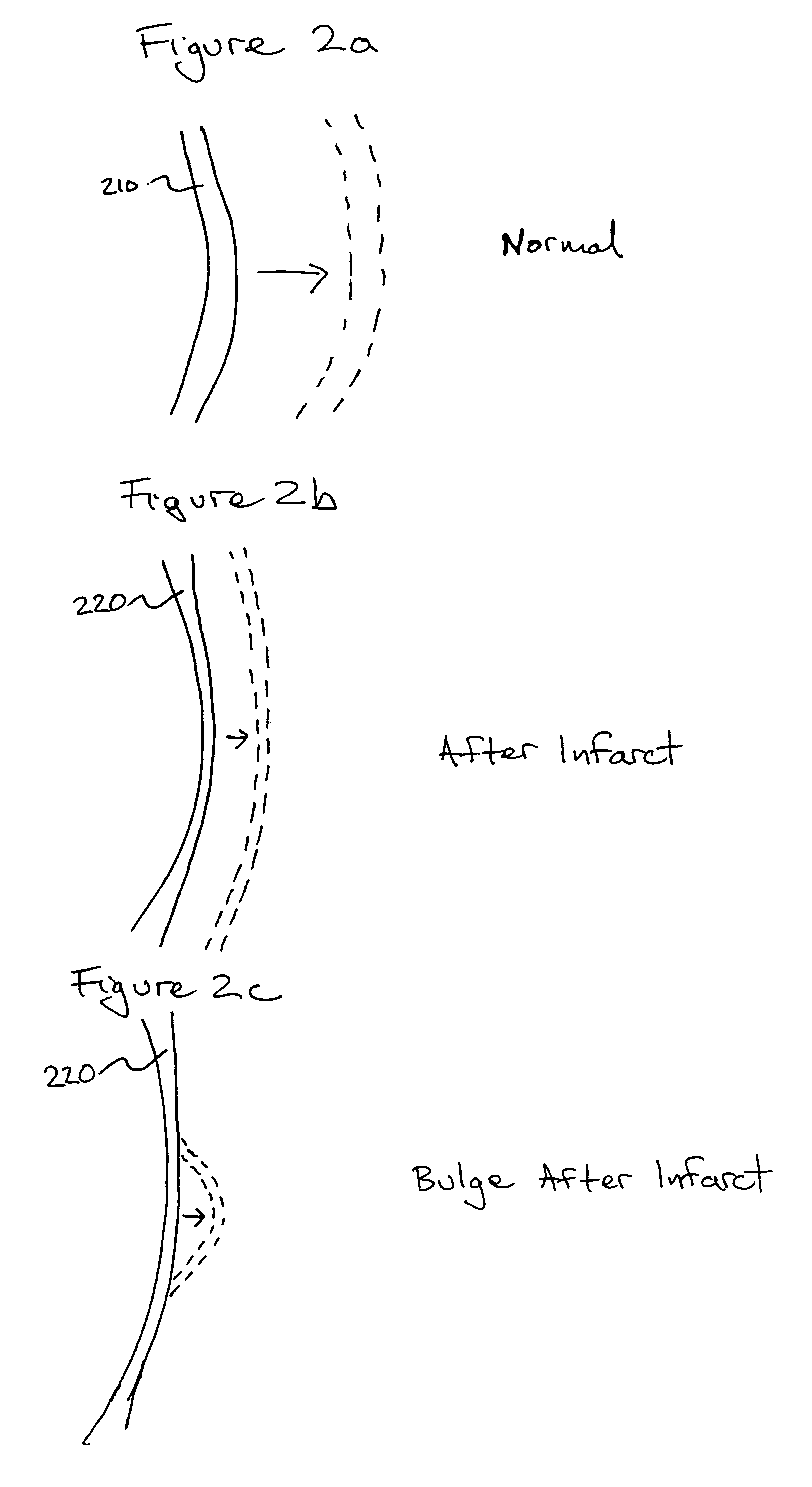
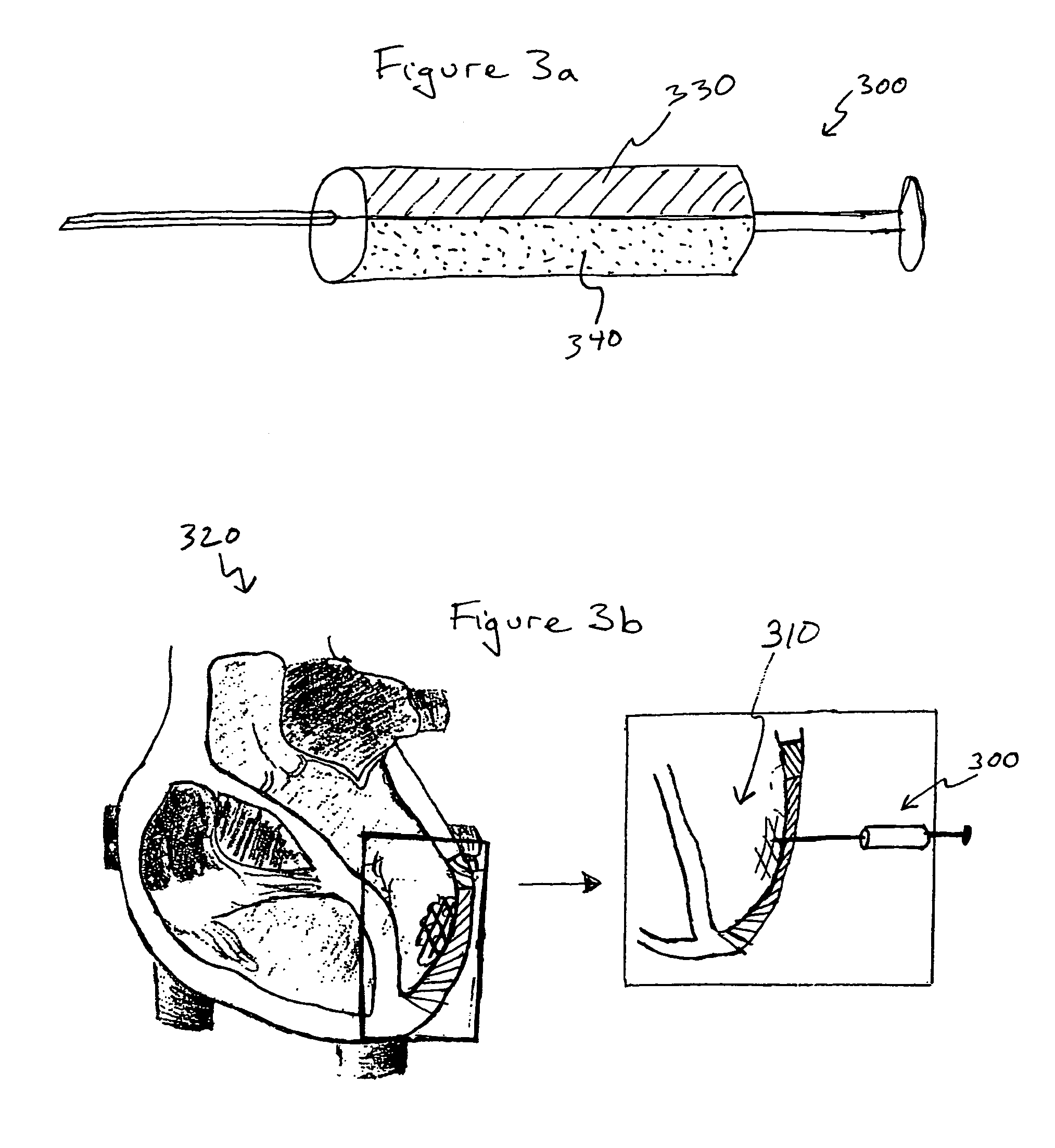
Bioscaffoldings formed of hydrogels that are crosslinked in situ in an infarcted region of the heart (myocardium) by a Michael's addition reaction or by a disulfide bond formed by an oxidative process are described. Each of the bioscaffoldings described includes hyaluronan as one of the hydrogel components and the other component is selected from collagen, collagen-laminin, poly-1-lysine, and fibrin. The bioscaffolding may further include an alginate component. The bioscaffoldings may have biofunctional groups such as angiogenic factors and stem cell homing factors bound to the collagen, collagen-laminin, poly-1-lysine, or fibrinogen hydrogel component. In particular, the biofunctional groups may be PR11, PR39, VEGF, bFGF, a polyarginine / DNA plasmid complex, or a DNA / polyethyleneimine (PEI) complex. Additionally, the hydrogel components may be injected into the infarct region along with stem cells and microspheres containing stem cell homing factors. The bioscaffolding may be formed on a stent or a cardiac medical device.
Owner:ABBOTT CARDIOVASCULAR
Stem cell growth factor-like polypeptides
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to a novel human and mouse secreted stem cell growth factor-like polypeptide. These polynucleotides comprise nucleic acid sequences isolated from cDNA libraries prepared from mouse bone marrow and human fetal liver spleen, ovary, adult brain, lung tumor, spinal cord, cervix, ovary, endothelial cells, umbilical cord, placental, lymphocyte, lung fibroblast, fetal brain, and testis. Other aspects of the invention include vectors containing processes for producing novel human secreted stem cell growth factor-like polypeptides, and antibodies specific for such polypeptides.
Owner:KIRIN PHARMA +1
Stem cell growth factor-like ployeptides
Owner:ARCA BIOPHARMA
Methods and compositions for obtaining hematopoietic stem cells derived from embryonic stem cells and uses thereof
InactiveUS20050221482A1Good conditionMammal material medical ingredientsArtificial cell constructsAutoimmune conditionAutoimmune disease
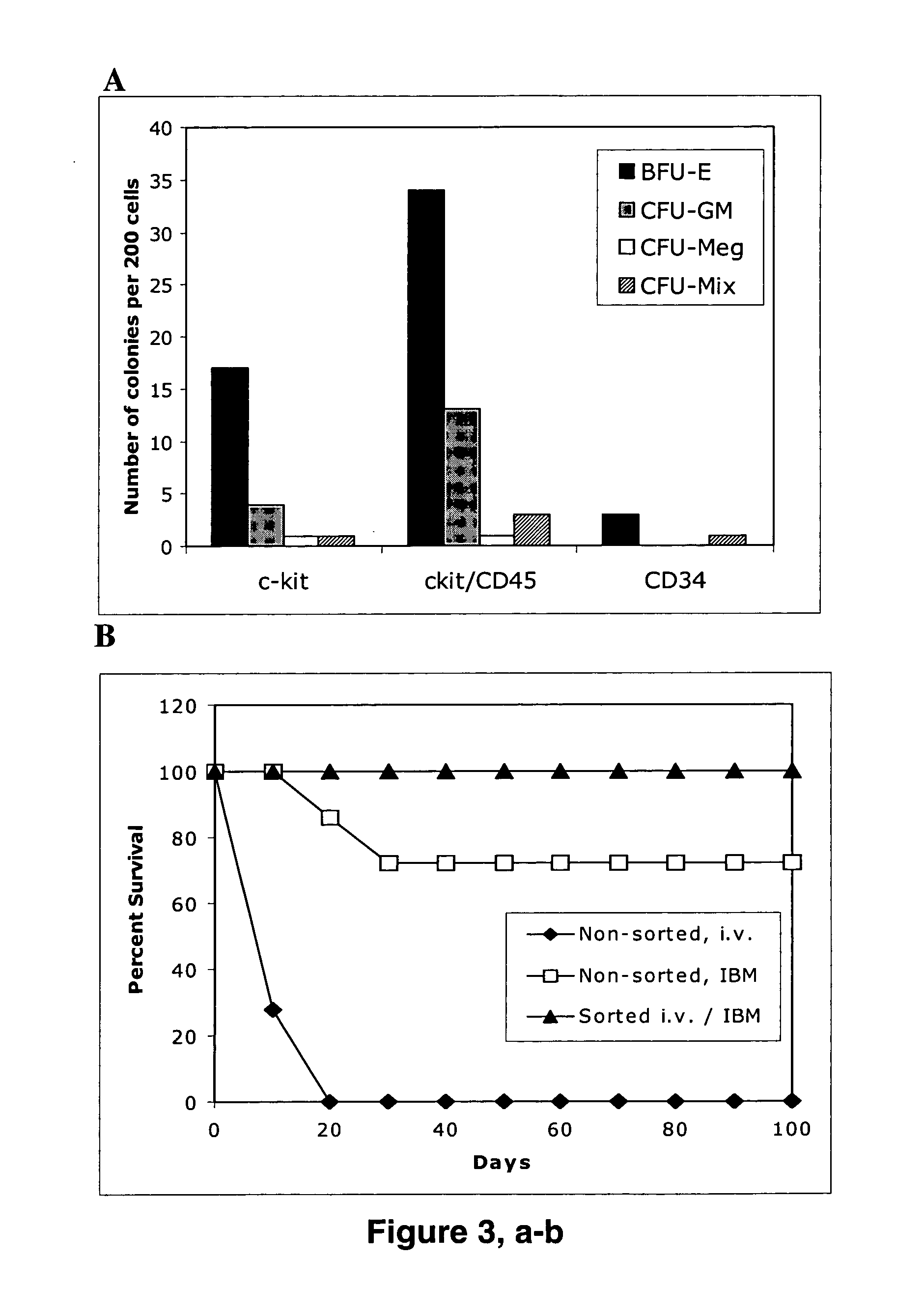
The present invention provides an isolated population of adult hematopoietic stem cells (HSC) derived from embryonic stem cells (ESC) induced to differentiate in vitro by culturing ESCs in a medium with stem cell factor, interleukin (IL)-3, and IL-6. HSC of immunophenotype c-kit+ or c-kit+ / CD45+ from this population are isolated and injected either intra bone marrow or intravenously into myeloablated recipient individuals. This method allows for the establishment of banks of allogeneic ESC-derived adult stem cells for treatments of autoimmune diseases, immune deficiencies and induction of immunotolerance during organ transplantation. These allogeneic ESC-derived adult hematopoietic stem cells (HSC) may be used in reconstituting bone marrow, without the development of teratomas or graft versus host disease, despite crossing histocompatibility barriers. Additionally, allogeneic ESC-derived HSC can be used to prevent the development of autoimmune diseases or organ rejection during transplantation.
Owner:NEWLINK GENETICS +1
Method for preparing stem cell active factor
ActiveCN106381284AHomogeneousIn line with biological characteristicsCosmetic preparationsToilet preparationsFreeze-dryingMesenchymal stem cell
The invention relates to a method for preparing a stem cell active factor, and on one hand, in particular relates to a method for preparing the stem cell active factor. The method comprises the following steps of: (1) acquiring umbilical cord placenta derived mesenchymal stem cells; (2) performing cell amplification; (3) performing cell identification detection; (4) preparing the stem cell active factor; and (5) performing optional freeze-drying. Furthermore, the invention relates to a stem cell active factor freeze-dried powder agent prepared by using the method demonstratively. The method provided by the invention has the beneficial effects as shown in the specification of the invention.
Owner:BOYALIFE
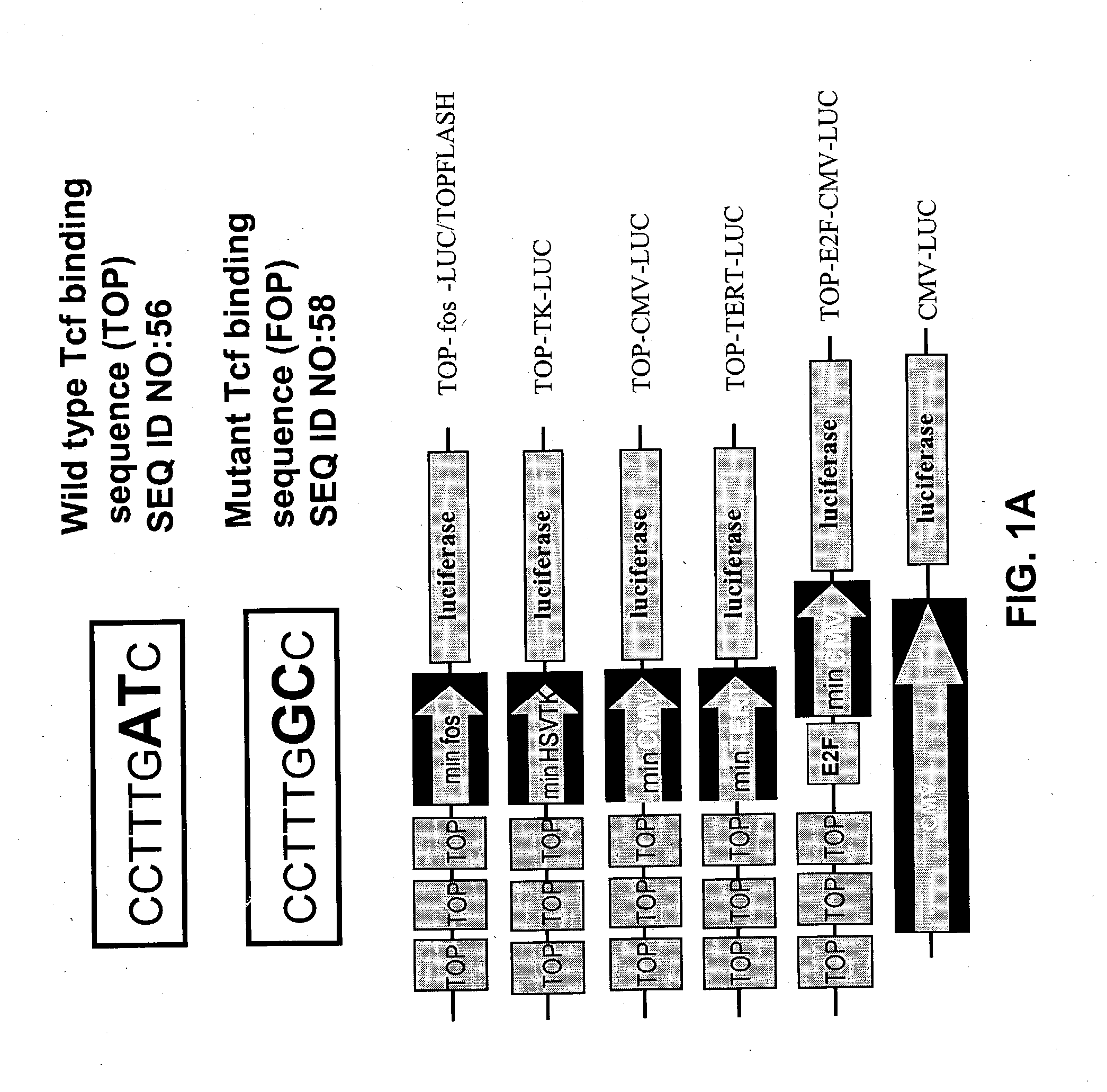
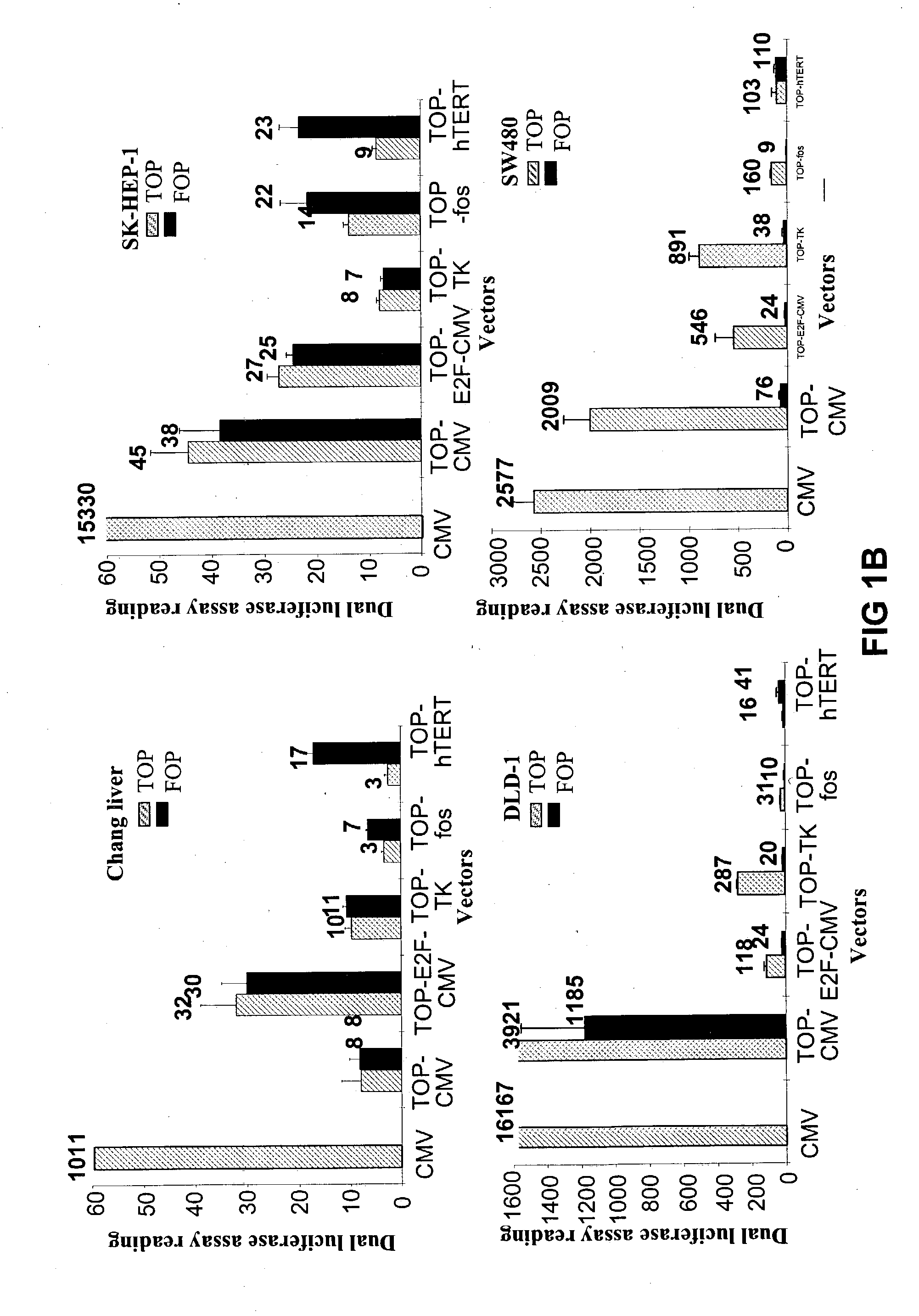
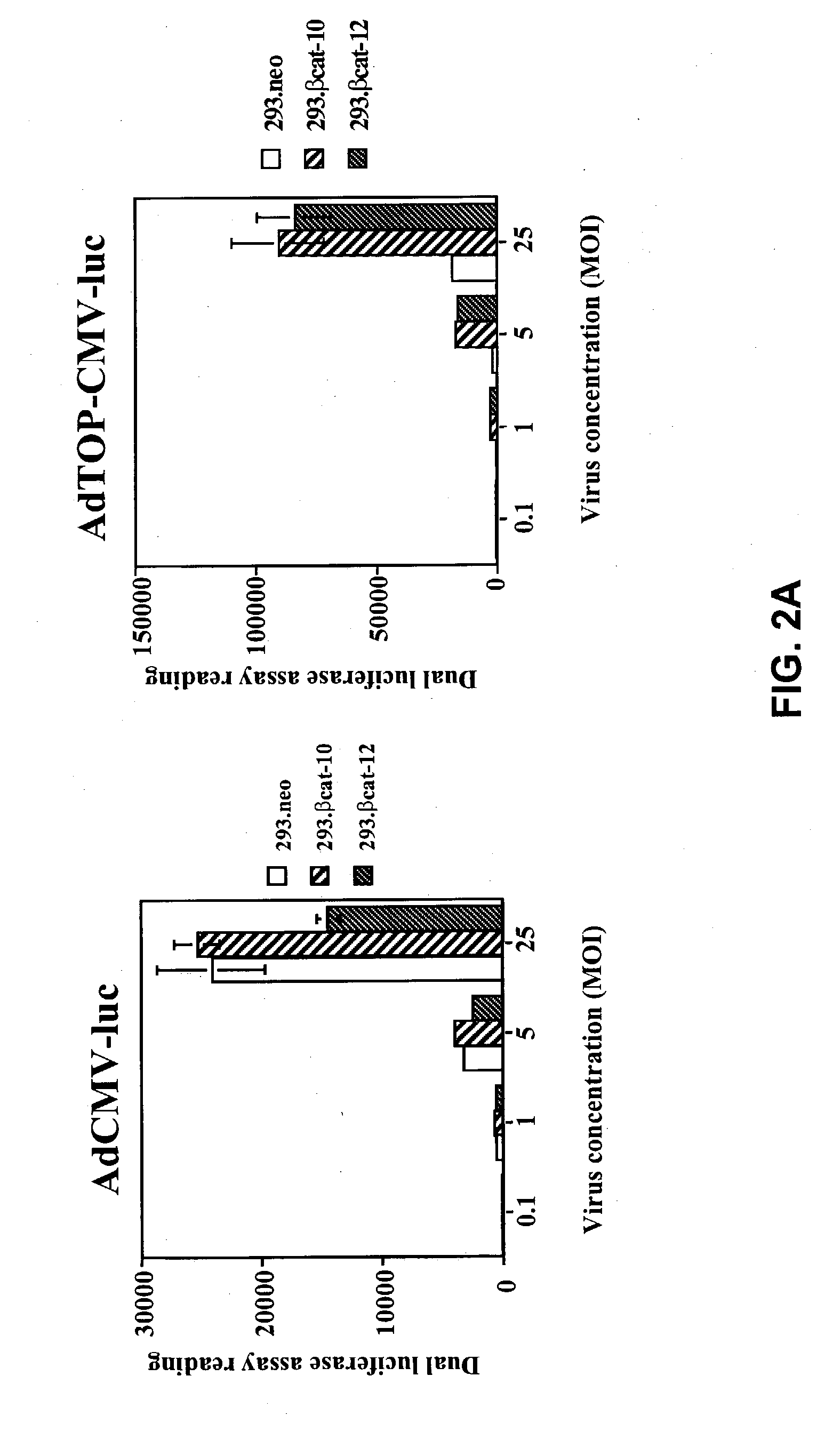
Bipartite T-cell factor (Tcf)-responsive promoter
InactiveUS20030228285A1High activityValid choiceBiocidePeptide/protein ingredientsBeta-cateninCancer therapy
The present invention is directed to methods and compositions for cancer therapy, particularly cancers resulting from a defective Wnt / beta-catenin signaling pathway. In specific embodiments, a T-cell factor (Tcf)-responsive promoter regulating expression of a therapeutic gene is administered to an individual having the cancer. In a specific embodiment, the Tcf-responsive promoter comprises a minimal CMV promoter and is present on an adenovirus vector. The promoter regulates expression of a therapeutic gene.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Beautifying and nursing essence and preparation method thereof
InactiveCN103908424AAvoid allergic reactionsNo side effectsCosmetic preparationsToilet preparationsCuticleUmbilical cord tissue
The invention discloses a beautifying and nursing essence and a preparation method thereof. The essence is prepared from an epidermis cell nutrient solution and contains a plurality of cell growth factors, amino acids and vitamins. The essence mainly comprises an epidermal growth factor, a fibroblast growth factor, collagen type I, a stem cell growth factor, a horn cell growth factor and amino acids and vitamins including gamma-aminobutyric acid, coenzyme Q, medical vitamin C, medical vitamin B5 and medical vitamin E. The preparation method for the essence comprises the following steps: acquisition of epidermis of umbilical cord tissue of a newborn; cell culture; collection of a cell nutrient solution; filtration sterilization; condensation of a volume; addition of active components; addition of glycerin and hyaluronic acid; etc. The beautifying and nursing essence provides a plurality of endogenous nutritional ingredients with physiological activity for the skin, can promote metabolism of epidermis cells and repair damaged cells, has a moisture retention effect and is applicable to skin beautifying and nursing.
Owner:奥思达干细胞有限公司
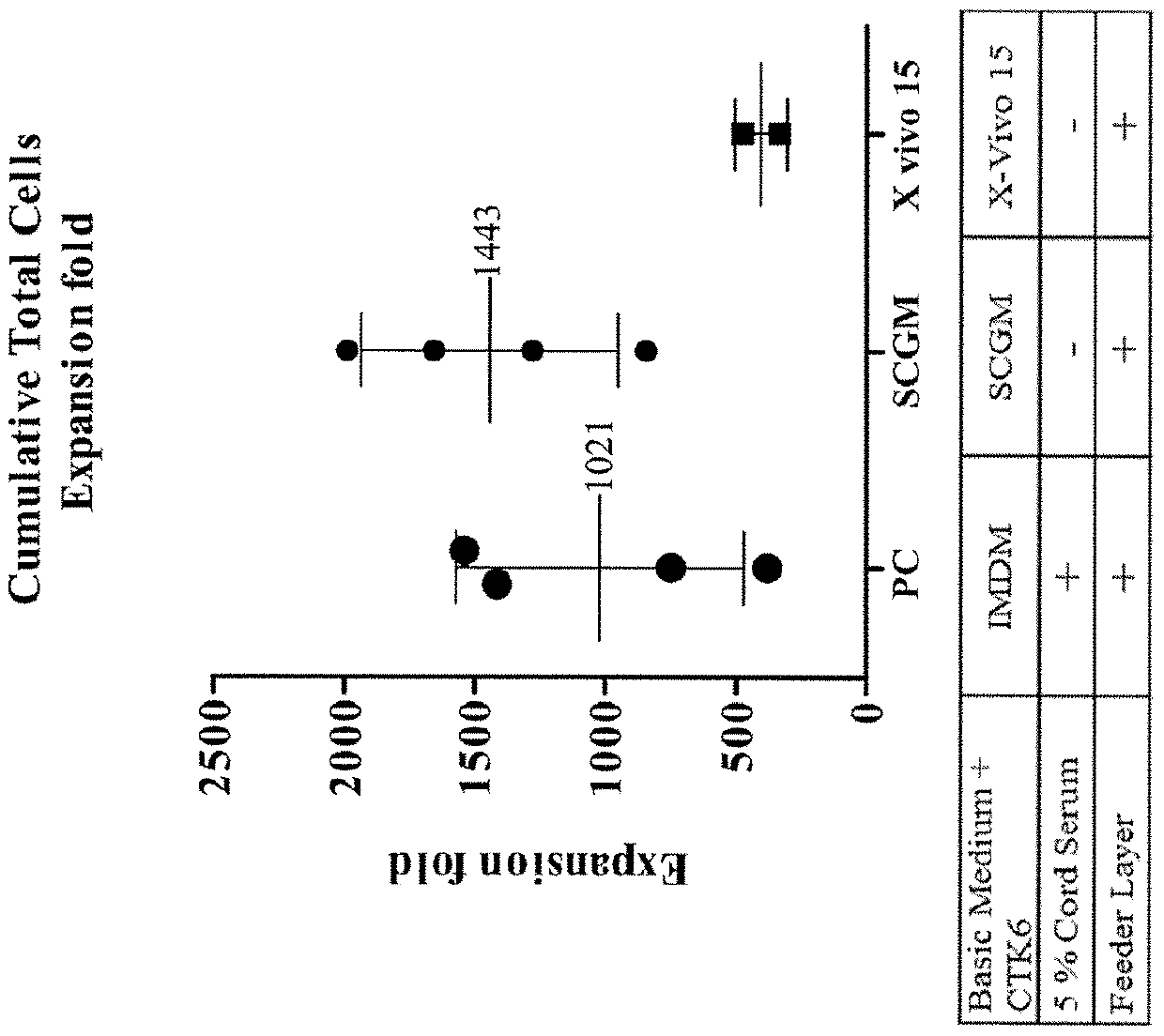
Serum-free culture medium and method for expanding hematopoietic stem cells
InactiveUS20180163177A1Culture processSkeletal/connective tissue cellsVitamin CHematopoietic growth factor
A serum-free culture medium for hematopoietic stem cell (HSC) expansion is provided. The serum-free culture medium includes a serum-free base medium, cytokines, an umbilical cord mesenchymal stem cell conditioned medium and supplemental components. The cytokines comprise stem cell factor, thrombopoietin and hematopoietic growth factor Flt3 ligand. The umbilical cord mesenchymal stern cell conditioned medium is derived from culturing human umbilical cord mesenchymal stem cells. The supplemental components comprise vitamin C, vitamin E or a combination of vitamin C and vitamin E.
Owner:HEALTHBANKS BIOTECH
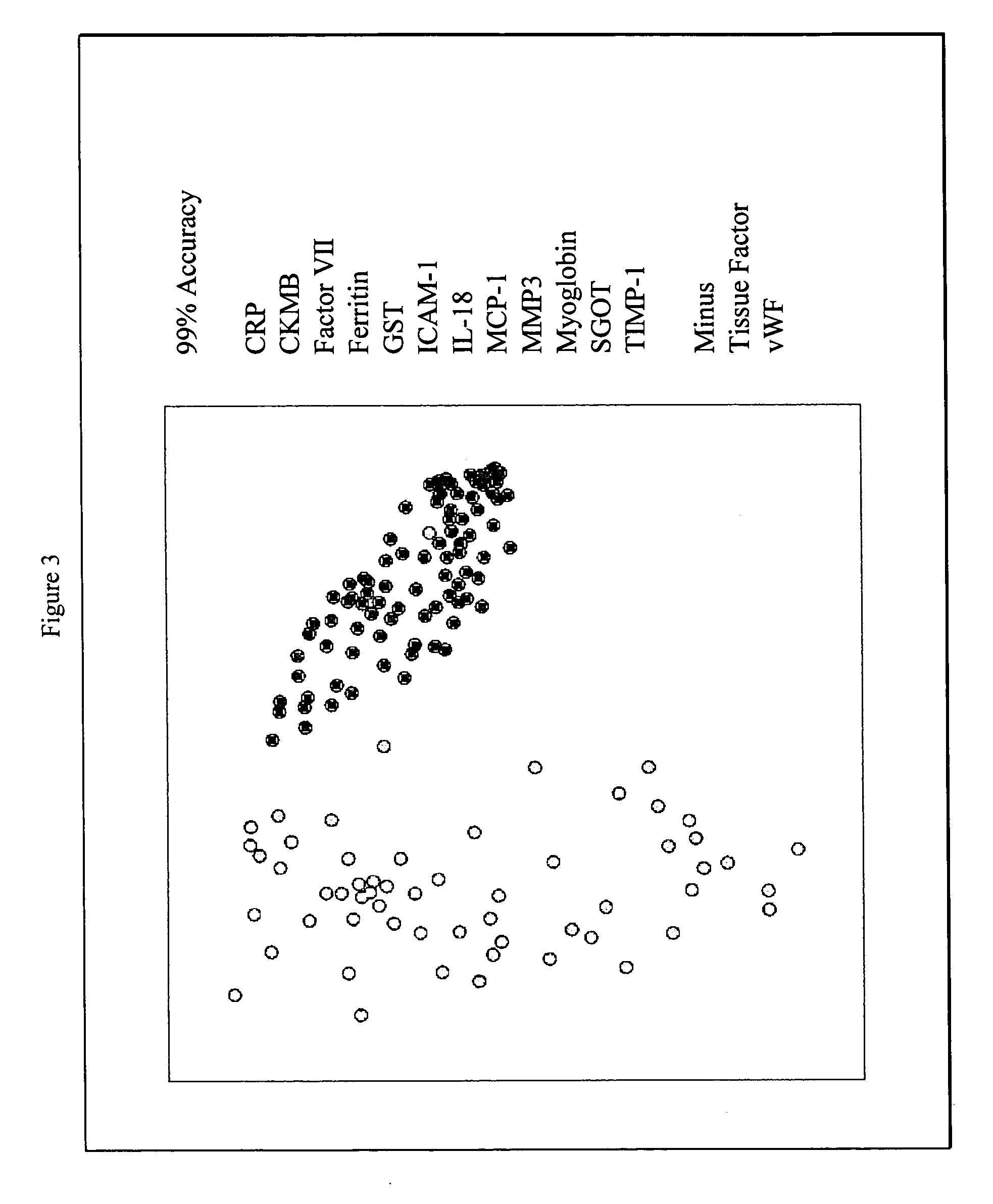
Methods and kits for the diagnosis of acute coronary syndrome
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
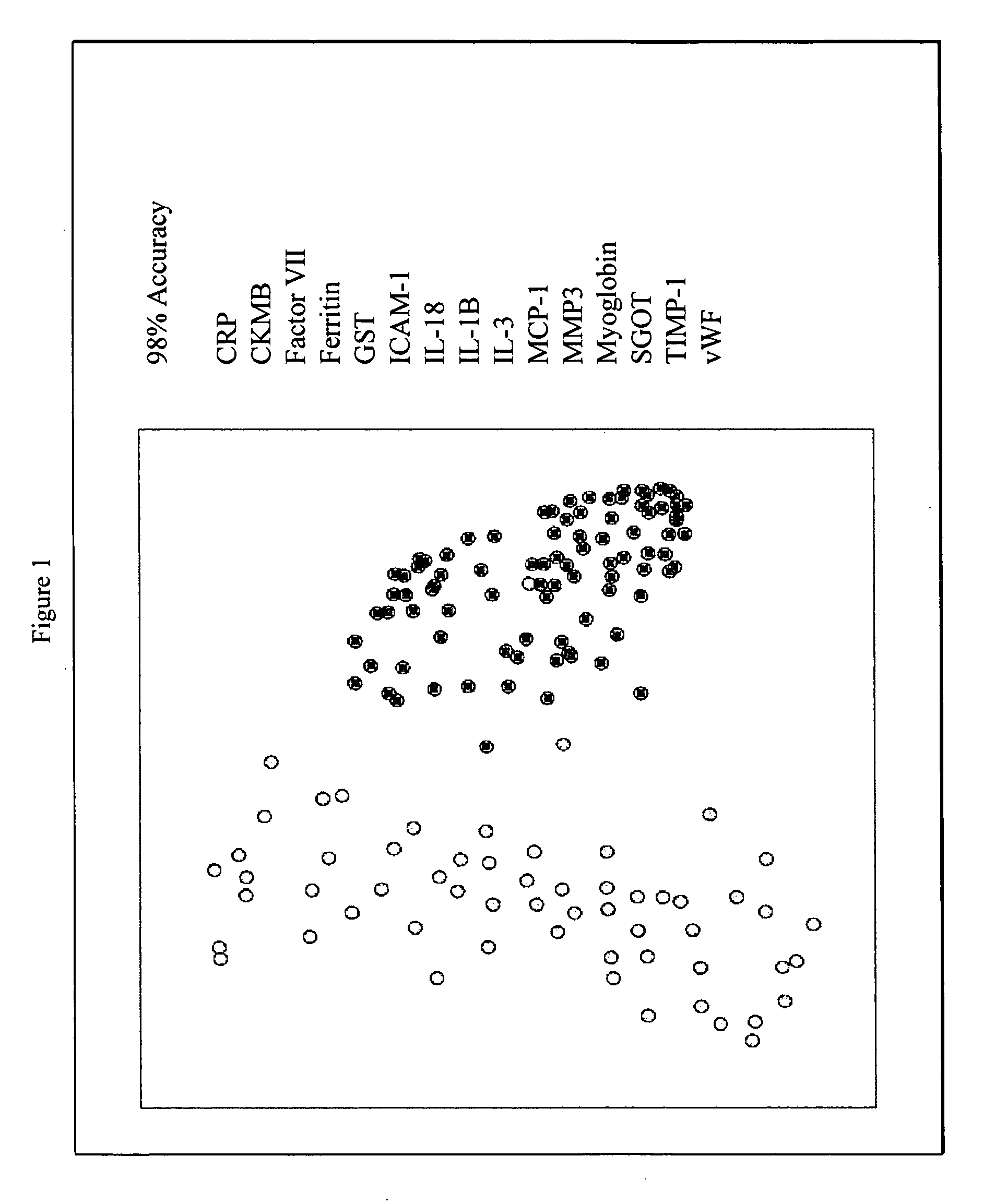
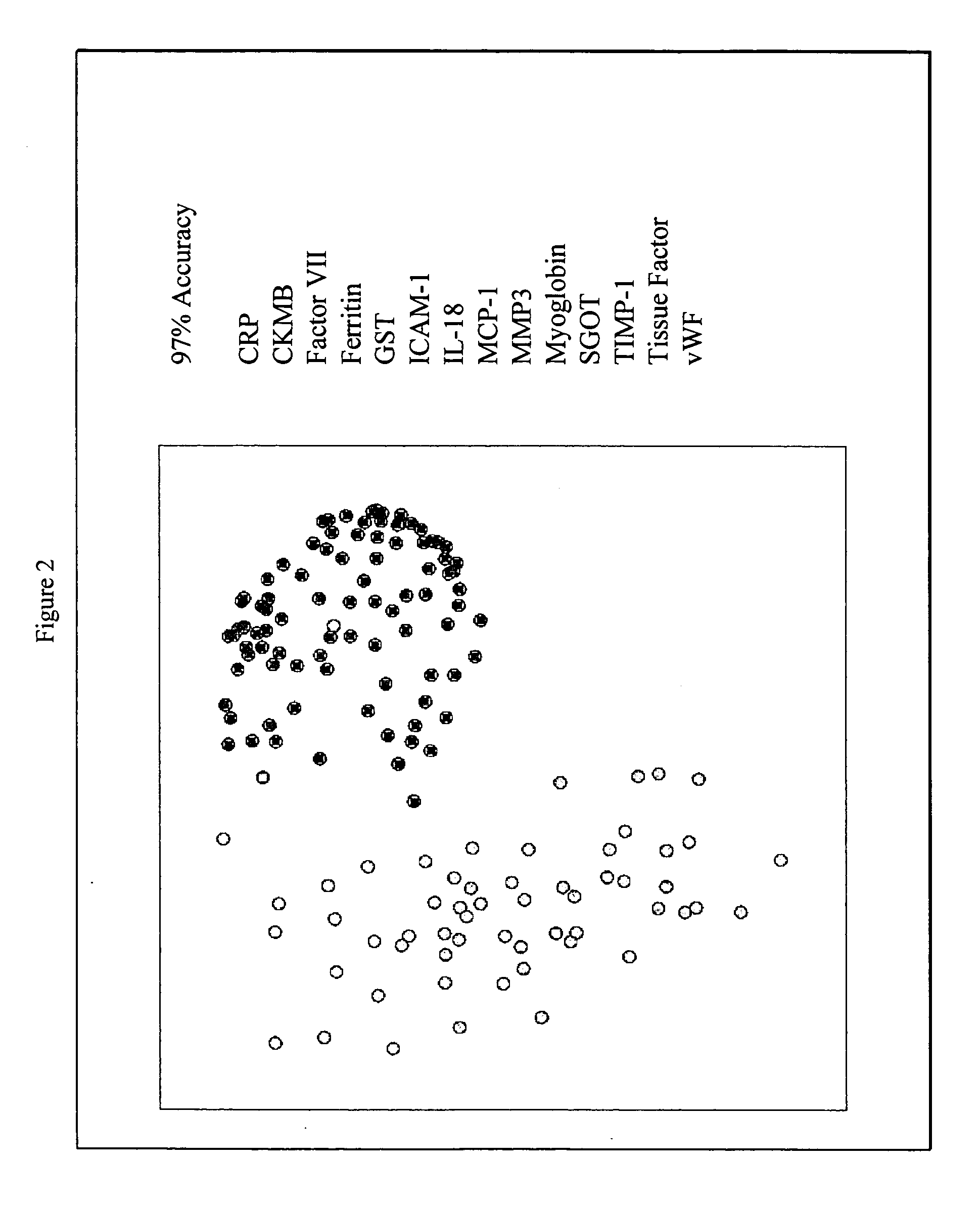
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Compositions for modulating growth of embryonic and adult kidney tissue and uses for treating kidney damage
InactiveUS20080090765A1Protection from damagePeptide/protein ingredientsBone-inducing factorNephronectinMammal
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Serum-free medium for human umbilical cord mesenchymal stem cells and preparation method thereof
InactiveCN105112365AIncrease proliferation rateSimple and fast operationSkeletal/connective tissue cellsVitamin CPancreatic hormone
The invention provides a serum-free medium for human umbilical cord mesenchymal stem cells and belongs to the technical field of stem cells. The serum-free medium comprises a DMEM basic medium and further comprises recombinant human insulin, human serum albumin, transferrin, fibronectin, vitamin C, biotin, stem cell growth factors and stem cell factors. The serum-free medium for human umbilical cord mesenchymal stem cells provided by the invention needs no enveloping of a culture flask, operating is convenient and simple, cell proliferation rate is high, good stem cell pedomorphism and stem cell characteristics are kept, the serum-free medium has a good potential for cell induced differentiation, and the cost is lowered.
Owner:广东美赛尔细胞生物科技有限公司
Medium and methods for culturing of avian primordial germ cells
InactiveUS20050282273A1Faster rateKeep for a long timeCulture processArtificial cell constructsInsulin-like growth factorPlant Germ Cells
The invention provides a novel culture medium useful for the proliferation and maintenance of avian primordial germ cells (PGCs) and encompassing a medium base, leukemia inhibitory factor, basic fibroblast growth factor, stem cell factor and insulin-like growth factor, and an avian serum. The invention also provides a method for the proliferation and maintenance of avian primordial germ cells for extended periods encompassing the steps of isolating a population of PGCs from an avian and culturing the PGCs in a culture medium containing the growth factors and avian serum. The invention further provides methods of producing chimeric avians by isolating and culturing PGCs in a culture medium containing avian serum, transferring the PGCs into a recipient avian embryo, and allowing the recipient avian to develop into a chimeric bird. Another aspect of the invention provides a culture of avian PGCs grown and maintained in the culture medium containing avian serum.
Owner:MERIAL LTD
Stem cell active factor and lyophilized powder thereof
ActiveCN106344492AHomogeneousIn line with biological characteristicsCosmetic preparationsToilet preparationsGlycineMANNITOL/SORBITOL
The invention relates to a stem cell active factor and lyophilized powder thereof, in particular to a stem cell active factor lyophilized powder on the one hand. The stem cell active factor lyophilized powder comprises a stem cell active factor and a lyophilization protecting agent, and the lyophilization protecting agent is selected from trehalose, mannitol, chitosan, dextran, glycine, arginine and glycine. The invention further relates to a preparation method for preparing the stem cell active factor lyophilized powder. The method has the beneficial effects as described in the specification of the invention.
Owner:BOYALIFE
Use of SCF and G-CSF in the treatment of cerebral ischemia and neurological disorders
InactiveUS20060153799A1Affect body weightAbility to focus on taskNervous disorderPeptide/protein ingredientsDiseaseNervous system
The present invention relates to the use of stem cell factor (SCF) polypeptide, alone and in combination with granulocyte colony stimulating factor (G-CSF) polypeptide, in the prevention or treatment of injury to the brain after cerebral ischemia or neurological disorder. More particularly, the invention provides methods of improving neurological function and outcome after stroke by the administration of SCF polypeptide, alone and in combination with G-CSF polypeptide. This treatment can be used alone or in combination with other well-known methods of treatment of cerebral ischemia and neurological disorders in a mammal.
Owner:NORTHWESTERN UNIV
Preparation method of antitumor adoptive immune cells and prepared immune cells
InactiveCN102321577AIncrease multiplierImprove tumor killing abilityBlood/immune system cellsImmunity activeCD28
The invention discloses a preparation method of antitumor adoptive immune cells. The method comprises the following steps of: sampling and separating a single peripheral blood karyocyte; and adding a cell factor for inducting and culturing to obtain a cytokine induced kill cell (CIK), wherein the cell factor comprises CD28. In a CIK induced system, the cell factor CD28 is induced, so that the inducing effect is enhanced, and the cell proliferation multiple is increased simultaneously.
Owner:深圳市中美康士生物科技有限公司
Preparation method of human skin stem cell factor nano-liposome-exosome complex
InactiveCN110859855AImprove stabilityGood biocompatibilityCell dissociation methodsCosmetic preparationsCell freeCytotoxicity
The invention discloses a preparation method of a human skin stem cell factor nano-liposome-exosome complex. According to the method, the exosome and the liposome are compounded, and through the synergistic effect between the exosome and the nano-liposome coated with the cytokine, the obtained compound system has good biocompatibility and immune escape performance, is free of cytotoxicity, not prone to being degraded by macrophages, good in stability and capable of continuously playing a role in vivo.
Owner:JINAN PANSHENG BIOTECH
Vaporized Stem Cell Derivatives for Topical and Other Therapeutic Uses
The invention provides compositions of vaporized stem cell derivatives and methods for their use and manufacture in the treatment of skin conditions and other therapeutic applications. Stem cell derivatives comprising vaporized stem cells, stem cell factors and / or stem cell microvesicles are disclosed and contemplated as being within the scope of the invention. The invention finds use in medical, rejuvenative and cosmetic applications.
Owner:STEMPROTEIN LLC
Recombinant virus with coexpression of anthropogenic glutamate decarboxylase and cell factor
InactiveCN101586096ANervous disorderPeptide/protein ingredientsGlutamate decarboxylaseAdeno associate virus
The invention relates to a recombinant virus containing a gene expression box expressing an anthropogenic GAD gene and a cell factor, in particular to a method for constructing a recombinant adeno-associated virus with the coexpression of an anthropogenic GAD 65 gene and a GDNF gene, an application and a meaning thereof. Anthropogenic GAD 65 gene and the GDNF gene protein products generated by the expression of the recombinant virus have favorable synergistic effect on the treatment of diseases relative to Parkinson's disease and the central nervous system and have important values for treatment of relative diseases.
Owner:王尚武 +2
SCF antibody compositions and methods of using the same
InactiveUS20050261175A1Peptide/protein ingredientsImmunoglobulins against growth factorsMethods of productionOligonucleotide
Novel stem cell factors, oligonucleotides encoding the same, and methods of production, are disclosed. Pharmaceutical compositions and methods of treating disorders involving blood cells are also disclosed.
Owner:BIOVITRUM AB (PUBL)
Silicosis treatment aerosol containing human mesenchymal stem cell exosome extract and preparation method thereof
InactiveCN107137426AIncrease concentrationPromote new lifeAntibacterial agentsDispersion deliveryHigh concentrationTissue Differentiation
The invention discloses a silicosis treatment aerosol containing a human mesenchymal stem cell exosome extract and a preparation method thereof, wherein the main component of the aerosol is the high-concentration mesenchymal stem cell exosome extract. The mesenchymal stem cells can secrete various cell factors in the culture process, and the cell factors can penetrate into the basal layer of an inner membrane, promote tissue differentiation of the inner membrane, angiogenesis and growth of granulation tissues, promote regeneration and repair of damaged skin tissue structures, reduce generation of scar connective tissues and reduce bacterial infection and inflammatory reaction of wound surface tissues. According to the aerosol and the preparation method thereof, a method of continuous starvation of the mesenchymal stem cells is adopted, a high-concentration human mesenchymal extract can be obtained in a short period of time, and a big amount of stem cell exosomes can be separated from the mesenchymal extract by an exosome extractor. The aerosol containing the high-concentration mesenchymal stem cell exosome extract disclosed by the invention is suitable for the restoration and treatment of silicosis.
Owner:TIANJIN PURUI SAIER BIOLOGICAL TECH CO LTD
Hydrogel bioscaffoldings and biomedical device coatings
Bioscaffoldings formed of hydrogels that are crosslinked in situ in an infarcted region of the heart (myocardium) by a Michael's addition reaction or by a disulfide bond formed by an oxidative process are described. Each of the bioscaffoldings described includes hyaluronan as one of the hydrogel components and the other component is selected from collagen, collagen-laminin, poly-l-lysine, and fibrin. The bioscaffolding may further include an alginate component. The bioscaffoldings may have biofunctional groups such as angiogenic factors and stem cell homing factors bound to the collagen, collagen-laminin, poly-l-lysine, or fibrinogen hydrogel component. In particular, the biofunctional groups may be PR11, PR39, VEGF, bFGF, a polyarginine / DNA plasmid complex, or a DNA / polyethyleneimine (PEI) complex. Additionally, the hydrogel components may be injected into the infarct region along with stem cells and microspheres containing stem cell homing factors. The bioscaffolding may be formed on a stent or a cardiac medical device.
Owner:ABBOTT CARDIOVASCULAR
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com