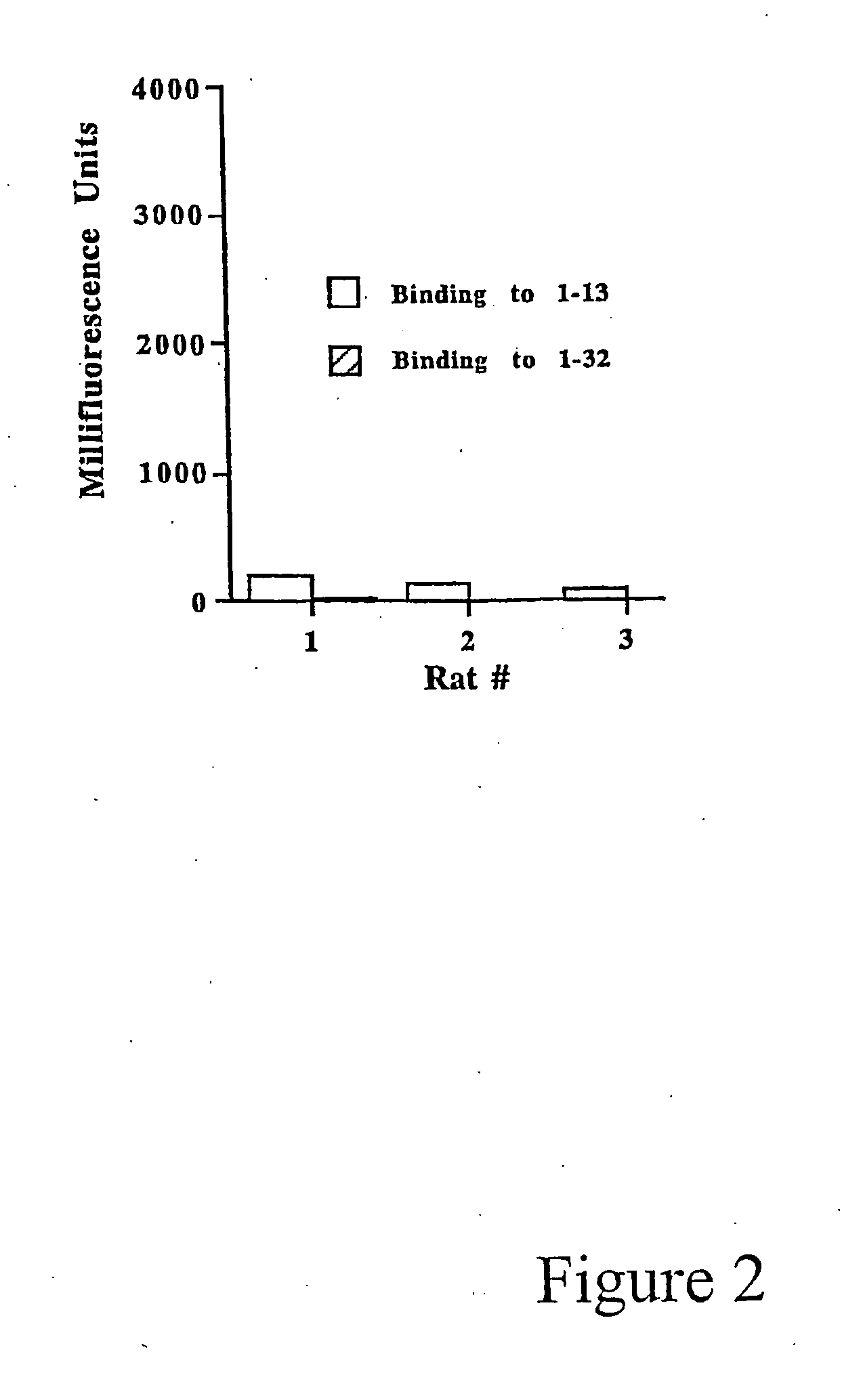
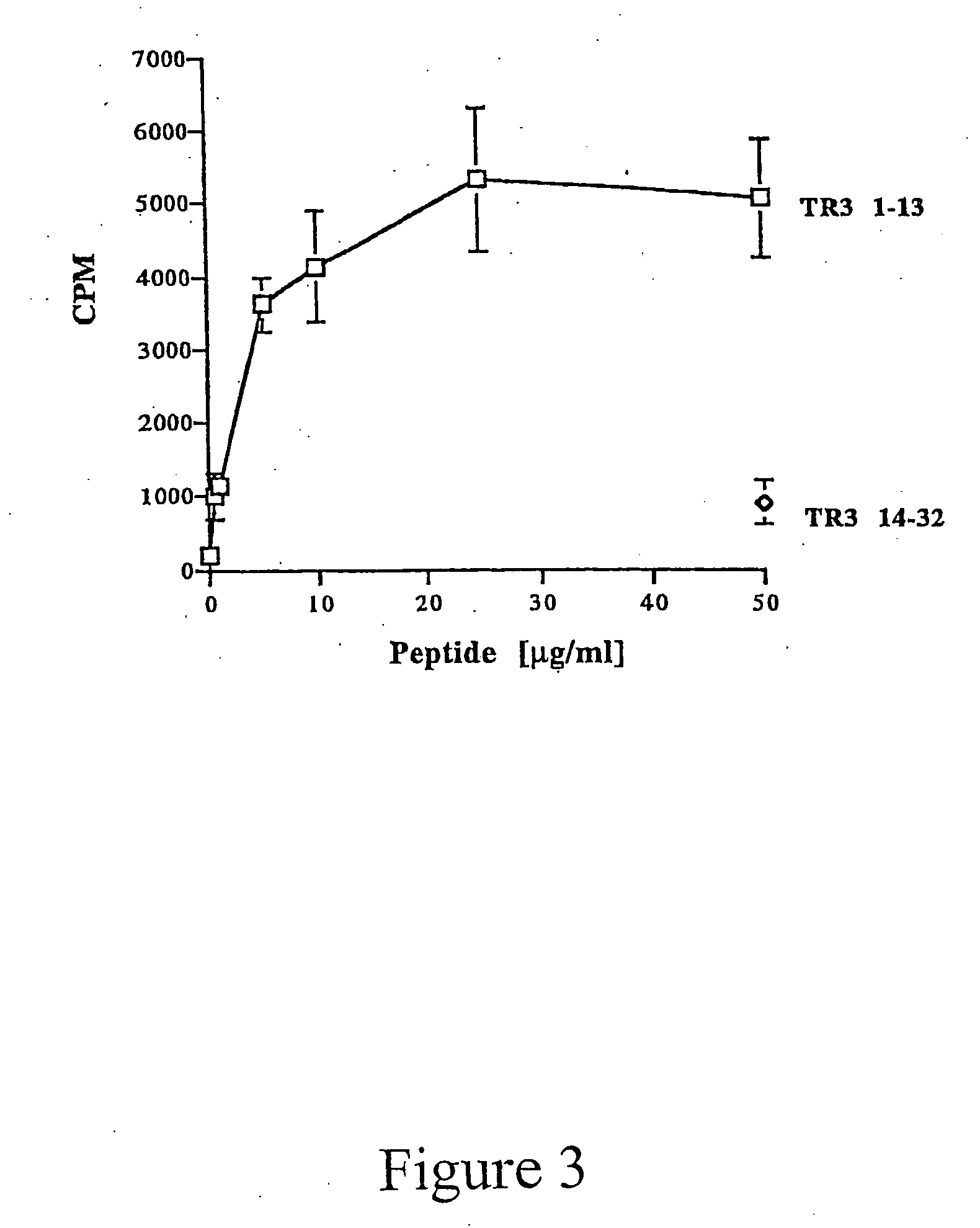
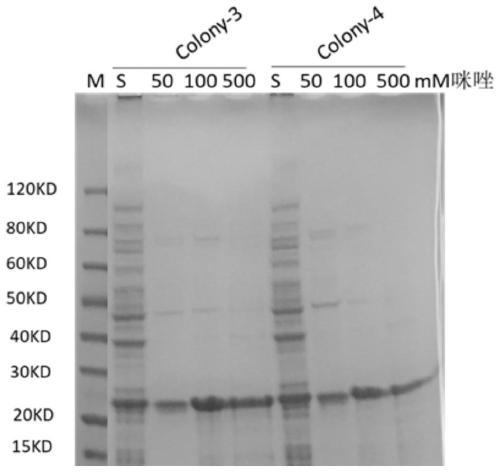
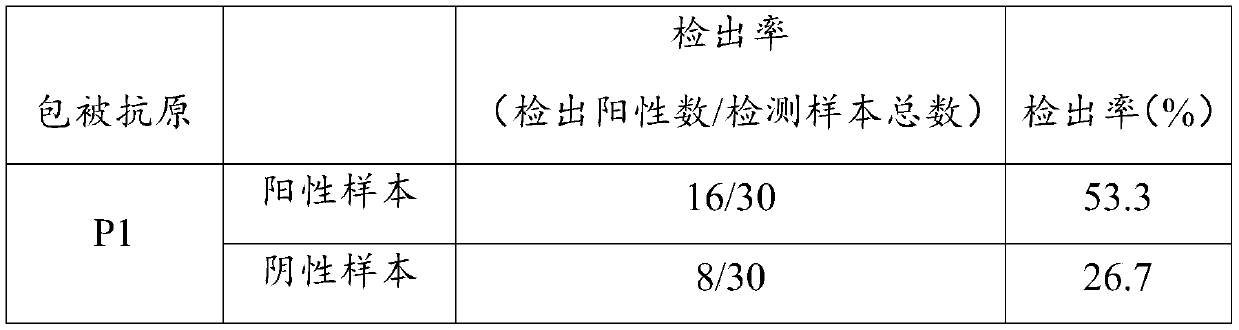
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153 results about "Immune serums" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term usually refers to blood serum, the clear, straw-colored, liquid portion of the plasma that does not contain fibrinogen or blood cells, and remains fluid after clotting of blood. Blood serum from persons or animals whose bodies have built up antibodies is called antiserum or immune serum.
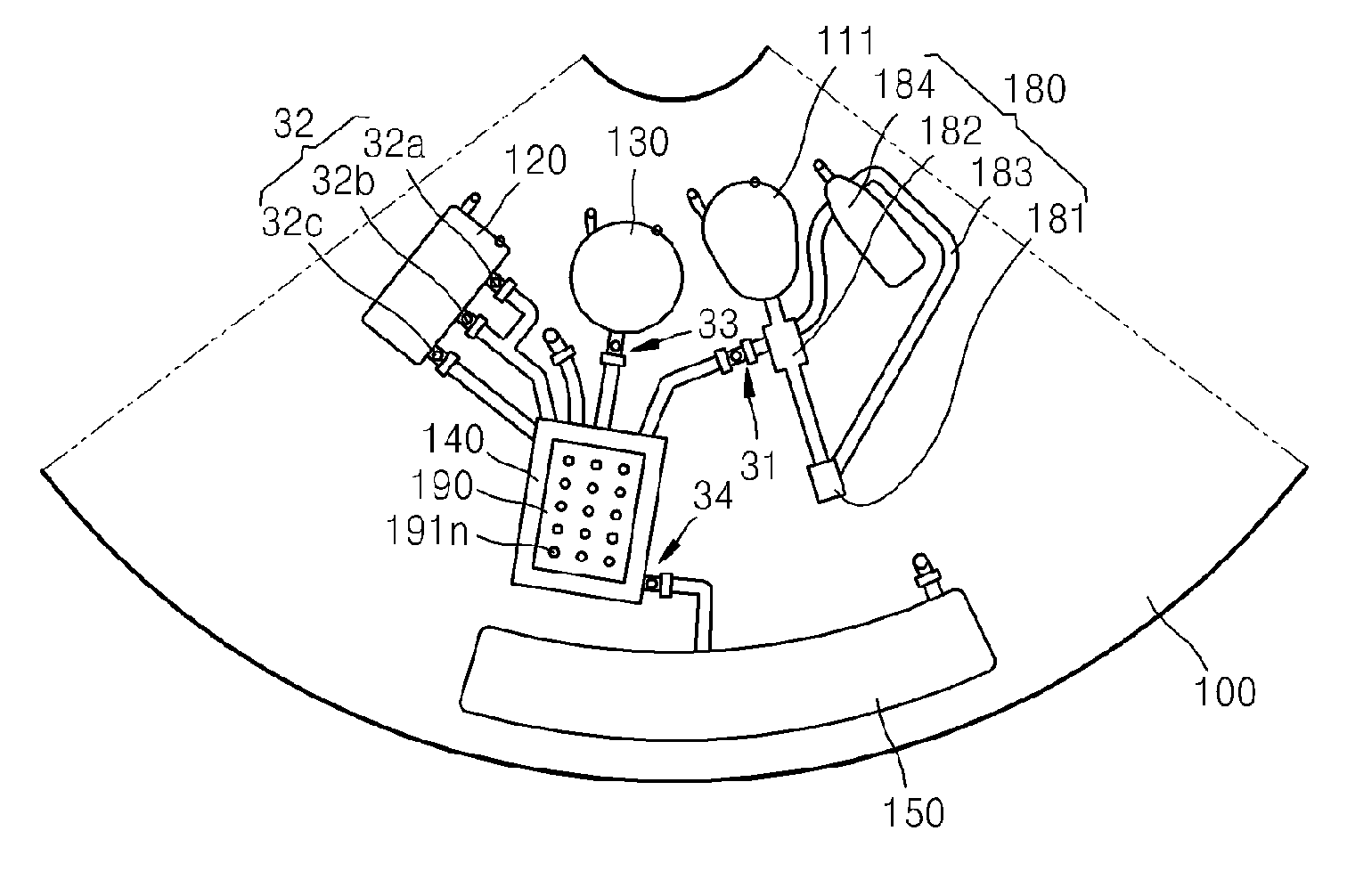
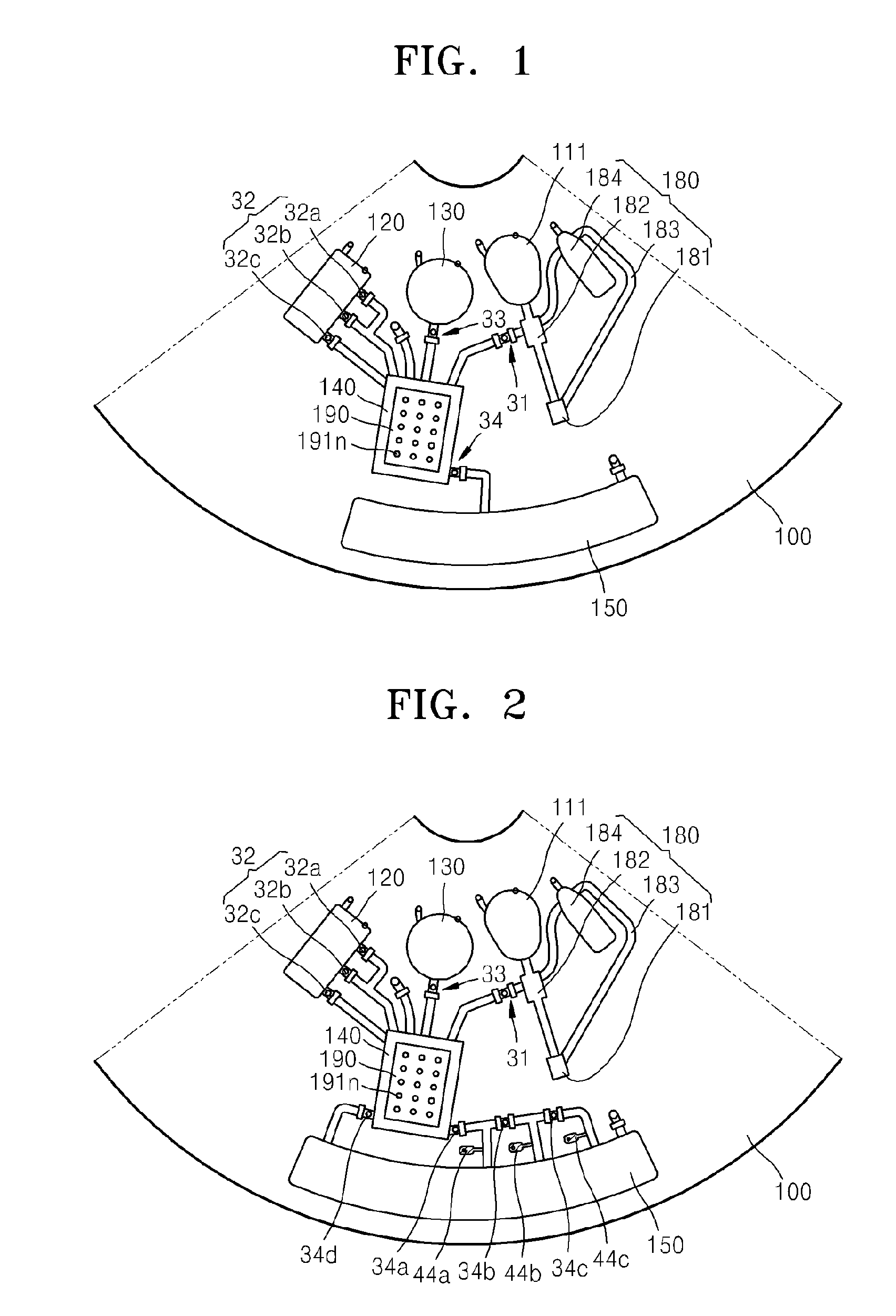
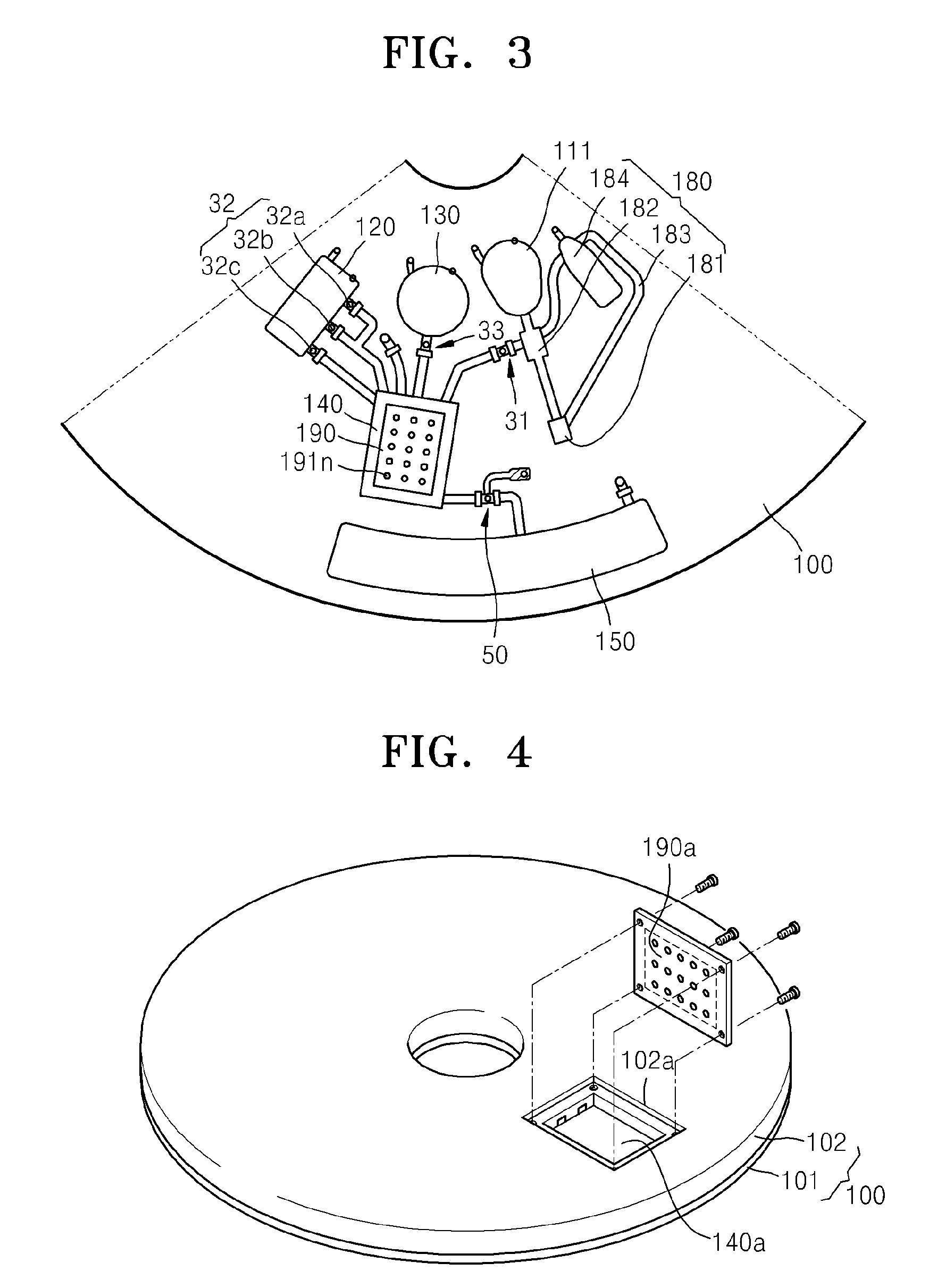
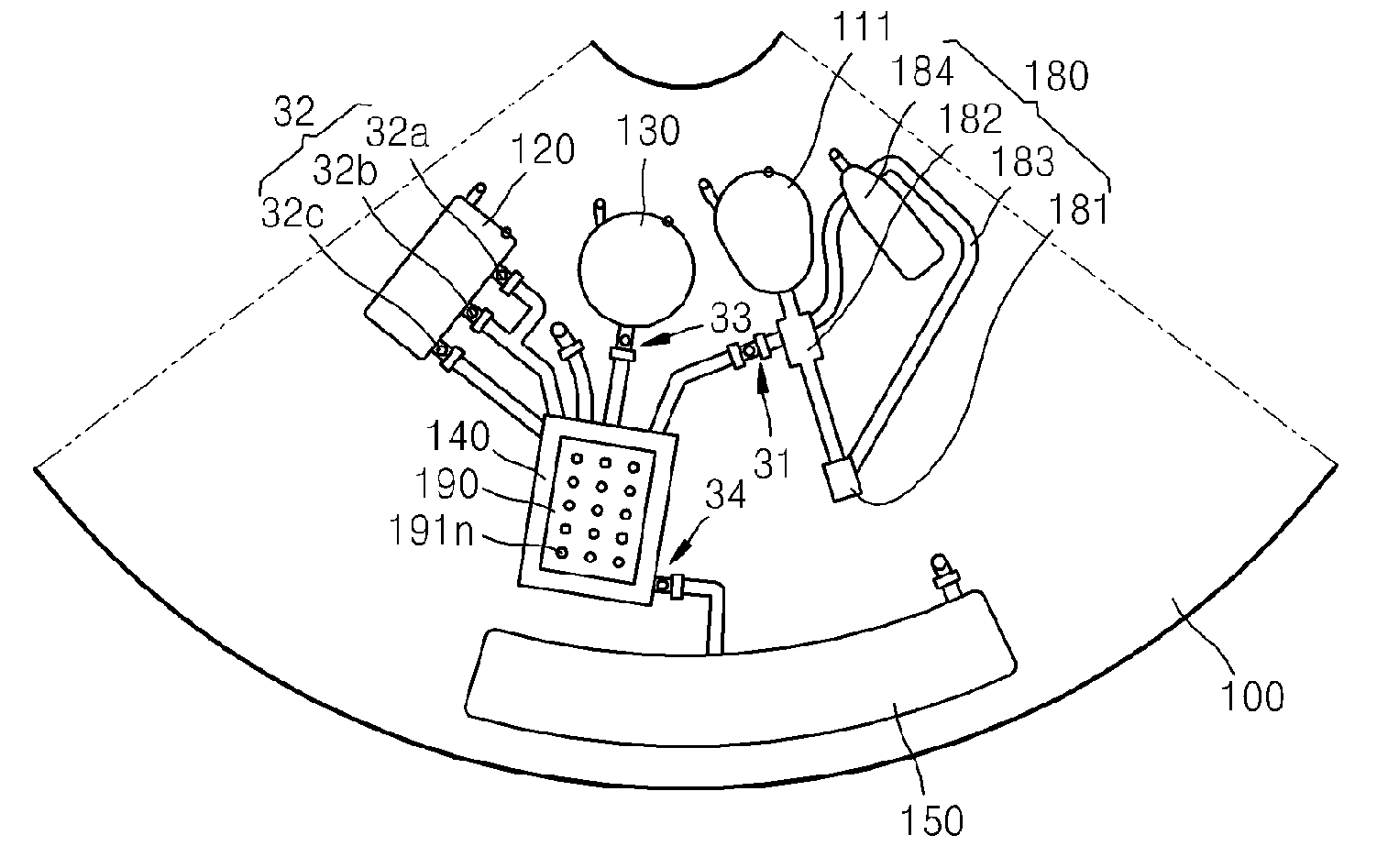
Microfluidic device using microfluidic chip and microfluidic device using biomolecule microarray chip
ActiveUS20090143250A1Guaranteed automatic operationDiagnosis be reducedBioreactor/fermenter combinationsBiological substance pretreatmentsControl flowEngineering
Disclosed is a microfluidic device including a microfluidic structure formed in a platform in which various examinations, such as an immune serum examination, can be automatically performed using the biomolecule microarray chip. The biomolecule microarray chip-type microfluidic device using a biomolecule microarray chip comprises: a platform which is rotatable; a microfluidic structure disposed in the platform, comprising: a plurality of chambers; a plurality of channels connecting the chambers each other; and a plurality of valves controlling flow of fluids through the channels, wherein the microfluidic structure controls flow of a fluid sample using rotation of the platform and the valves; and a biomolecule microarray chip mounted in the platform such that biomolecule capture probes bound to the biomolecule microarray chip contact the fluid sample in the microfluidic structure.
Owner:PRECISIONBIOSENSOR INC
Microfluidic device using microfluidic chip and microfluidic device using biomolecule microarray chip
ActiveUS7988915B2Diagnosis be reducedReduce the numberBioreactor/fermenter combinationsBiological substance pretreatmentsControl flowEngineering
Disclosed is a microfluidic device including a microfluidic structure formed in a platform in which various examinations, such as an immune serum examination, can be automatically performed using the biomolecule microarray chip. The biomolecule microarray chip-type microfluidic device using a biomolecule microarray chip comprises: a platform which is rotatable; a microfluidic structure disposed in the platform, comprising: a plurality of chambers; a plurality of channels connecting the chambers each other; and a plurality of valves controlling flow of fluids through the channels, wherein the microfluidic structure controls flow of a fluid sample using rotation of the platform and the valves; and a biomolecule microarray chip mounted in the platform such that biomolecule capture probes bound to the biomolecule microarray chip contact the fluid sample in the microfluidic structure.
Owner:PRECISIONBIOSENSOR INC
Preparation of troponin I specific locus antibody and detection kit thereof
The invention relates to preparation of troponin I specific locus antibody and a detection kit thereof. A fixed point hydrolysis method is adopted to obtain the required three peptide sections of human cardiac troponin I (cTnI); the three peptide section are orderly subjected to immunogenicity modification and animal immunization so as to obtain high titer immune serum; the three peptide sections extracted in the hydrolysis are taken as the ligand and the polyacrylamide is taken as genin to prepare an affinity column; and finally the obtained high titer immune serum is subjected to immunoaffinity chromatography so as to obtain polyclonal antibodies having specific recognition on the three peptide sections. The antibodies have the advantages that: the affinity of the antibodies is higher than that of a corresponding monoclonal antibody, the specificity is equal to that of a corresponding monoclonal antibody, moreover, the preparation cost is lower, and the preparation process is simpler, compared to that of a monoclonal antibody. The obtained three polyclonal antibodies can be made into an immunity latex kit for a semi-automatic or automatic generation analysis instrument for detecting the content of cardiac troponin I (cTnI) in serum.
Owner:南陵县建设投资有限责任公司
Porcine epidemic diarrhea virus resistant hyper-immune serum and preparation method thereof
ActiveCN103705918AResist attackStable virulenceAntiviralsAntibody medical ingredientsSerum igeCure rate
The invention discloses hyper-immune serum prepared by immunizing a hybridized pig with the age of 50-60 days by taking an inactivated vaccine of porcine epidemic diarrhea virus ZJ08 strain CGMCC No.7806 as an immunogen due to immunization for three times. The prepared hyper-immune serum is high in properties, high in specificity and high in safety and is not polluted by bacteria, moulds, mycoplasma and exogenous viruses, and the artificial infection cure rate is 100 percent. The neutralization titer of the serum is over 26, the serum is separately filled at the temperature of minus 70 DEG C, and the retention period is 12 months.
Owner:兆丰华生物科技(南京)有限公司 +3
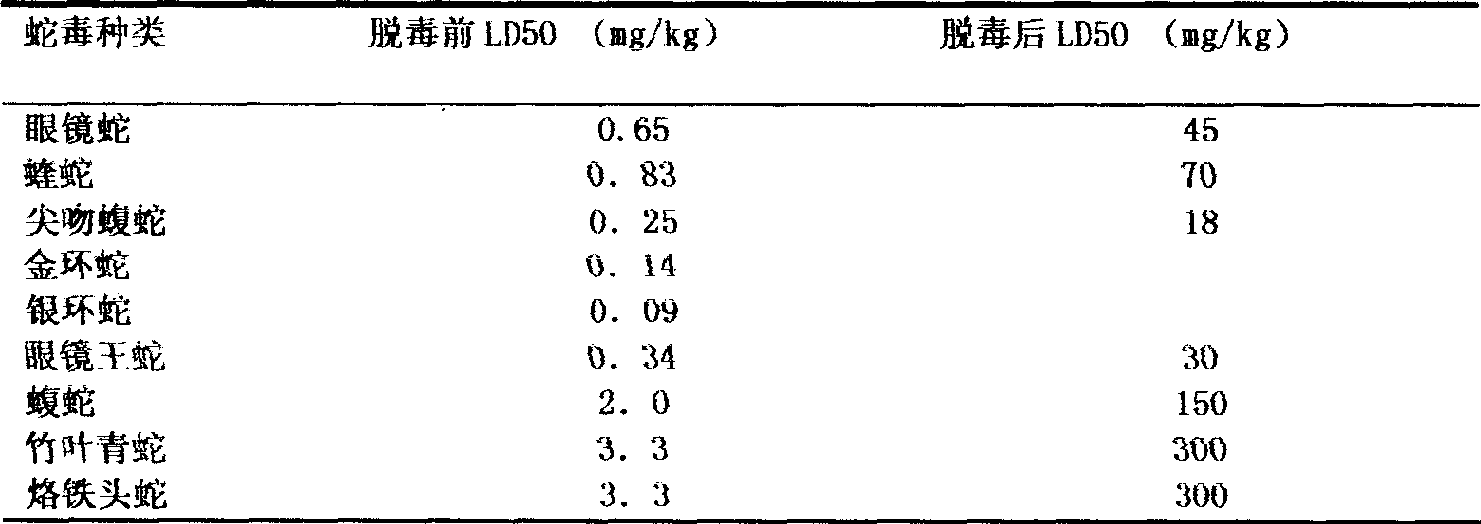
Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
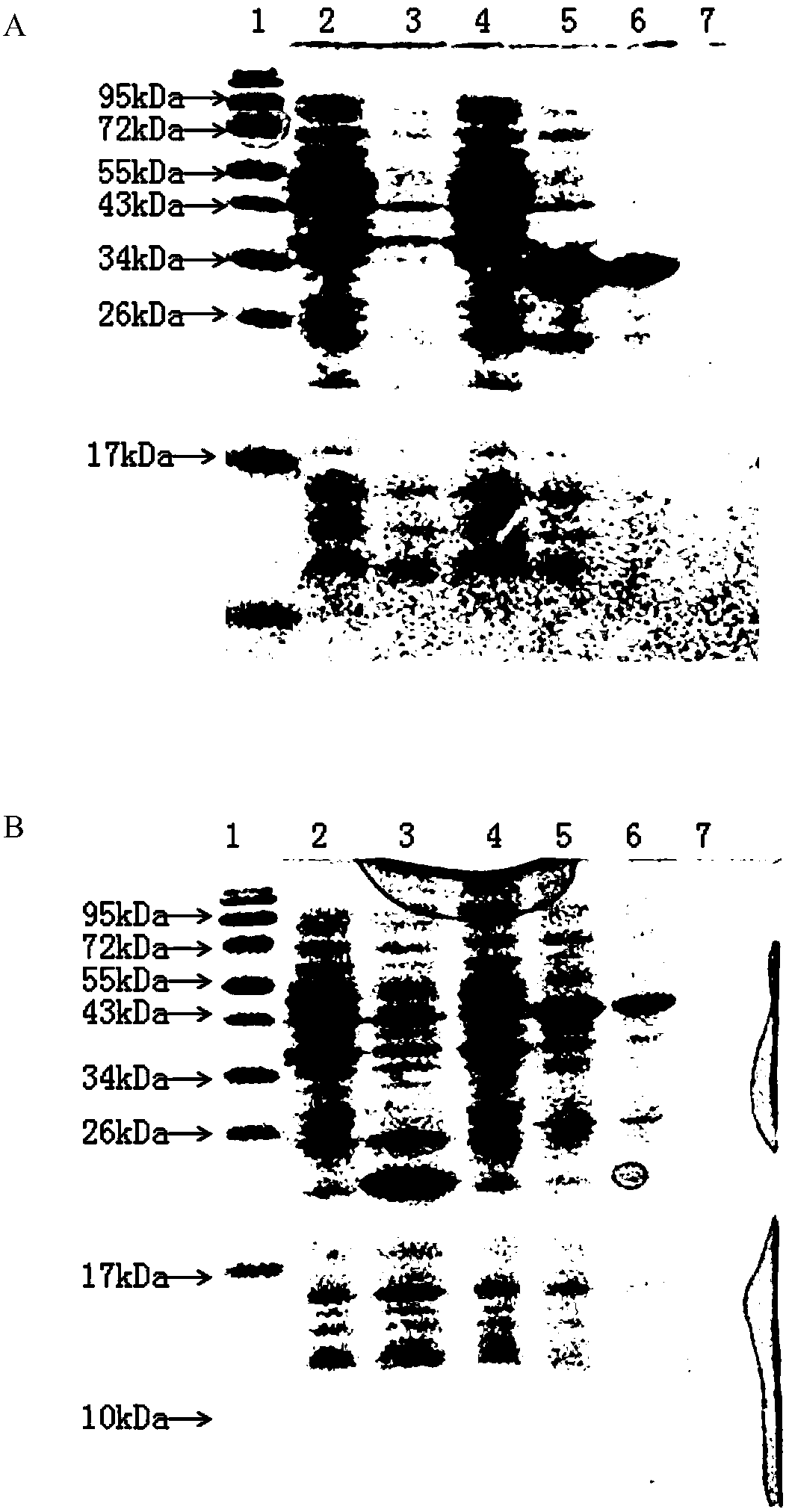
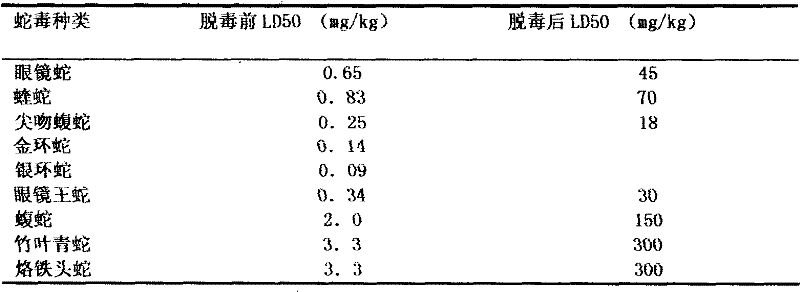
InactiveCN101385855AImprove immunityQuality improvementAntibody ingredientsDermatological disorderVirulent characteristicsFreeze-drying
The invention discloses a method for refining a polyvalent ASV (anti-snake venom) lyophilized serum, pertaining to an immune serum containing antibody which aims at least two kinds of antigens. The serum takes the venom of Ancistrodon acutus, Agkistrodon halys, Pit-vipers, bamboo snakes, adders and the like as the immune antigen which is inoculated into the bodies of animals after degerming and dialysis, and then titer immune serum after purification is used for collecting the antibody which is then desalted and lyophilized; mice are used for evaluating the LD50 of the virulence of the venom and the titer of the ASV serum; the antibody inoculated into the bodies of the animals is one of the venom, toxoid or snake venom or the composition thereof, and glutaraldehyde or formaldehyde is used for toxin detoxication; before immunity, anti-tetanic toxoid condensed antibody for human beings is used for carrying out minim gradual immunization on the animals, or an Emuade adjuvant is used for immunization purpose. The polyvalent ASV lyophilized serum complies with the biological product standards upon the tests and inspections of purity and specific activity, safety, pyrogen, hypersusceptibility, dissolution and the like; moreover, the serum can cure sufferers who are bitten by vipers with the advantages of high purity, low foreign protein, easy preservation and quick dissolving capacity.
Owner:KUNMING INST OF MILITARY MEDICINE CHENGDU MILITARY REGIONS CENT FOR DISEASE CONTROL & PREVENTION
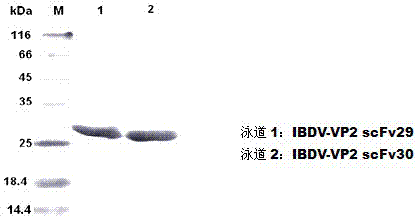
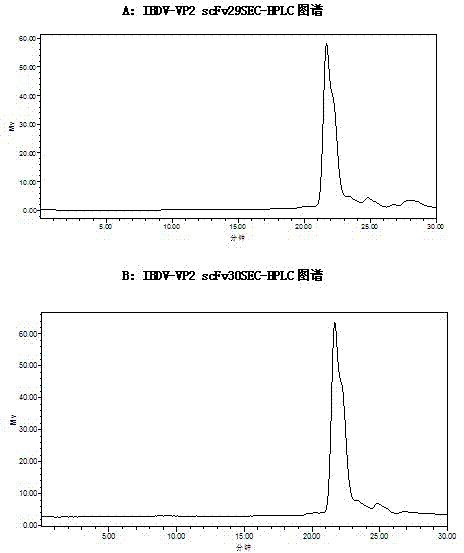
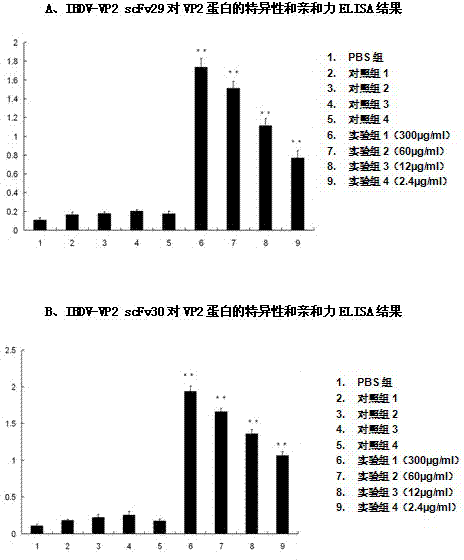
Two ScFv (Single Chain Variable Fragment ) antibodies, encoding genes and application thereof for preparing preparation for treating or preventing infectious bursal disease of chicken
InactiveCN103483449AStrong specificityGood treatment effectBacteriaImmunoglobulins against virusesAnimal virusDisease
The invention discloses two types of ScFv (Single Chain Variable Fragment) antibodies, encoding genes of the antibodies and applications of the antibodies for preparing the preparations for treating or preventing infectious bursal disease of chicken. Two single-chain antibodies (namely, the ScFv antibodies) are provided; and the ScFv antibodies can be specially bound with the protein 2 (VP2) (Virus Protein 2) in an IBDV (Infectious Bursal Disease Virus) structure and a plurality of IBDV strains to block the cytopathic effect (CPE) of the chicken embryo fibroblast by the IBDV so as to protect the young chicken infected with IBDV. The immune serum and egg yolk antibody are tedious in preparation, high in production cost, unstable in effect, difficult to control industrialized production quality, capable of causing horizontal disease spread and the like in a use process; the ScFv antibodies disclosed by the invention can overcome the above disadvantages and have the advantages of strong specificity, good treatment effect, controllable industrialized production quality, capability of preventing the horizontal disease spread caused by the egg yolk antibody and the like; and therefore, by virtue of the ScFv antibodies, a new situation is opened up in the IBDV prevention and treatment history, and even in the entire prevention and treatment history for animal virus diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application
InactiveCN102399806AReduce colonizationPromote accumulationBacteriaGenetic material ingredientsBacillus megateriumCell wall
The invention discloses a porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application. An antigen fusion gene shown as SEQ ID No. 1. is obtained by connecting an antigen locus of porcine epidemic diarrhea virus (PEDV) S glycoprotein and a cell wall anchoring sequence. The invention further constructs a secretion expression vector containing the antigen fusion gene, and secretion expression vector is converted into the bacillus megaterium to express recombinant protein on the cell wall or surface of the bacillus megaterium. Immunoblotting experiments indicate that the expressed recombinant protein can react with PEDV immune serum and has the same antigenicity as PEDV natural antigens. Immunofluorescent tests of live bacteria which are subjected to induced expression indicate that the expressed recombinant protein is positioned to the surfaces of the bacteria. Experiment results indicate that the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating porcine epidemic diarrhea.
Owner:WUHAN HUAYANG ANIMAL PHARMA
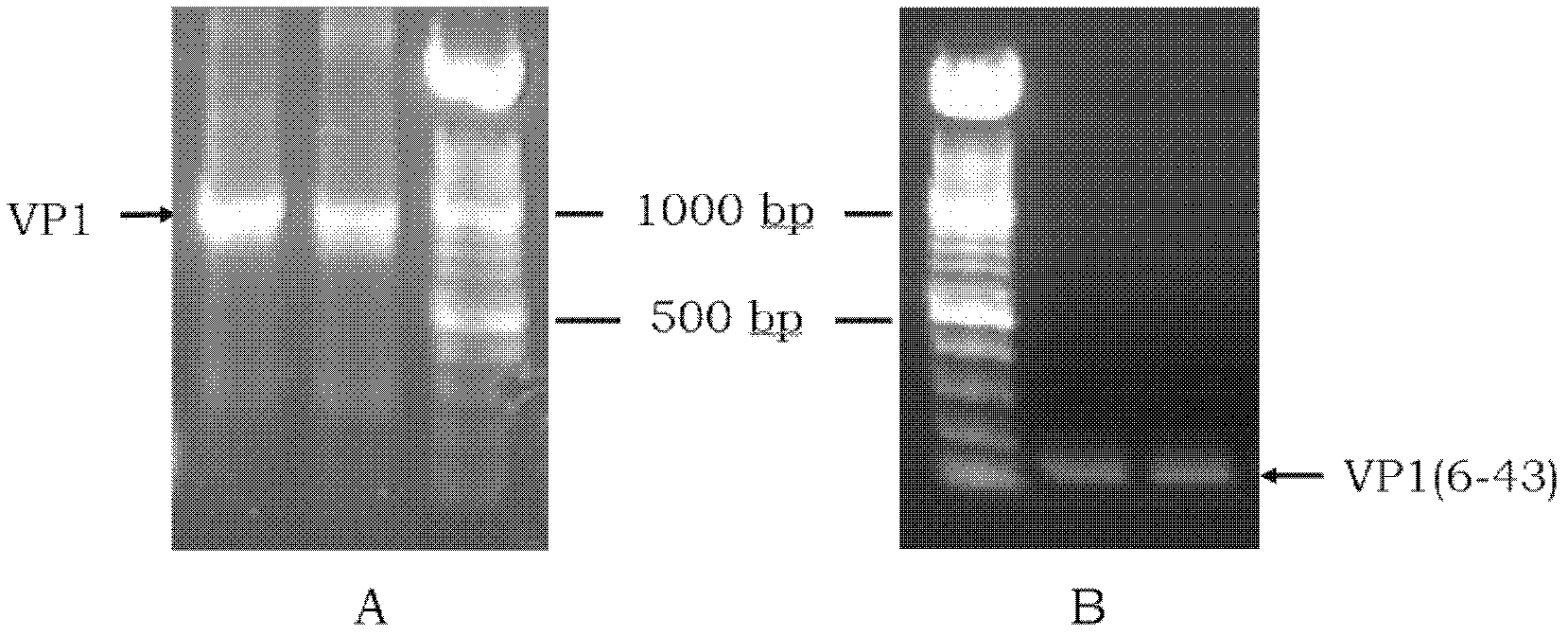
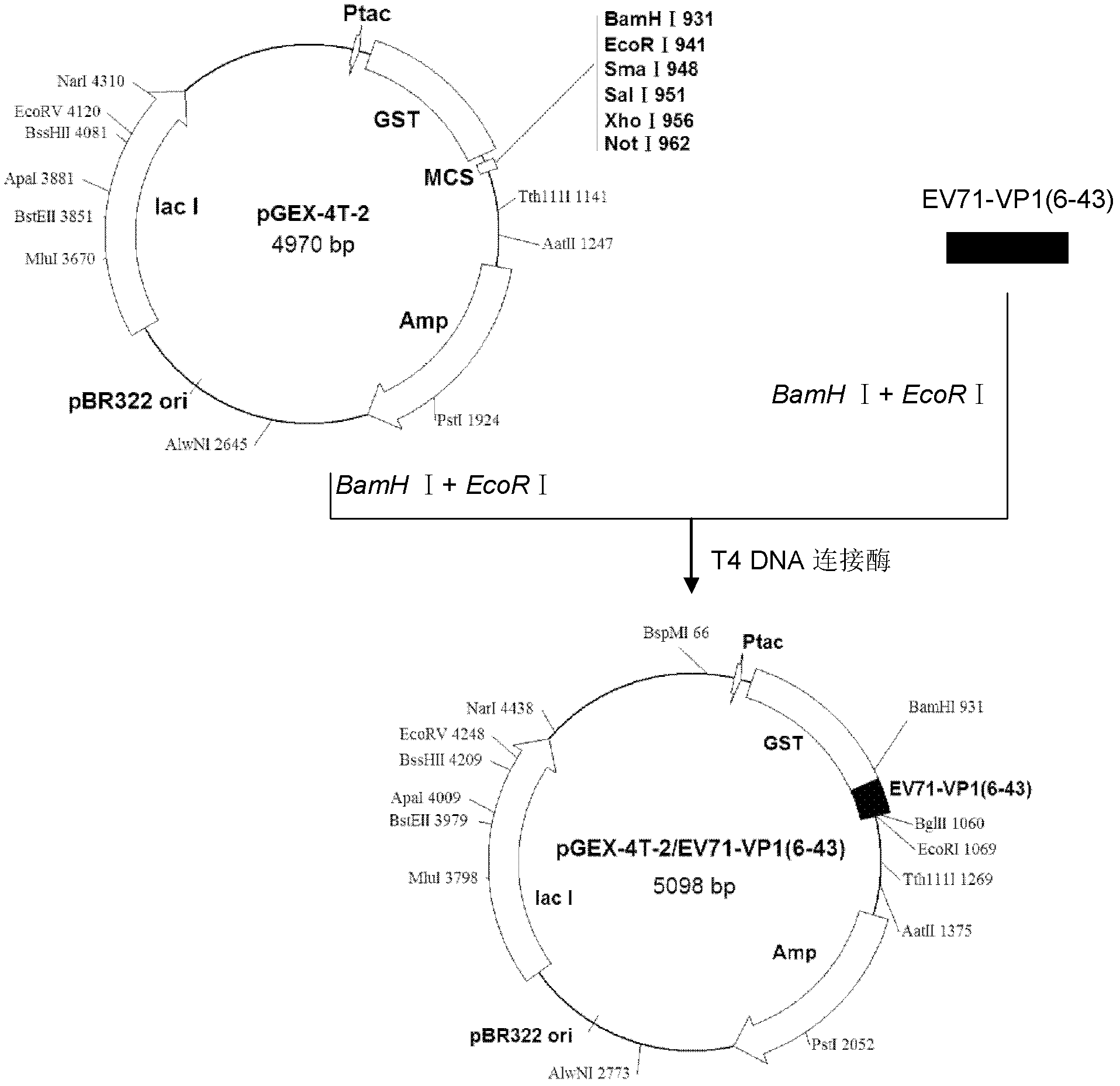
Enterovirus 71 type specific recombinant protein antigen and application thereof
InactiveCN102558313AIncreased sensitivityImprove featuresBacteriaMicroorganism based processesSerodiagnosesType specific
Owner:SOUTHEAST UNIV
Porcine epizootic diarrhea virus S1 protein fusion gene and recombinant bacillus megaterium, and their use
The invention discloses a porcine epizootic diarrhea virus S1 protein fusion gene and recombinant bacillus megaterium, and their use. An S1 antigen site of an S glycoprotein of the porcine epizootic diarrhea virus is connected to an anchor sequence of a cell wall so that the porcine epizootic diarrhea virus S1 protein fusion gene shown in the formula of SEQ ID No.1 is obtained. The invention further discloses a secrete expression vector containing the porcine epizootic diarrhea virus S1 protein fusion gene. The secrete expression vector containing the porcine epizootic diarrhea virus S1 protein fusion gene is transferred into the bacillus megaterium and a recombinant protein is expressed on the cell wall or the surface of the bacillus megaterium. A result of a western blot experiment shows that the expressed recombinant protein can react with PEDV immune serum so that it is proved that the expressed recombinant protein has the antigenicity the same as antigenicity of the PEDV natural antigen. A post-inducible expression living bacterium immuno-fluorescence test proves that the expressed recombinant protein is positioned on the surface of the recombinant bacillus megaterium. An experiment result shows that the recombinant protein provided by the invention can be prepared into a safe and effective mucosal immune live bacterium vaccine for preventing and treating porcine epizootic diarrhea.
Owner:WUHAN HUAYANG ANIMAL PHARMA
A method of indentifying brucella natural infection or immunifaction for livestock
A method of indentifying brucella natural infection or immunifaction for livestock is provided. According to the method, Omp31 protein or recombinant Omp31 protein containing a His label is adopted as interspecific difference identification protein, and Bp26 protein or recombinant Bp26 protein containing a His label is adopted as positive control protein. For livestock positive to a brucella infection serum SAT, the Bp26 protein or the recombinant Bp26 protein containing the His label reacts with serums infected by different strains of brucella, while the Omp31 protein or the recombinant Omp31 protein containing the His label only reacts with serums infected by brucella except the bovine brucella, and therefore the livestock which is positive to the SAT is naturally infected or is subjected to immunifaction. Through Western Blot detection, the two kinds of protein can identify the bovine brucella immune serums and non-bovine brucella immune serums, and can identify natural infection and immunifaction of bovine. The Omp31 protein and the Bp26 protein can be used for indentifying nonspecific agglutination reactions of other microorganisms with a brucella SAT antigen.
Owner:ICDC CHINA CDC
Process for preparing high-immune serum for main epidemic disease of dog and formulation thereof
InactiveCN1729996ASimple production processDisappear quicklySerum immunoglobulinsAntiviralsDiseaseBacteroides
The invention provides a process for preparing high-immune serum for main epidemic disease of dog and formulation, which comprises subjecting one or several pathogens causing serious diseases of canine animals to culture and propagation of primary or passage cell or bacteria culture media of dogs, monkeys and cats, charging adjuvant to obtain immunogens, immuning the canine animals and separating serum, eradicating virus, finally making single or combined serum or antibody preparation.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Amantadine artificial hapten and artificial antigen as well as preparation method and application of amantadine artificial hapten and artificial antigen
ActiveCN104402753AStrong specificityConformational stabilityOrganic compound preparationCarboxylic acid amides preparationNew Zealand white rabbitCarrier protein
The invention discloses an amantadine artificial hapten and an artificial antigen as well as a preparation method and application of the amantadine artificial hapten and the artificial antigen. The molecular structural formula of the amantadine artificial hapten is as shown in the formula (I), and the molecular structural formula of the amantadine artificial antigen is as shown in the formula (II). The application is to utilize the amantadine artificial antigen to prepare an amantadine antibody. The amantadine artificial hapten disclosed by the invention retains the feature structure of amantadine to the largest extent, comprises active groups capable of being linked and coupled with carrier protein as an antigen determinant; the prepared and obtained amantadine artificial antigen can be immunized to obtain the amantadine antibody with high affinity, high sensitivity and high specificity; the titer of immune serum obtained by immunizing a New Zealand white rabbit is up to 1:70000; the amantadine artificial hapten and the artificial antigen can be used for quick and accurate immunodetection and immunoassay to amantadine.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES +1
Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
InactiveCN101385855BEasy to storeImprove securityAntibody ingredientsDermatological disorderFreeze-dryingTetanus toxoids
The invention discloses a method for refining a polyvalent ASV (anti-snake venom) lyophilized serum, pertaining to an immune serum containing antibody which aims at least two kinds of antigens. The serum takes the venom of Ancistrodon acutus, Agkistrodon halys, Pit-vipers, bamboo snakes, adders and the like as the immune antigen which is inoculated into the bodies of animals after degerming and dialysis, and then titer immune serum after purification is used for collecting the antibody which is then desalted and lyophilized; mice are used for evaluating the LD50 of the virulence of the venom and the titer of the ASV serum; the antibody inoculated into the bodies of the animals is one of the venom, toxoid or snake venom or the composition thereof, and glutaraldehyde or formaldehyde is used for toxin detoxication; before immunity, anti-tetanic toxoid condensed antibody for human beings is used for carrying out minim gradual immunization on the animals, or an Emuade adjuvant is used for immunization purpose. The polyvalent ASV lyophilized serum complies with the biological product standards upon the tests and inspections of purity and specific activity, safety, pyrogen, hypersusceptibility, dissolution and the like; moreover, the serum can cure sufferers who are bitten by vipers with the advantages of high purity, low foreign protein, easy preservation and quick dissolving capacity.
Owner:KUNMING INST OF MILITARY MEDICINE CHENGDU MILITARY REGIONS CENT FOR DISEASE CONTROL & PREVENTION
Protein chip for lyme disease flagellin antigen immunoserology diagnosis and preparation method and application of protein chip
InactiveCN104374921AHigh sensitivityStrong specificityBiological testingAgainst vector-borne diseasesSerodiagnosesADAMTS Proteins
The invention discloses a protein chip for lyme disease flagellin antigen immunoserology diagnosis and a preparation method and application of the protein chip. The protein chip is characterized in that borrelia burgdorferi recombination flagellin antigen probes are fixed on the surface of a solid phase carrier in a dot matrix mode; the solid phase carrier is a 16-amino-1-hexadecyl mercaptan modified gold foil chip which is combined with 4-(N-maleinimide methyl) cyclohexane-1-carboxylic acid succinimide ester and 4-(dimethylamino) pyridine. The protein chip disclosed by the invention can accurately detect an anti-flagellin antigen IgG antibody and an anti-flagellin antigen IgM antibody in serums of lyme disease patients, the operation is simple, and the detection result is stable.
Owner:ANHUI MEDICAL UNIV
Streptococcus protective antigen SAP and preparation method thereof
InactiveCN106008680AHighly conservativeStrong specificityDepsipeptidesNucleic acid vectorProtective antigenEscherichia coli
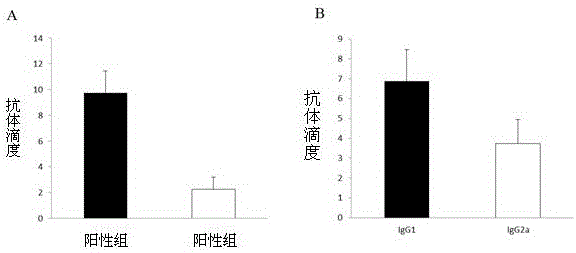
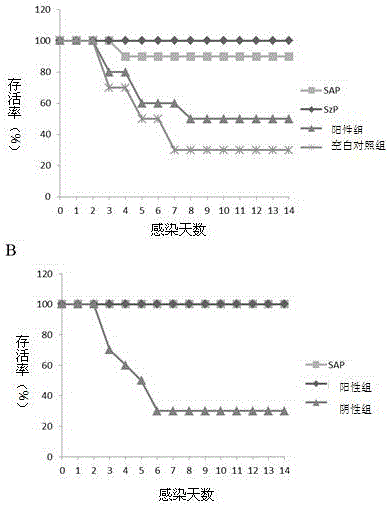
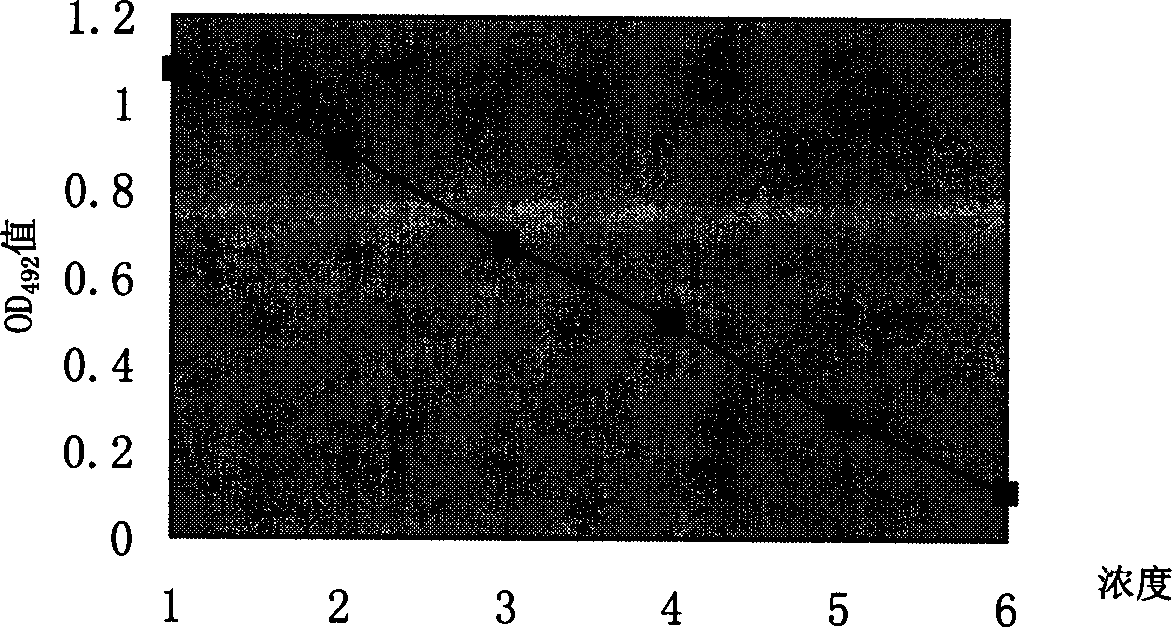
The invention provides streptococcus protective antigen SAP and a preparation method thereof. The antigen SAP is SAP recombination protein of SEZ. The preparation method comprises the following steps: firstly obtaining a target gene through a PCR technology, connecting the target gene to a carrier and converting escherichia coli, and performing purification on an expression product induced by escherichia coli, so as to finally obtain target recombination protein. The streptococcus protective antigen SAP has high conservatism, vaccine prepared from the protein is high in specificity, a series of bioengineering technologies and mouse experimental analysis prove that the SAP can provide higher protective efficacy after immunization, anti-SAP mice second immune serum has obvious passive immune protection to mice, and high-level antibody titer is expressed in mice serum of the SAP after immunization. The transcriptional level of SAP gene in mice body infected by SEZ is higher than that of SAP gene cultured in vitro, and an SAP antibody can induce high-level bactericidal ability.
Owner:FOSHAN UNIVERSITY
Hyperimmune serum against Vibrio parahaemolyticus, its preparation and inspecotion for astacin pathogenic bacterium
Owner:NATIONAL MARINE ENVIRONMENTAL MONITORING CENTRE
Animal composite immune globulin production method
ActiveCN1763096ASolve problemsWith immune functionSerum immunoglobulinsAntiviralsImmune effectsSerum ige
The production process of composite immune globulin for animal includes the following steps: preparing immune blood serum and packing immune blood serum at -20 deg.c inside storing container; separating immune blood serum to extract immune globulin IgG, IgA and IgM; purifying immune globulin IgG, IgA and IgM; mixing immune globulin IgG, IgA and IgM in the ratio of 8-10 to 1 to 1-2; and nanofiltering. The composite immune globulin thus produced includes three kinds of immune globulin, IgG, IgA and IgM, and has three kinds of immune functions, high immune effect of preventing animal viral diseases and easy absorption.
Owner:DALIAN SANYI ANIMAL MEDICINE CO LTD +2
Preparation method of spleen byproducts for producing homology anti-serum blood and transfer factor from fox, raccoon dog, mink
InactiveCN101422485AEasy to manufactureControl spreadAntibacterial agentsPeptide/protein ingredientsDiseaseBlood collection
The invention relates to a preparation method for obtaining blood used for preparing homologous antiserums and a spleen byproduct used for preparing transfer factors from the bodies of fur bearing animals such as foxes, raccoon dogs and minks; the furs of the foxes, the raccoon dogs and the minks are taken concentratedly in December and confirmed according to the fur-taking dates of different areas; healthy individuals are picked up 45 days before a predicated fur-taking date; the booster immunization of univalent vaccines or polyvalent vaccines of canine distemper, Canine parvovirus enteritis, adenovirus disease, corona virus laxness, parainfluenza, and pasteurellosis is carried out for 3 times by purified and condensed antigens on the foundation of carrying out immunization for once in summer; when the furs are taken, aseptic anticoagulation blood collection is carried out to a heart to improve the blood serum yield; and the spleens of the animals are collected for extracting specific or common transfer factors simultaneously. The blood can be conveniently prepared into the blood serums which resist communicable diseases, and the transfer factors can be extracted the spleen, thus providing guarantee for the healthy development of the breeding in the next year; the invention is used for curing the corresponding communicable diseases of the wild animals of the same species; and when a small amount of communicable disease cases appear in a breeding crowd, the corresponding pathogeny hyper-immune serums and transfer factors are mainly injected to a supposed healthy crowd after the pathogeny is confirmed so as to cure the affected animals in the early period of disease and control the spreading of the epidemic situations.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Efficient cascade amplification antibody testing method
InactiveCN101833000ARaise the response signalLow background signalMaterial analysisSorbentAntigen-antibody reactions
The invention relates to an efficient cascade amplification antibody testing method which adopts the enzyme-linked immuno sorbent assay (ELISA). The method comprises the following steps: performing coating treatment, namely adsorbing the known antigen or antibody on the surface of solid phase carrier; adding specific second antibody labeled with enzyme to perform antigen antibody reaction on the surface of solid phase carrier; and adopting washing method to remove free components in liquid phase, wherein during the coating treatment, high purity immune serum is used as sealing agent. The method of the invention performs further improvements on the basis of ELISA, thus increasing the response signal, reducing the background signal and effectively amplifying the signal of a substance to be tested in the sample. Therefore, the method can be used to test the low-content substances or trace substances in the biological sample.
Owner:北京北方北方有限责任公司
Preparation process of autogenous high-immunity serum for treating post-weaning multi systemic wasting syndrome of baby pig
InactiveCN1970082AImprove immunityImprove the quality of immune serumAntiviralsAntibody ingredientsMortality rateWasting Syndrome
The invention discloses a house hyper-immune serum making method to treat multi-system weaning exhaustion syndrome PMWS of pigling, which is characterized by the following: adopting multiple PCR technique to detect main pathogen PCV2 in the organ and the content of synergic infection PPV and PRRSV virus; selecting relative high-virus content organ as raw material to make house organ vaccine; proceeding multi-point injection strengthening immune through inactivated organ vaccine; detecting serum antibody level of immune pig; separating serum through high-antibody level pig; detecting the serum after biological safety as treating hyper-immune serum.
Owner:SHANGHAI JIAO TONG UNIV
Cell strain 16, monoclonal antibody produced by cell strain 16 and use of monoclonal antibody produced by cell strain 16
ActiveCN103911348AHigh sensitivityStrong specificityMicroorganism based processesTissue cultureBovine serum albuminAmmonium sulfate precipitation
The invention relates to a cell strain 16, a monoclonal antibody produced by the cell strain 16 and a use of the monoclonal antibody produced by the cell strain 16. A preparation method of the cell strain 16 comprises the following steps of coupling hapten 1 (H1) 3-[cyano[cis-3-[2-chloro-3,3,3-trifluoroethyl-2, dimethyl]cyclopropane-phenoxy]phenylpropionic acid and hemocyanin KLH by an active ester method, coupling hapten 2 (H2) cyano-[3-(4-aminophenoxy)phenyl-cis-3-[2-chloro-3,3,3-trifluorovinyl-2,2-dimethyl]cyclopropionate and bovine serum albumin by a diazotization method to obtain a conjugate as a coating antigen, and carrying out animal immunization, serum determination, cell fusion, screening and subcloning to obtain the cell strain 16. Through a mouse internal ascites induced-production method, an ascitic fluid is produced, and then through a caprylic acid-ammonium sulfate precipitation method, the ascitic fluid is purified so that the corresponding monoclonal antibody is produced. The monoclonal antibody has high sensitivity, median inhibitory concentration (IC50) of 13.26+ / -1.23ng / mL and strong singularity, provides a technology for detection of high-efficiency cyhalothrin residue in domestic agricultural products and has a good market prospect.
Owner:HENAN UNIV OF SCI & TECH
Utilization of mhc class II binding motifs in immunization to produce immune, serum, monoclonal antibodies and vaccines
This invention provides compositions and methods for raising humoral antibody responses. The compositions are peptides containing major histocompatibility Class 11 antigen binding motifs (ABM) either native or inserted into the peptide sequence. The ABM can be at the carboxy or amino terminus of the peptide and is shown to provide a T cell epitope thereby assuring adequate T cell help. Associated with the ABM is an extended peptide that rests outside the Class 11 molecule and that is recognized by the B cell, a B cell epitope. This B cell epitope can be a contiguous peptide sequence either at the amino or carboxy terminus. The extended peptide can be irrelevant and can serve as a bridge to an attached B cell epitope such as a hapten. These haptens can be any chemical structure such as a fluorescein molecule or a carbohydrate. The compositions and methods of this invention provide inexpensive vaccines to raise antibodies.
Owner:TITTLE THOMAS +1
Mycoplasma pneumoniae antigen as well as preparation method and application thereof
ActiveCN111253478AStrong specificityHigh sensitivityNucleic acid vectorDepsipeptidesMycoplasma antigenImmuno detection
The invention relates to the field of in-vitro diagnostic immunoassay, and in particular provides a mycoplasma pneumoniae antigen as well as a preparation method and application thereof. The mycoplasma pneumoniae antigen provided by the invention is protein composed of an antigenic protein sequence P1M:residues 1340-1518, specifically an amino acid sequence shown in SEQ ID NO.1, or protein which is obtained by performing substitution and / or deletion and / or addition on the amino acid sequence shown in the SEQ ID NO.1 by one or more amino acid residues and has same function. The antigen is verified by an immunoserological detection technology to have strong specificity and high sensitivity, and the antigen is easy to culture and purify, is more conducive to industrial production and saves costs; and in addition, the antigen is suitable for preparation of MP antibody detection products, can be used for any forms of products in the field of in-vitro immunodiagnosis, and has broad market prospects.
Owner:ZHUHAI LIVZON DIAGNOSTICS
New application of malaria erythrocytic stage inactivated whole organism vaccine
InactiveCN104771752APlay a role in preventioPlay a therapeutic roleAntiparasitic agentsAntibody medical ingredientsWhole OrganismMicrobiology
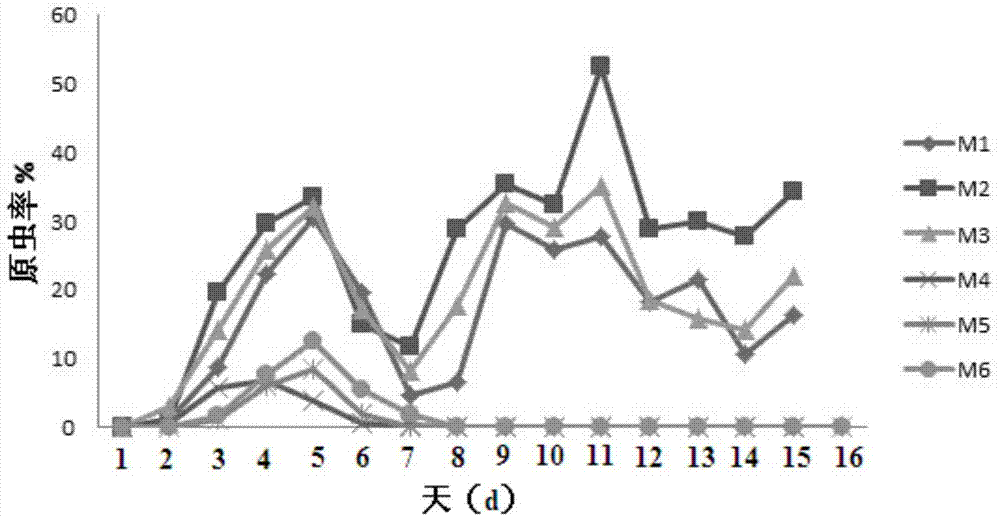
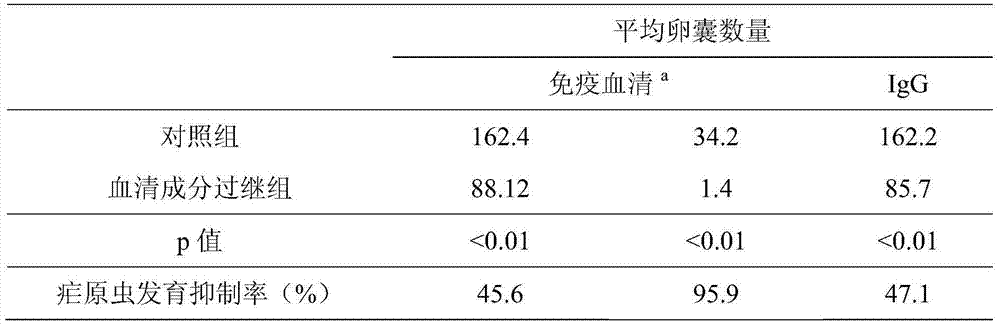
The invention discloses a new application of a malaria erythrocytic stage inactivated whole organism vaccine, and in particular relates to an application of the malaria erythrocytic stage inactivated whole organism vaccine serving as a transmission blocking type vaccine. According to the new application disclosed by the invention, a plasmodium erythrocytic stage immune serum is applied to a plasmodium mosquito stage transmission stage, and a whole organism vaccine used at the erythrocytic stage is discovered to show a certain effect of blocking mosquito stage transmission, so that the application range of the whole organism vaccine is enlarged, the preventing and curing effects on an immune individual are achieved, and the transmission opportunity of malaria among groups can be greatly reduced.
Owner:ARMY MEDICAL UNIV
Foot-and-mouth disease virus non-structural protein 3B epitope peptide and application thereof
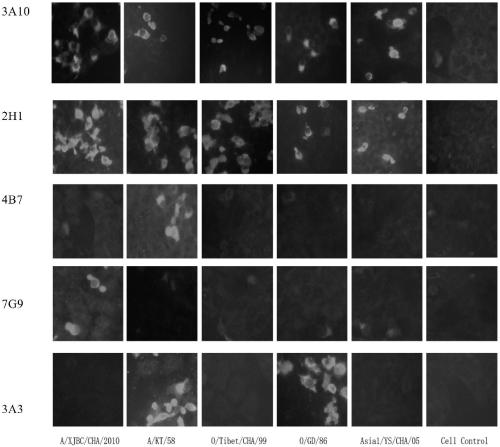
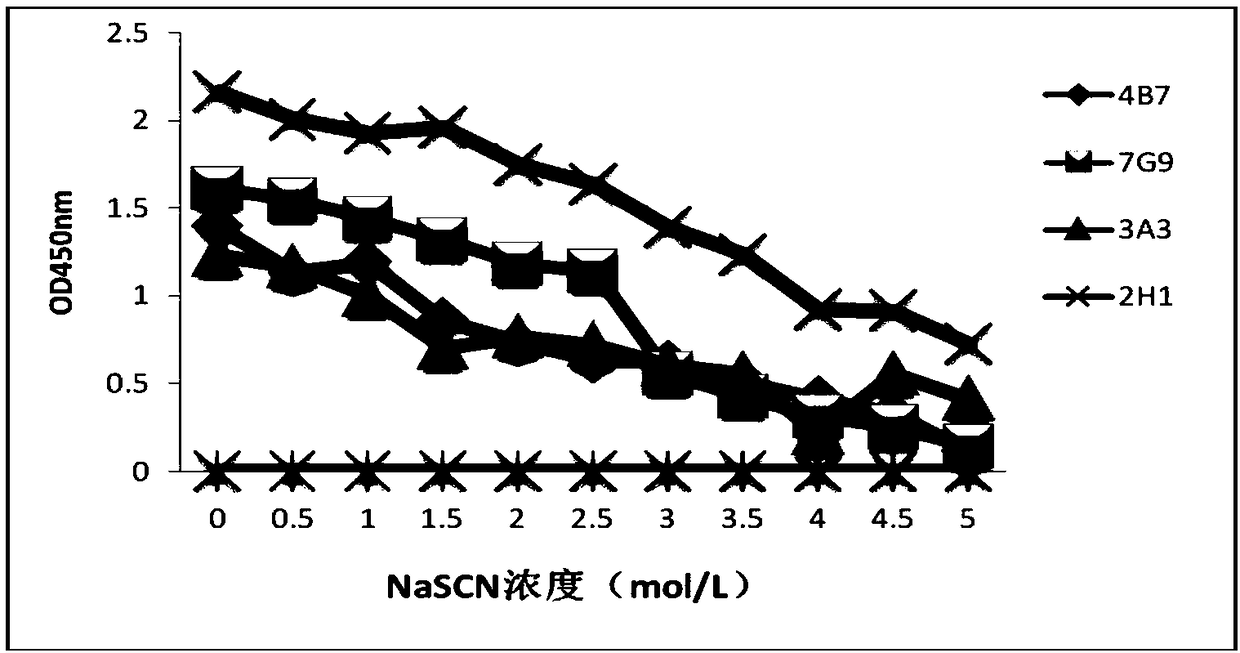
ActiveCN109293747AHighly conservativeImprove reliabilitySsRNA viruses positive-senseVirus peptidesStructural proteinMutant
The invention discloses a foot-and-mouth disease virus non-structural protein 3B epitope peptide and an application thereof. A hybridoma cell line 2H1 is obtained through screening using a cell fusiontechnology, and a monoclonal antibody secreted by the hybridoma cell line 2H1 has a broad spectrum and a high affinity, and is highly competitive with serum. The antigen epitope recognized by the monoclonal antibody 2H1 is further identified accurately, and the amino acid sequence of the monoclonal antibody 2H1 is represented by SEQ ID No.2; and the antigen epitope peptide is mutated to obtain aseries of mutants. The antigen epitope peptide and the mutants thereof have good reaction ability with FMDV antibody positive serums of foot-and-mouth disease infected pigs, cattle and sheep, and an antibody ELISA detection kit established by using the antigen epitope peptide as envelope antigens can specifically distinguish FMDV infected serum and antigen immunized serum, can be adopted to detectthe serum of any animal species, spans the inter-species barrier, and has the advantages of high reliability, wide application range, good broad spectrum, strong competitiveness, and quickness and convenience in detection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Swine transmissible gastroenteritis S/N protein fusion gene, recombinant lactococcus lactis and application
InactiveCN102399807AAntibacterial and antibacterialGood immune protectionBacteriaGenetic material ingredientsStreptococcus pyogenesSwine Transmissible Gastroenteritis
The invention discloses a swine transmissible gastroenteritis S / N protein fusion gene, recombinant lactococcus lactis and application. The invention provides an antigen fusion gene obtained by connecting A and D antigen loci in swine transmissible gastroenteritis virus (TGEV) S protein and an N321 antigen locus in N protein and a streptococcus pyogenes cell wall anchoring sequence, and a nucleotide sequence of the antigen fusion gene is shown as SEQ ID No. 1. The invention further provides an expression vector containing the antigen fusion gene and the recombinant lactococcus lactis strain containing the recombinant expression vector. The recombinant lactococcus lactis strain is induced by using nisin, the fusion gene can be expressed on the surface of the bacterial cell wall. Immunoblotting experiments indicate that the expressed recombinant protein can react with TGEV immune serum and has the same antigenicity with TGEV natural antigens, and the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating swine transmissible gastroenteritis.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Swine transmissible gastroenteritis virus S/N protein fusion gene and recombinant lactococcus lactis, and their use
ActiveCN103194471ABacteriaGenetic material ingredientsStreptococcus pyogenesSwine Transmissible Gastroenteritis
The invention discloses a swine transmissible gastroenteritis virus S / N protein fusion gene and recombinant lactococcus lactis, and their use. A and D antigen sites of a S protein of the swine transmissible gastroenteritis virus and an N321 antigen site of an N protein of the swine transmissible gastroenteritis virus are connected to a cell wall anchor sequence of streptococcus pyogenes so that the swine transmissible gastroenteritis virus S / N protein fusion gene is obtained and has a nucleotide sequence shown in the formula SEQ ID No.1. The invention further discloses an expression vector containing the swine transmissible gastroenteritis virus S / N protein fusion gene and the recombinant lactococcus lactis containing the expression vector. The recombinant lactococcus lactis is induced by Nisin so that the swine transmissible gastroenteritis virus S / N protein fusion gene can be expressed on the bacterial cell wall surface. A result of a western blot experiment shows that the expressed recombinant protein can react with TGEV immune serum so that it is proved that the expressed recombinant protein has the antigenicity the same as antigenicity of the TGEV natural antigen and thus the expressed recombinant protein can be prepared into a safe and effective mucosal immune live bacterium vaccine for preventing and treating swine transmissible gastroenteritis.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Method for constructing haemophilus parasuis genome library and screened immune protein
InactiveCN102251289ABest immune protection peptideMicroorganism based processesFermentationEnzyme digestionSerotype
The invention belongs to the field of biological engineering, and provides a method for constructing a haemophilus parasuis genome library. The method comprises the following steps of: extracting bacterial genome DNA of haemophilus parasuis with collection number CCTCC NO. M2010063, performing enzyme digestion on the haemophilus parasuis bacterial genome, connecting enzyme digestion fragments of the haemophilus parasuis bacterial genome and vectors and transforming, and performing polymerase chain reaction (PCR) identification and positive cloning. The haemophilus parasuis with collection number CCTCC NO. M2010063 is screened by using the genome library, antiserum is prepared in the screening process, enzyme-linked immunosorbent assay (ELISA) is performed on the antiserum, and the library is repeatedly screened by using the immune serum to obtain 5 positive clones. The positive clones obtained by repeated screening provide a choice for researching antigenic peptide for ELISA detection, and 15 serum type protein antigens of the haemophilus parasuis can be detected at one time.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Identification method of deer blood active crystal preparation
InactiveCN103421882AComplete and scientifically reliable identification methodMicrobiological testing/measurementMaterial analysis by electric/magnetic meansBlood componentImmunoprecipitation
The invention discloses an identification method of a deer blood active crystal preparation. The method comprises a PCR amplification test identification of specific DNA sequence of deer blood in the deer blood active crystal preparation, if a 405 bp stripe is amplified out, indicating that the preparation contains components of sika deer blood and red deer blood, if a 317 bp stripe is amplified out, indicating that the preparation contains components of sika deer blood; an immunoprecipitation test identification, if an immunoprecipitation line appears between each non-deer blood antibody immune serum and the deer blood active crystal preparation, indicating that the tested deer blood active crystal preparation contains other non-deer blood components; and an SDS-PAGE test identification for specific protein components contained in the deer blood active crystal preparation, if the processed gel appears feature spectrum bands, indicating that the deer blood active crystal preparation contains deer blood specific protein components. The method mentioned above provides a complete and scientific identification method for distinguishing true or false and high-quality or low-quality of deer blood crystal products in present markets.
Owner:苏州红冠庄国药股份有限公司
Recombinant lactobacillus casei for expressing infectivity pancreatic necrosis virus VP2 protein and production method thereof
InactiveCN101508969AEffective stimulationImprove the level ofBacteriaMicroorganism based processesProtein targetInfectious pancreatic necrosis
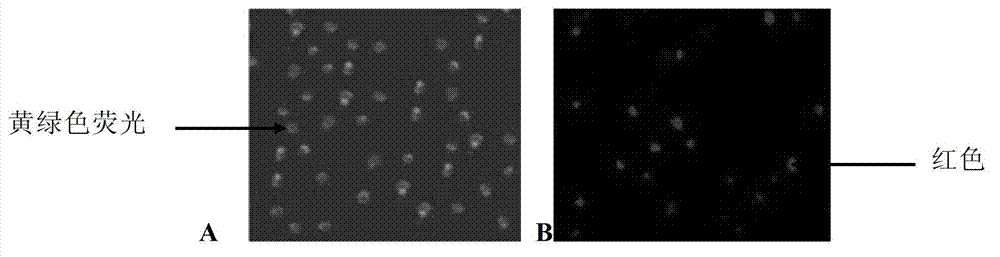
The invention provides a recombinant Lactobacillus casei for expressing the infectious pancreas necrosis virus (IPNV) VP2 protein and a preparation method thereof. According to the whole gene sequence of the IPNV VP2 protein and the gene fusion characteristic of expression vector plasmid, a pair of primers are designed to perform PCR to obtain target segment of 1095bp containing the main antigen site of the IPNV VP2 protein, the IPNV VP2 protein obtained through amplification is linked to the surface expressed carrier plasmid pPG1 and enters the cell of the host strain Lactobacillus casei 393 through electrotransformation, and the Lactobacillus casei containing positive recombinant plasmid pPG1-IPNV VP2 is named pPG1-IPNV VP2 / L. casei 393. The recombinant pPG1-IPNV VP2 / L. casei 393 is expressed under the induction of lactose. In the invention, the Lactobacillus casei expression carrier system of the IPNV VP2 protein is constructed and the target protein of around 40 KDa containing the main antigen site of the IPNV VP2 protein is expressed, and according to the Western blot experiment and indirect immunofluorescence experiment, the expressed foreign protein is capable of reacting with the IPVN immune serum, which shows that the recombinant VP2 protein and the IPNV natural antigen have the same antigenicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com