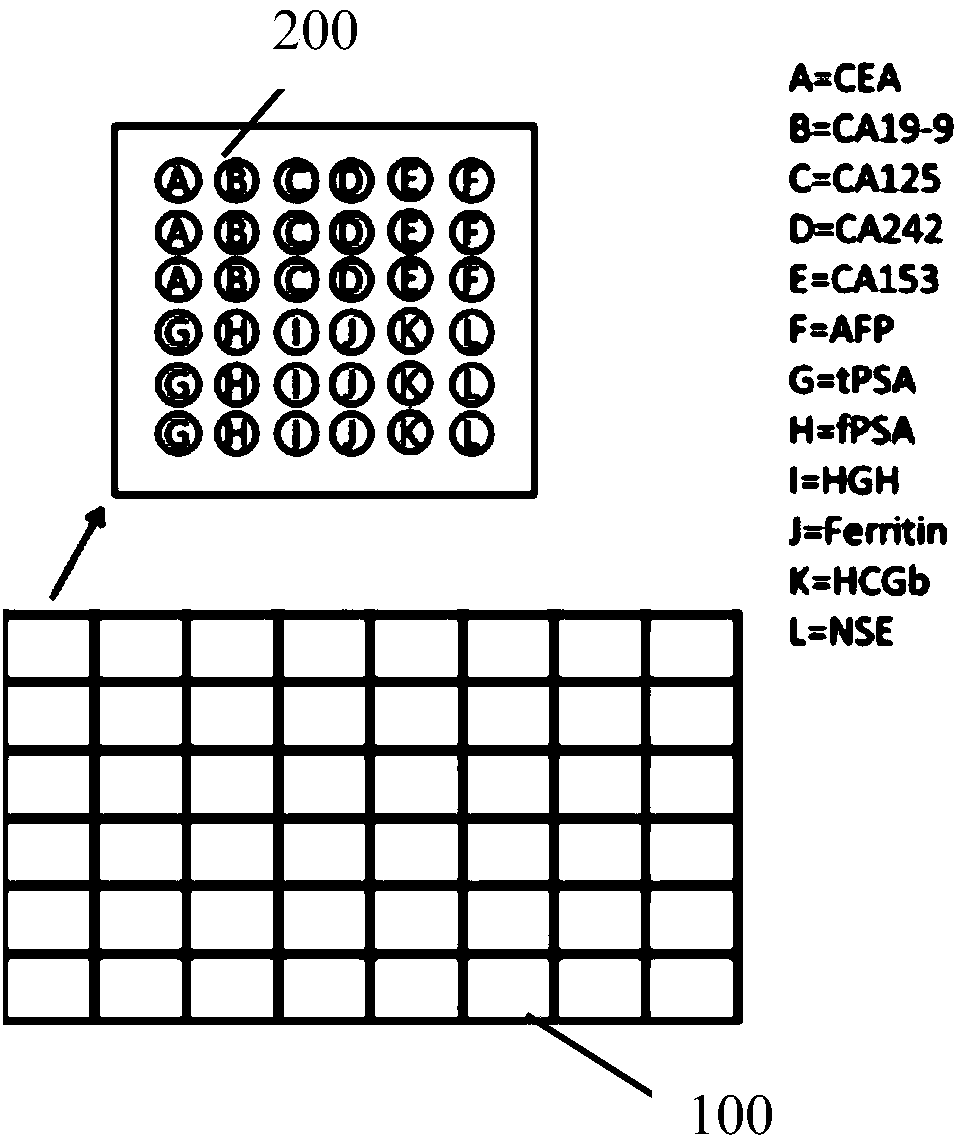
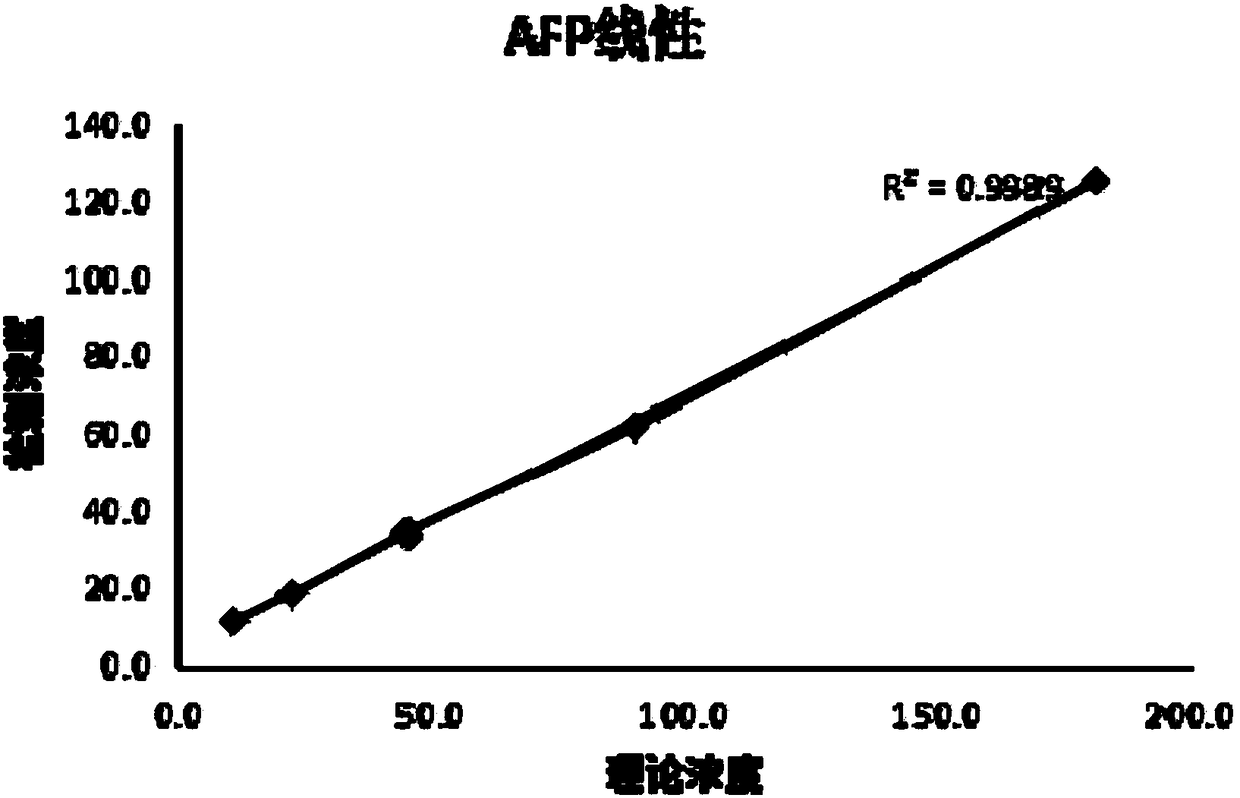
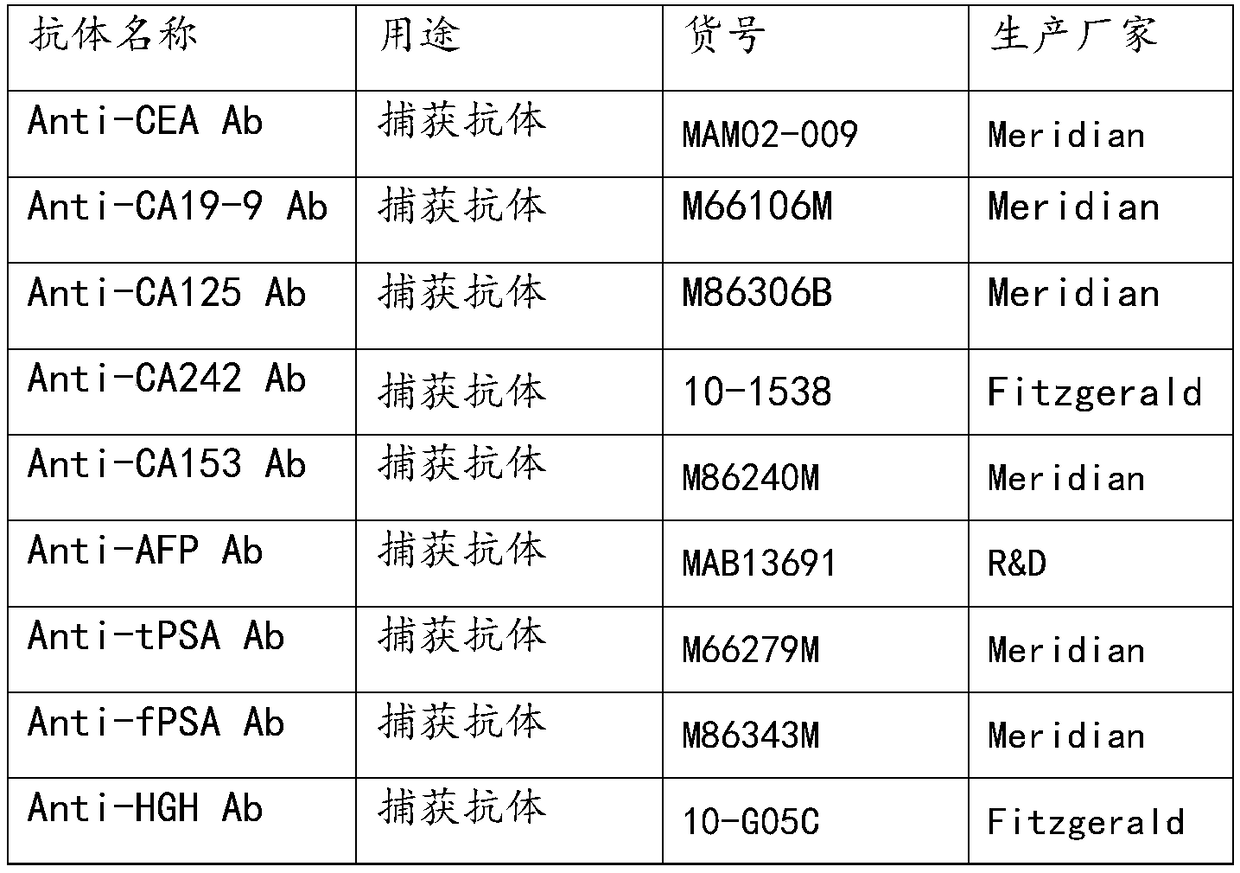
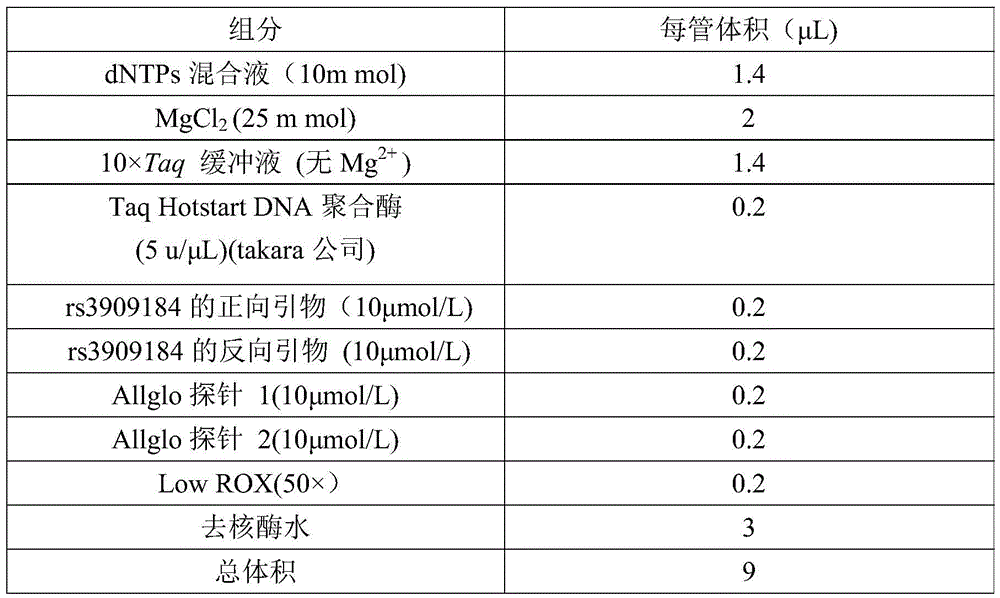
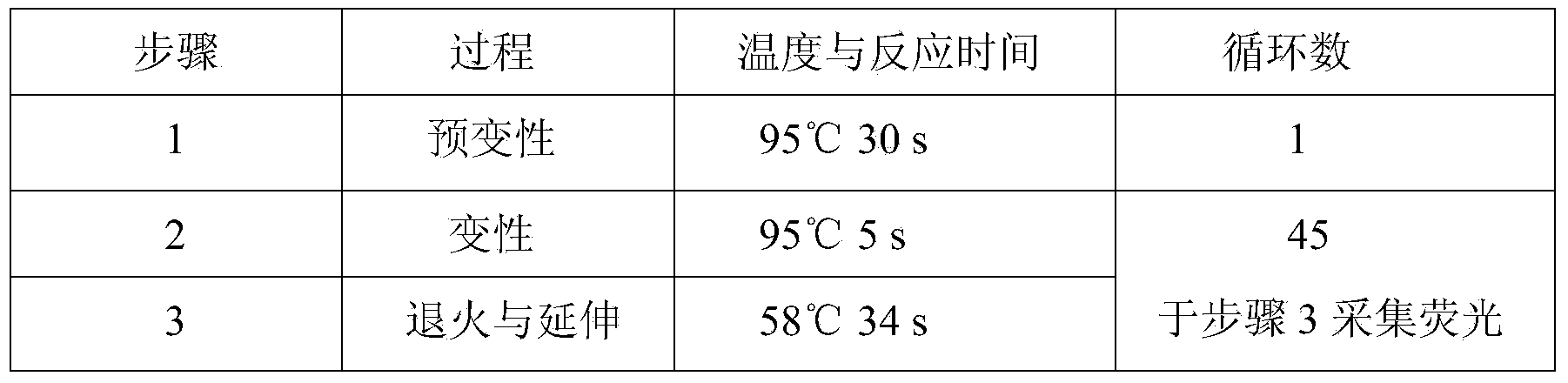
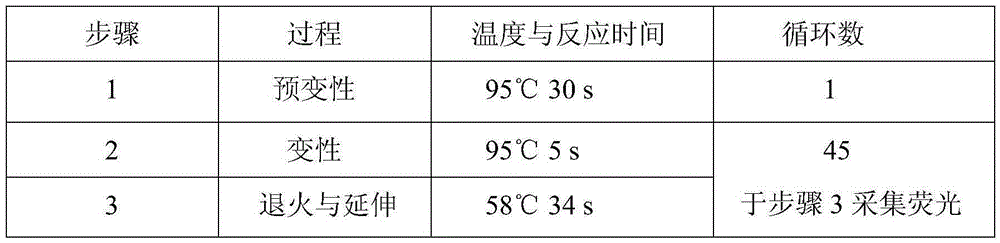
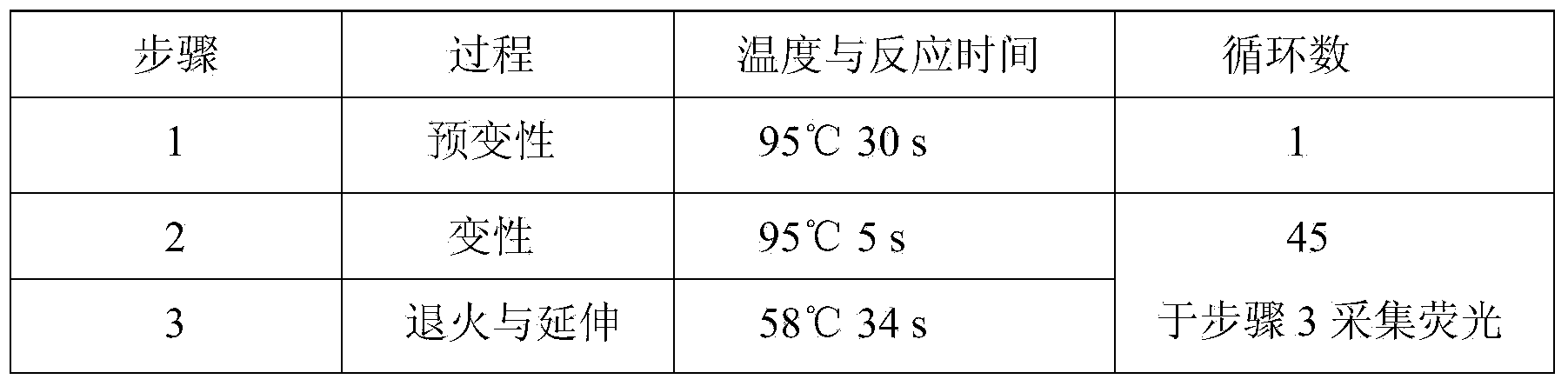
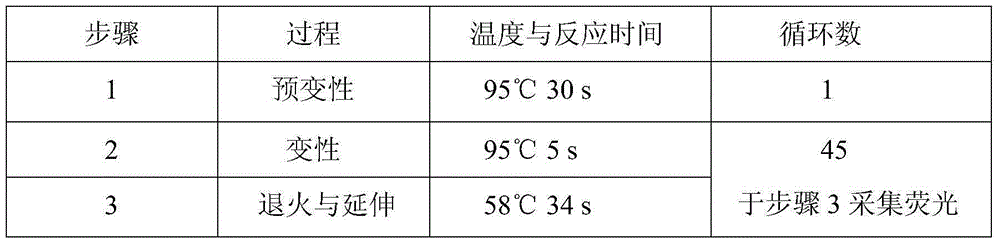
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55results about How to "Low background signal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
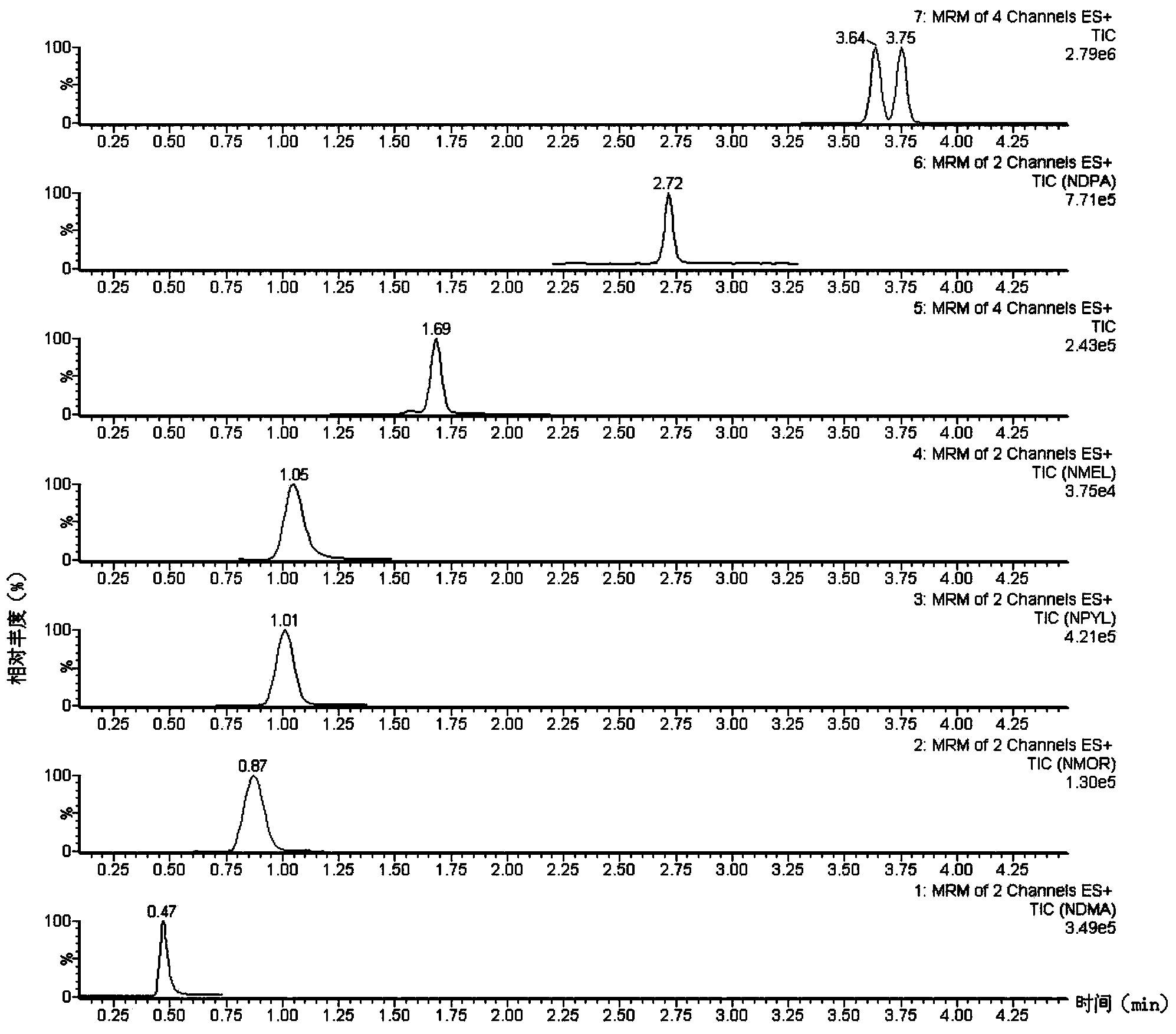
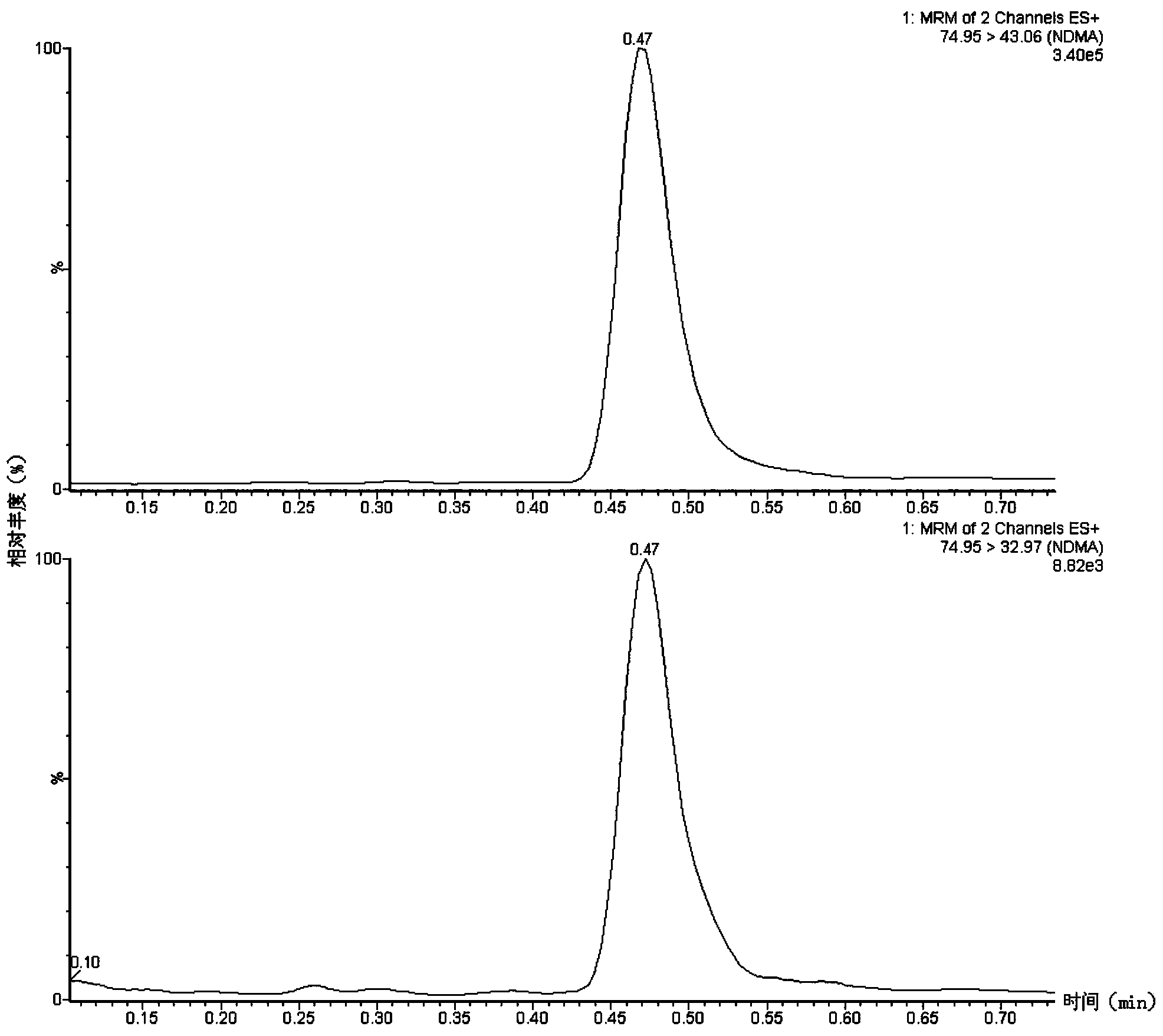
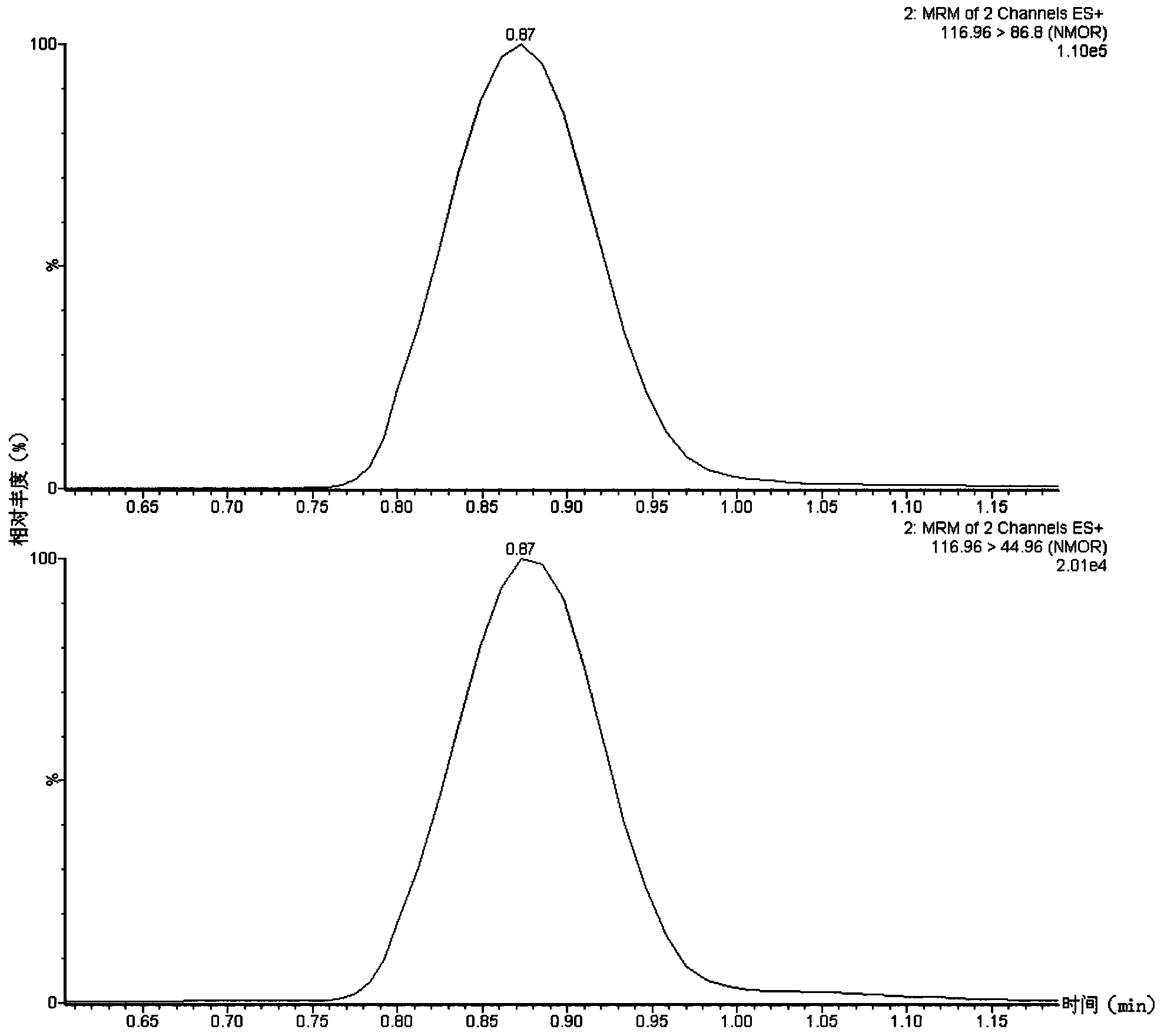
Method suitable for simultaneously detecting 9 N-nitrosamines in food contact rubber products
InactiveCN104076106AQualitatively accurateQuantitatively accurateComponent separationNitrosoN-nitrosamine
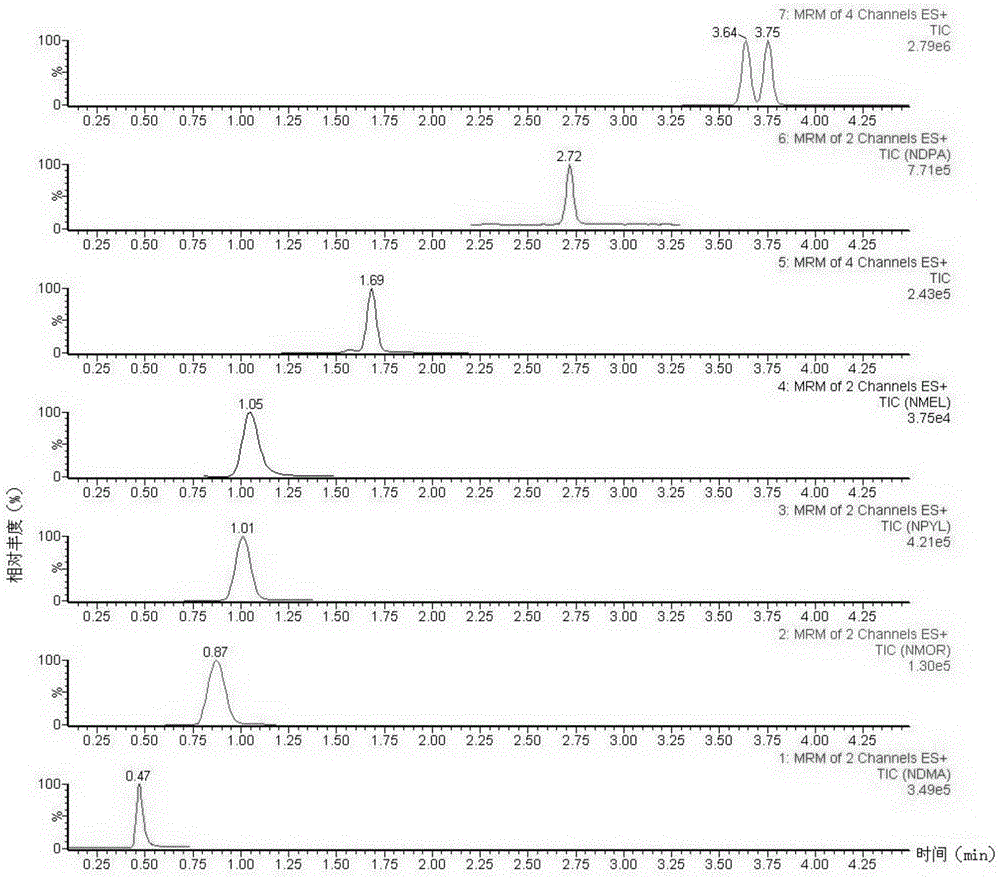
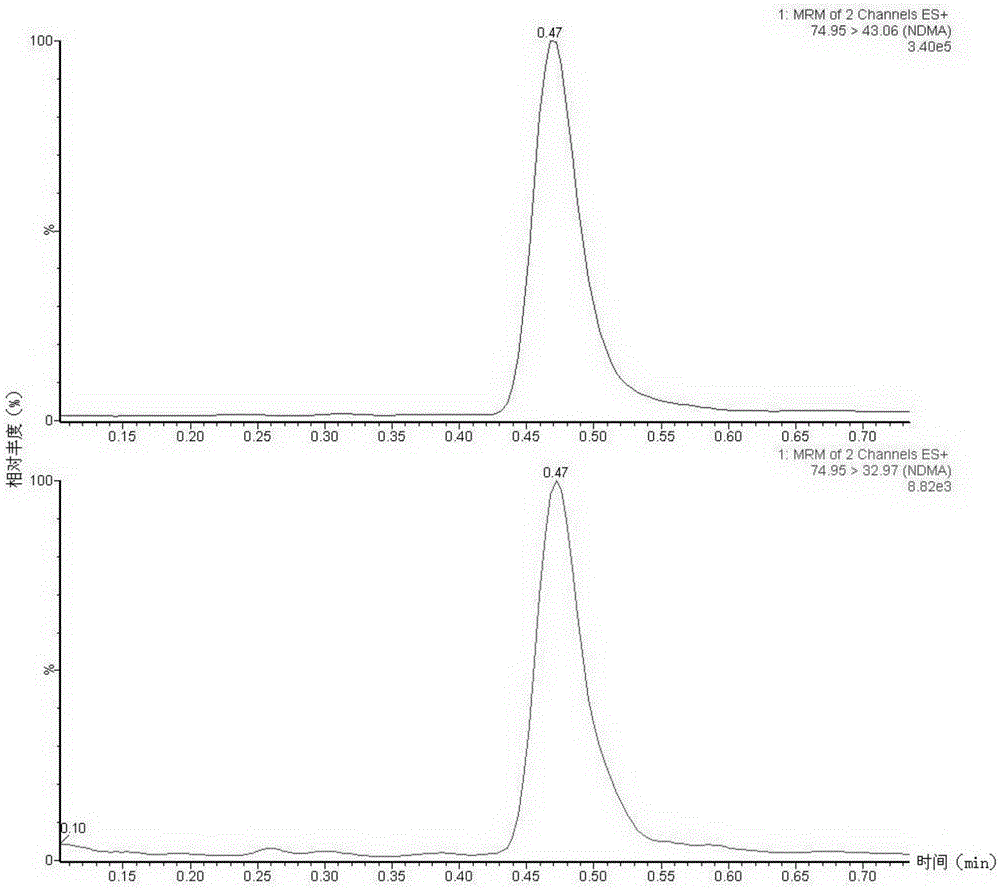
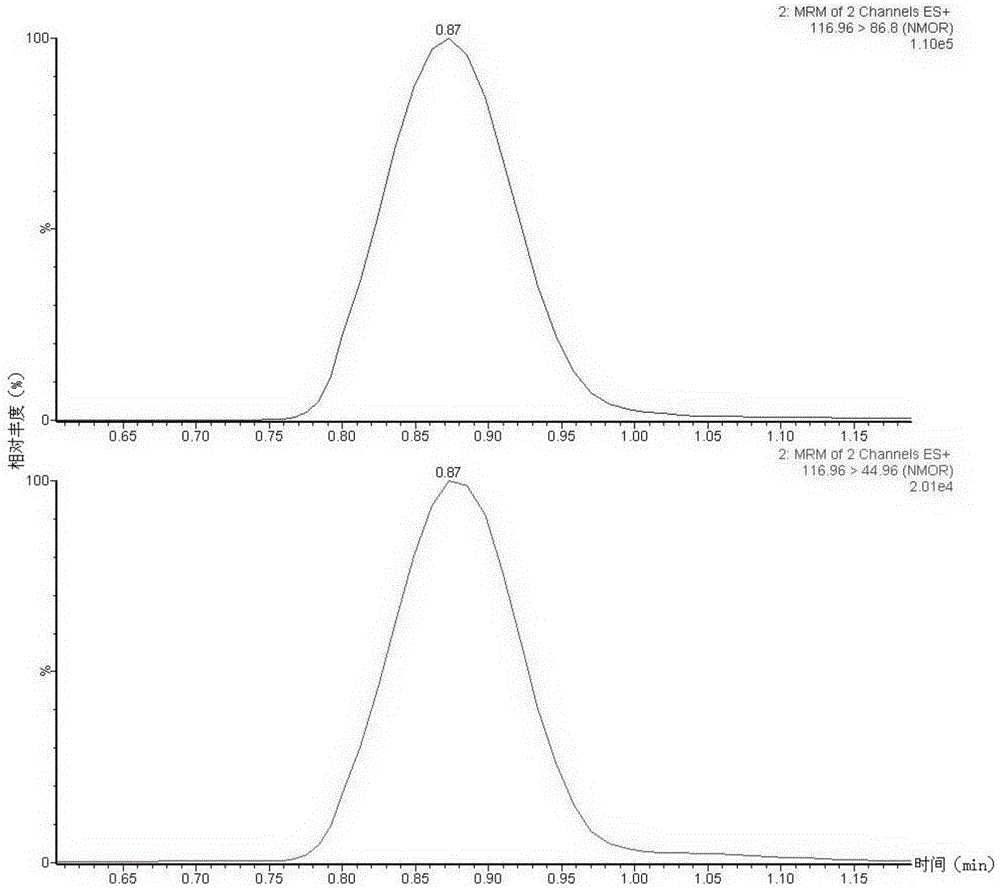
The invention discloses a method suitable for simultaneously detecting 9 N-nitrosamines in food contact rubber products. The method comprises the following steps: utilizing a sample to be tested to establish a migration system to obtain a migration extract; utilizing the migration extract to prepare a sample solution to be tested to obtain a supernatant / filtrate; preparing 9 N-nitrosamines into a standard solution to further prepare a standard working solution with the gradient of 0.01-0.40 [mu]g / mL; injecting the gradient standard solution and the supernatant / filtrate into a liquid Chromatogram-tandem mass spectrograph, and testing the cation multi-reaction monitoring model to finally obtain the respective contents of 9 N-nitrosamines in the sample to be tested. The 9 N-nitrosamines are N-Nitrosodimethylamine, N-Nitroso-ethyl methylamine, N-Nitrosomorpholine, N-Nitrosopiperidine, N-Nitrosopyrrolidine, N-Nitroso-diethylamine, N-Nitrosodibutylamine, N-Nitrosodi-n-propylamine and N-Nitroso-diphenylamine.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method suitable for detecting residues of eleven defoliating agents in cotton
InactiveCN104090058AFully automatedHarm reductionComponent separationSpectrometerTandem mass spectrometry
The invention discloses a method suitable for detecting residues of eleven defoliating agents in cotton. The method is characterized by comprising the following steps: (1) preparing a sample solution to be detected, namely weighing cotton to be detected, and extracting by utilizing an accelerated solvent extraction device; by taking methanol as an extraction agent, operating according to a set extraction program; performing vacuum concentration after extraction is finished, fixing the volume and centrifuging; obtaining the supernatant; (2) preparing a standard solution, namely preparing a 0.01-0.30mu g / mL of gradient standard working solution of eleven defoliating agent pesticides; (3) injecting the gradient standard solution into an ultrahigh pressure liquid chromatography-tandem mass spectrometer, measuring in a positive and negative ion multiple-reaction monitoring mode, and making a standard curve equation; (4) measuring the supernatant obtained in the step (1) according to a method in the step (3) to finally obtain the content of each defoliating agent pesticide in the sample to be detected.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Quantitative pet imaging of tissue factor expression using 18f-labled active site inhibited factor vii
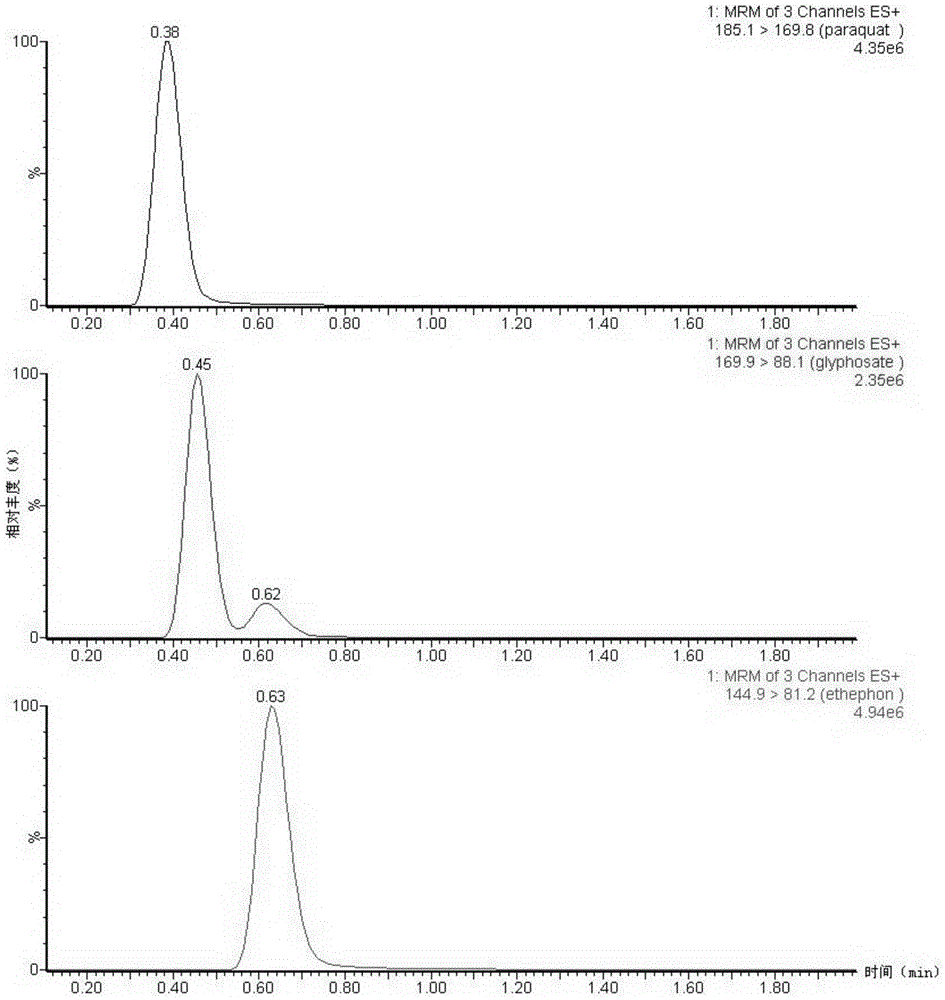
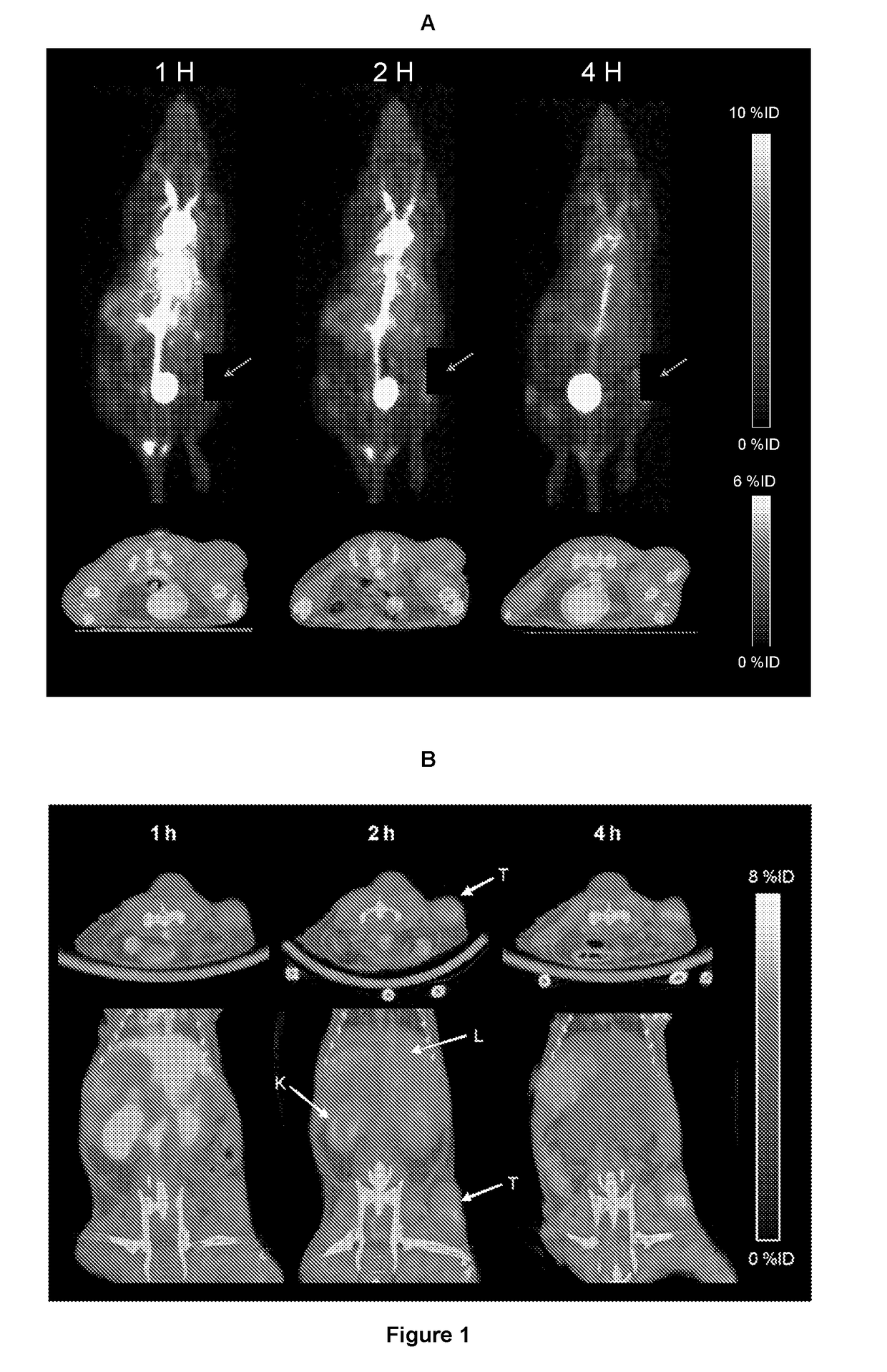
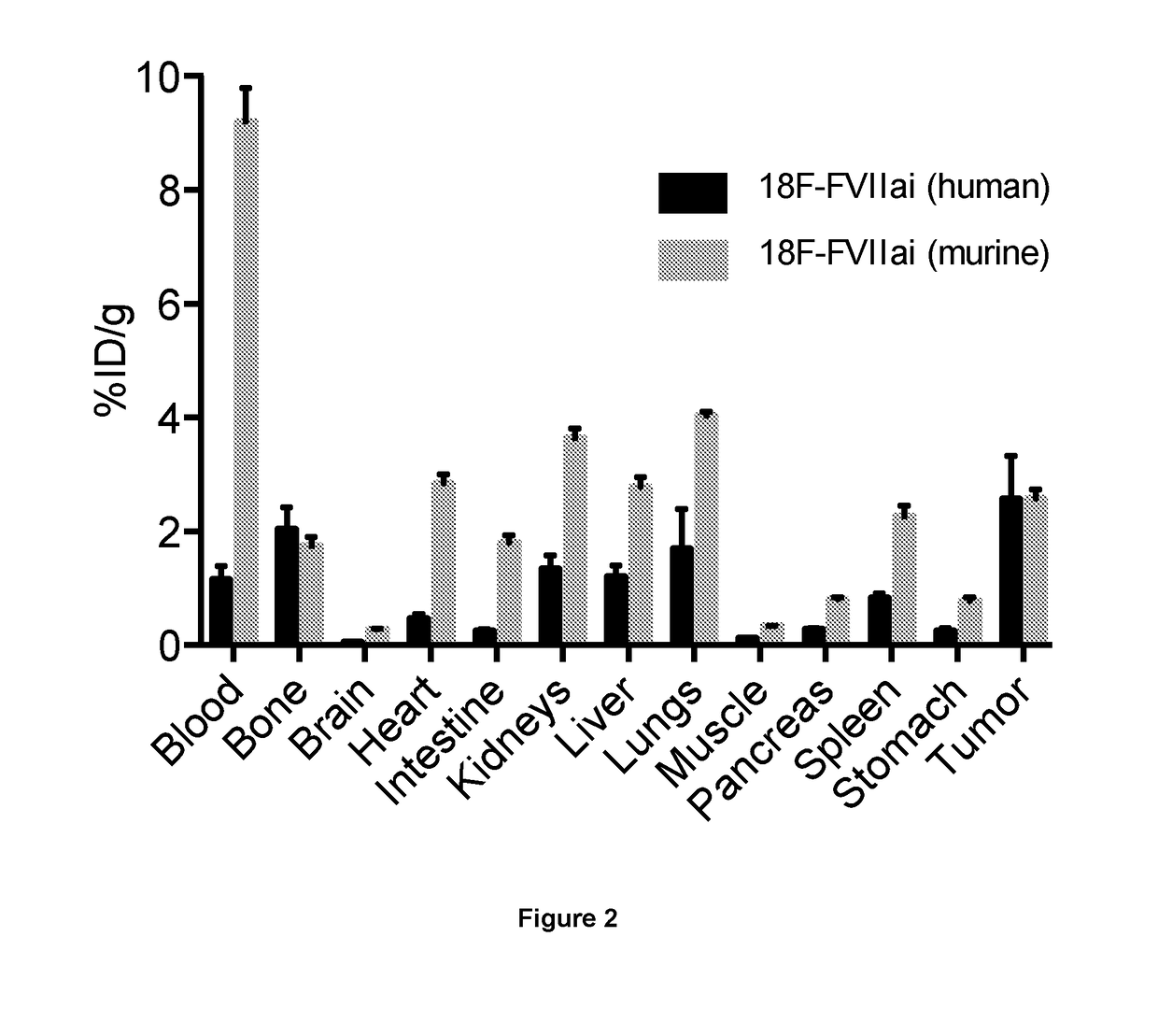
PendingUS20190015532A1Low background signalPeptide/protein ingredientsHydrolasesNon invasiveLymphatic Spread
Owner:RIGSHOSPITALET
Efficient cascade amplification antibody testing method
InactiveCN101833000ARaise the response signalLow background signalMaterial analysisSorbentAntigen-antibody reactions
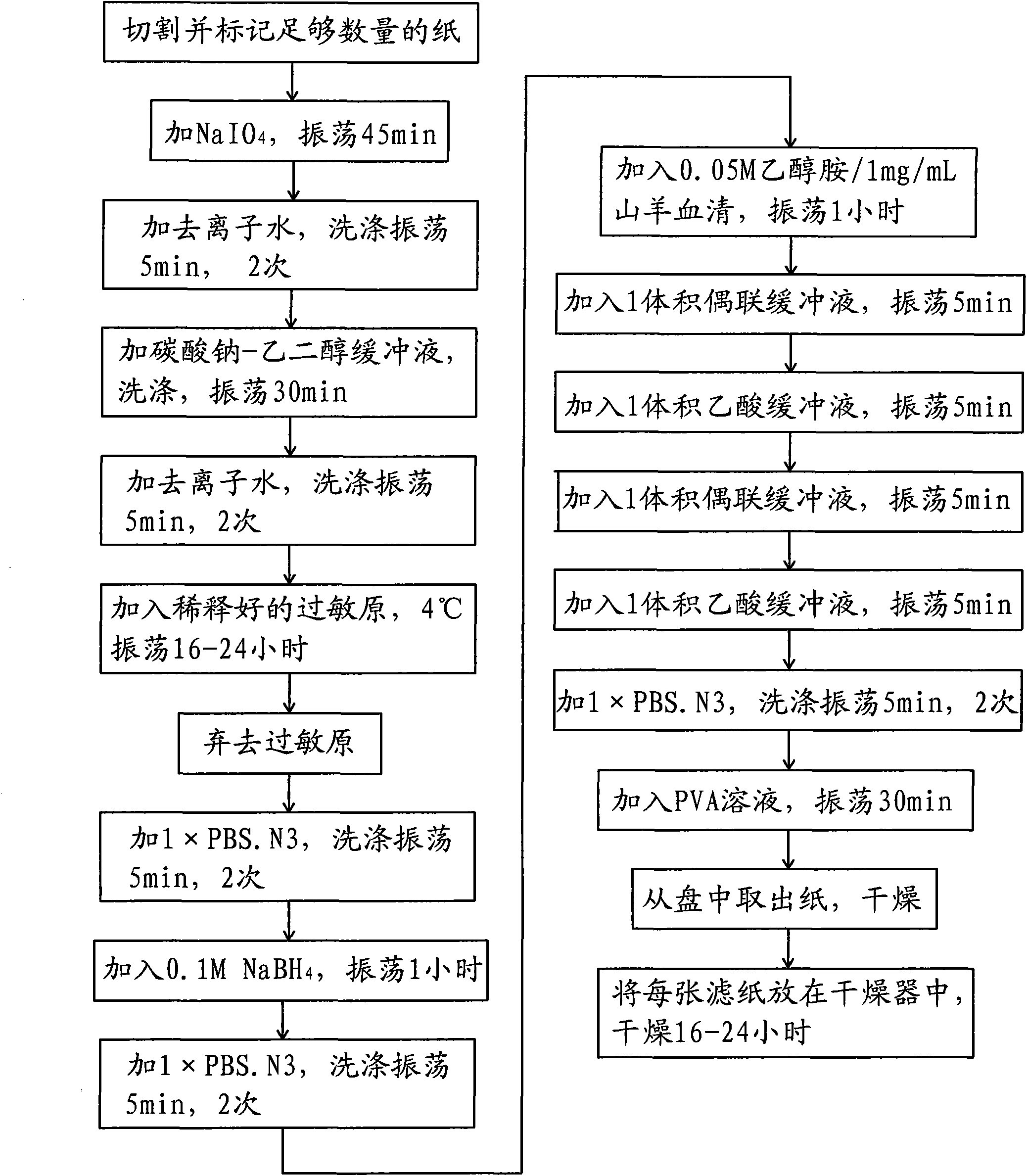
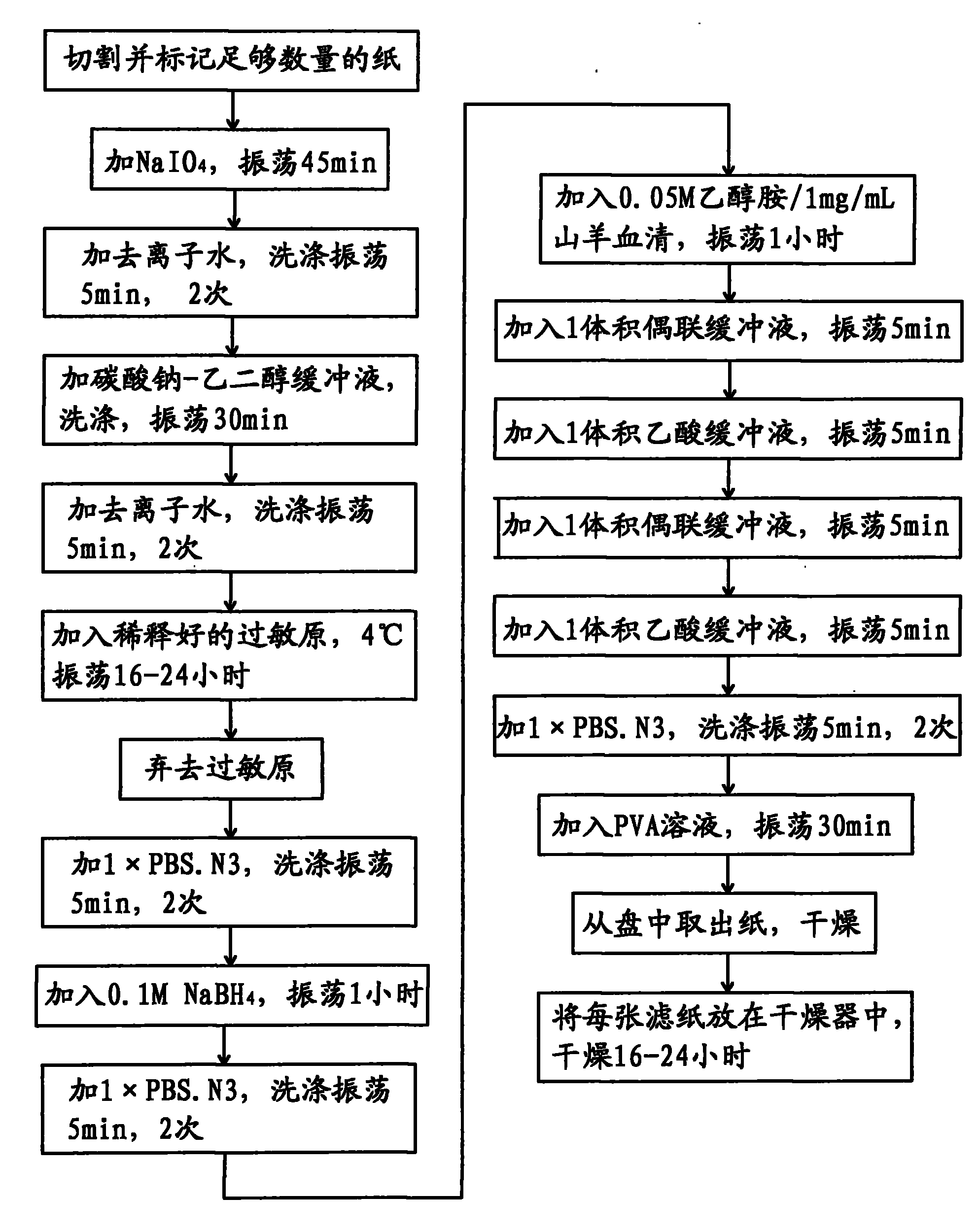
The invention relates to an efficient cascade amplification antibody testing method which adopts the enzyme-linked immuno sorbent assay (ELISA). The method comprises the following steps: performing coating treatment, namely adsorbing the known antigen or antibody on the surface of solid phase carrier; adding specific second antibody labeled with enzyme to perform antigen antibody reaction on the surface of solid phase carrier; and adopting washing method to remove free components in liquid phase, wherein during the coating treatment, high purity immune serum is used as sealing agent. The method of the invention performs further improvements on the basis of ELISA, thus increasing the response signal, reducing the background signal and effectively amplifying the signal of a substance to be tested in the sample. Therefore, the method can be used to test the low-content substances or trace substances in the biological sample.
Owner:北京北方北方有限责任公司
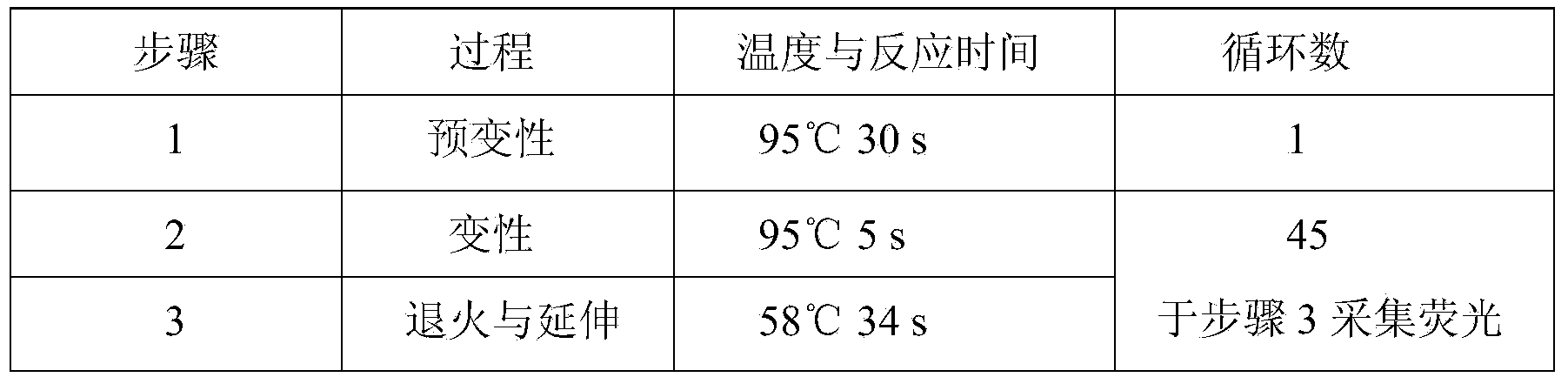
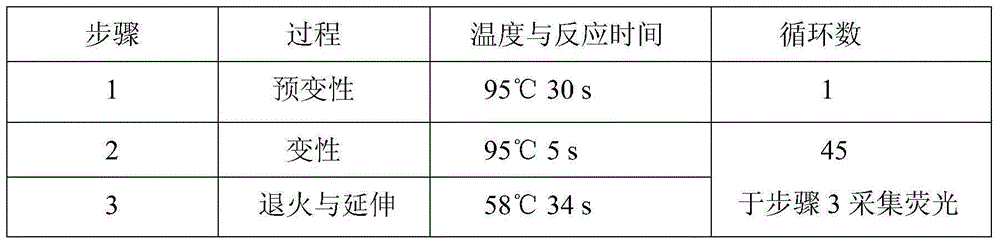
Hsa-miR-188-5p detection kit based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and detection method thereof
InactiveCN103757122ALow costReduce sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsPlasma samplesTissue sample
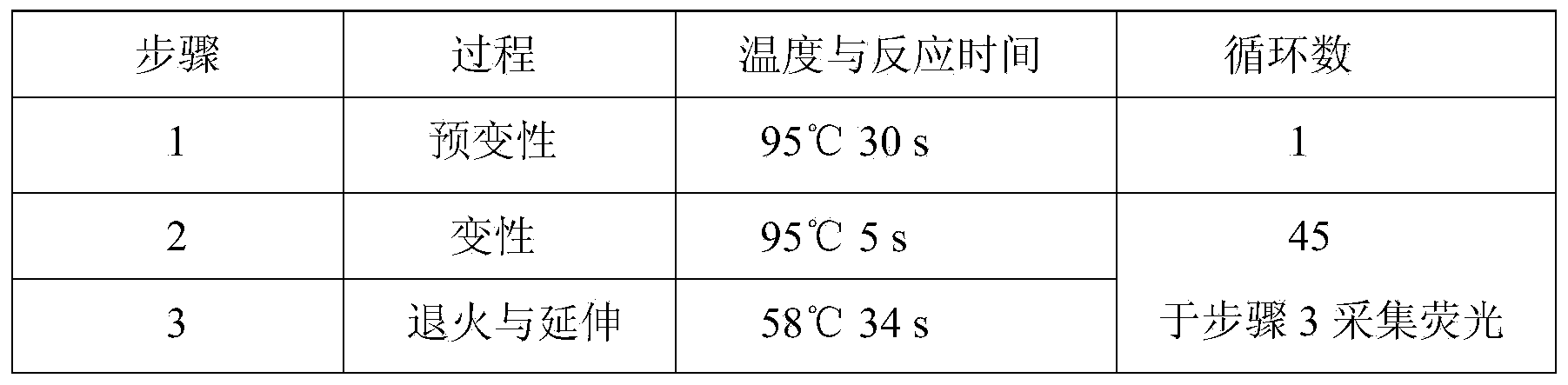
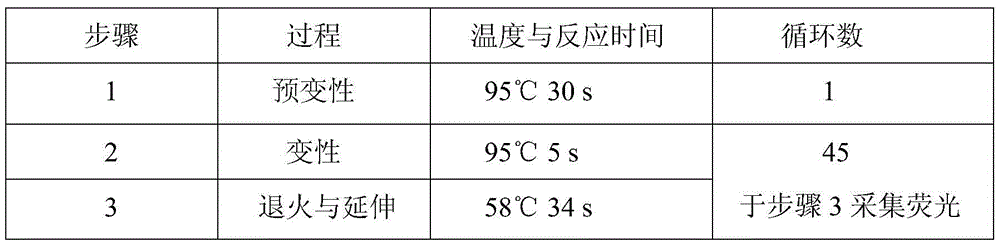
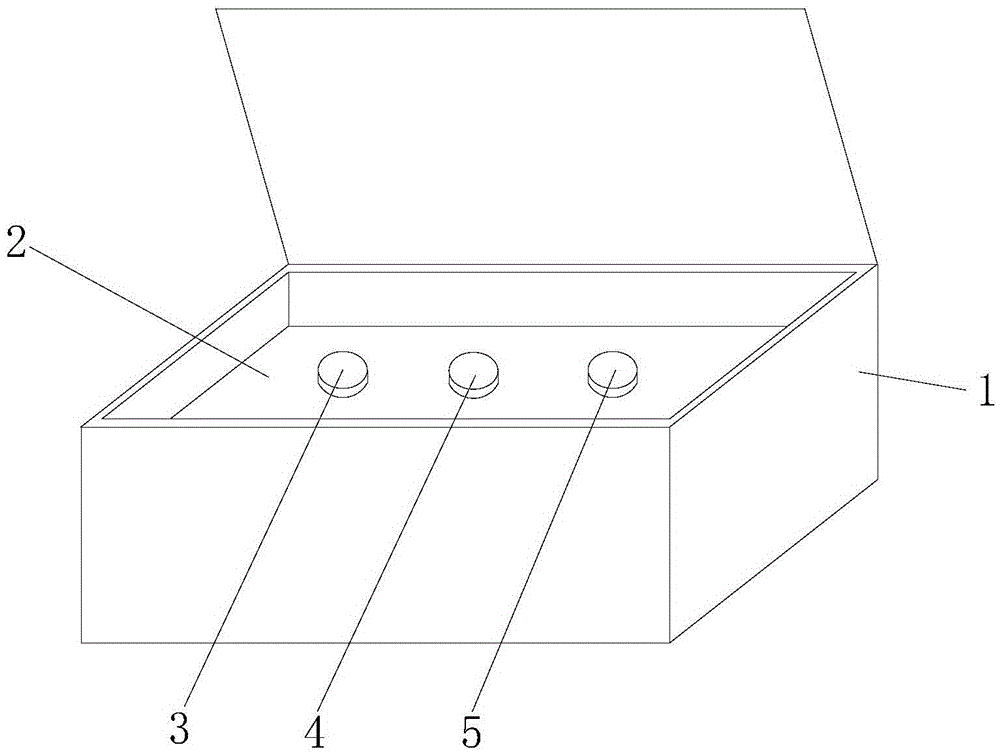
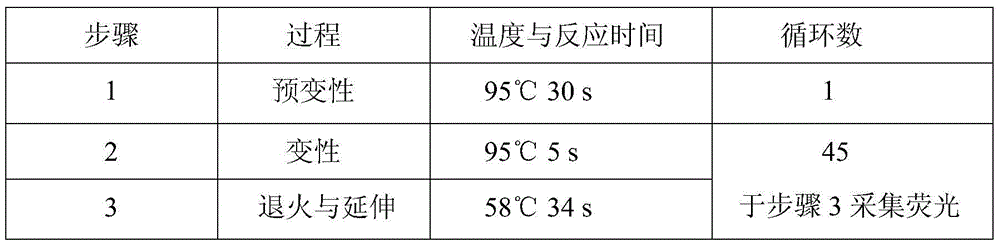
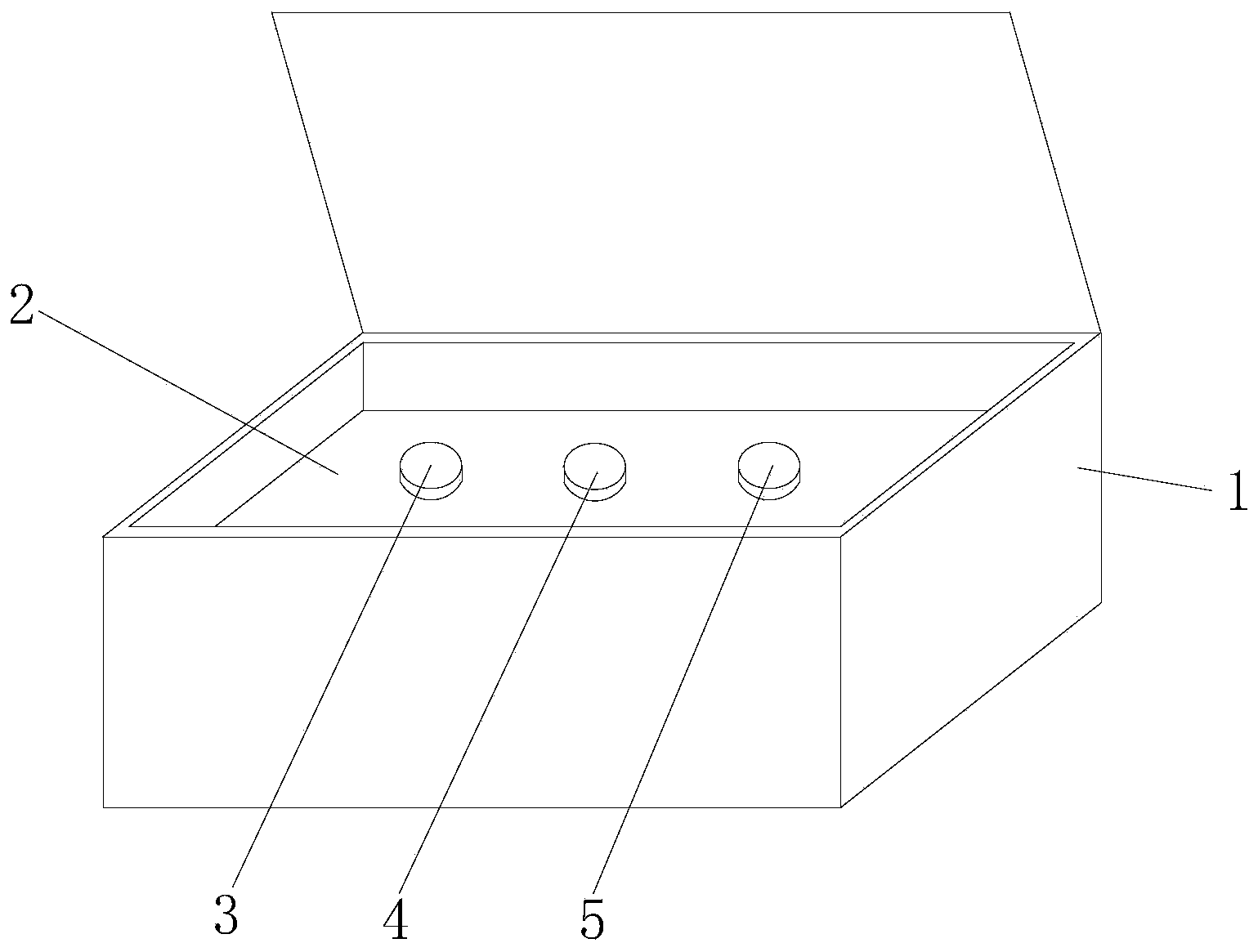
A hsa-miR-188-5p detection kit based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and a detection method of the hsa-miR-188-5p detection kit relate to MicroRNA. The detection kit is provided with a kit body, a clapboard, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle, and a real-time fluorescent quantitative PCR reagent bottle. The miRNA in a sample is extracted, and if the sample is a serum / plasma sample or other liquid samples, then 5 mu L exogenous reference cel-miR-39 with the concentration of 5nmol, provided by the reagent bottle, is added after the sample is fully split, and the mixture is oscillated in vortex; but if the sample is a cell or tissue sample, then exogenous reference cel-miR-39 is not added; a stem-loop reverse transcription reagent provided by the reagent bottle is used to reversely transcribe miRNA into cDNA; a real-time fluorescent quantitative PCR reagent provided by the reagent bottle is used to conduct real-time PCR amplification; various data provided by an analytical instrument are integrated together, and reasonable thresholds and reference lines are set for result analysis.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Antibody chip kit for early screening and diagnosis of tumors
The invention discloses an antibody chip kit for early screening and diagnosis of tumors. The detection is realized based on an enzyme lined immunosorbent assay (ELISA); the antibody chip kit comprises an antibody chip and an antigen standard substance mixture of multiple tumor markers, wherein the antibody chip comprises a base membrane and capture antibodies, fixed on the base membrane, of the multiple tumor markers; the base membrane is a polydimethylsiloxane (PDMS) membrane having a surface containing hydrophilic groups. On the premise of maintaining the characteristics of high sensitivity, large flux, much information and the like, the base membrane adopted by the invention is good in stability, strong in biological compatibility, good in flexibility, easy to clean and convenient to use; chip substrates of the capture antibodies of the tumor markers are fixed.
Owner:上海铭源数康生物芯片有限公司
Rs 3909184 detection genotyping kit based on AllGlo probe and genotyping method thereof
InactiveCN105483279AIncrease the Tm valueImprove signal-to-noise ratioMicrobiological testing/measurementPositive controlFluorescence
The invention relates to single nucleotide polymorphism, in particular to an rs 3909184 detection genotyping kit based on an AllGlo probe and a genotyping method thereof. The kit comprises a real-time fluorescence quantification PCR (polymerase chain reaction) reagent, positive control and negative control, and rs 3909184 is an SNP (single nucleotide polymorphism) locus serial number provided by NCBI(National Center of Biotechnology Information). The detection genotyping method includes the steps that DNA in an EDTA anticoagulant whole blood sample is extracted by adopting a conventional method; the real-time fluorescence quantification PCR reagent provided by the rs 3909184 detection genotyping kit based on the AllGlo probe is used for conducting real-time fluorescence quantification PCR amplification on DNA; the rs 3909184 SNP locus is genotyped according to detected fluorescence signals. On the premise of keeping high specificity and sensibility of the AllGlo probe, the detection price is lower than that of a direct sequencing method, the process is simpler, and consumed time is shorter.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Modified fluorescent probe and application thereof
ActiveCN111961667AImprove target site recognition specificityImprove bindingMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesNucleotide
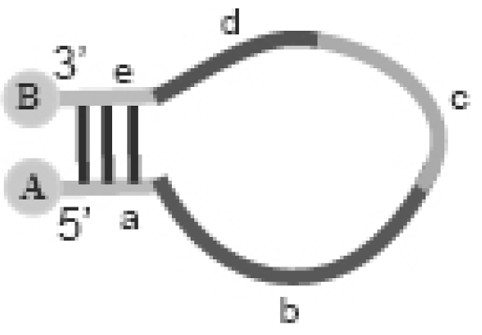
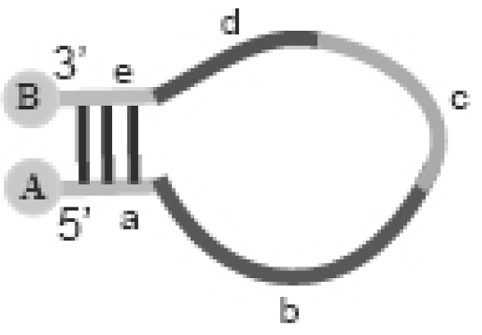
The invention provides an oligonucleotide probe. The oligonucleotide comprises an oligonucleotide molecule and a marker binding to the oligonucleotide molecule, wherein the oligonucleotide molecule comprises a self-complementary region which can form a hairpin structure, a target nucleic acid recognition region and a target nucleic acid recognition analogue region; and the marker provides a detectable signal when the probe is in a non-hybridized form, but weakens or substantially does not provide a detectable signal when the probe hybridizes with a complementary nucleic acid, or the marker provides a detectable signal when the probe hybridizes with a complementary nucleic acid, but weakens or substantially does not provides a detectable signal when the probe is in a non-hybridized form.
Owner:SINGLERA HEALTH TECH SHANGHAI LTD
Hsa-miR-629-5p detection kit based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and detection method thereof
ActiveCN103757124AAccurate detectionImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsPlasma samplesTissue sample
A hsa-miR-629-5pdetection kit based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and a detection method of the hsa-miR-629-5pdetection kit relate to MicroRNA. The detection kit is provided with a kit body, a clapboard, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle, and a real-time fluorescent quantitative PCR reagent bottle. The miRNA in a sample is extracted, and if the sample is a serum / plasma sample or other liquid samples, then 5 mu L exogenous reference cel-miR-39 with the concentration of 5nmol, provided by the reagent bottle, is added after the sample is fully split, and the mixture is oscillated in vortex; but if the sample is a cell or tissue sample, then exogenous reference cel-miR-39 is not added; a stem-loop reverse transcription reagent provided by the reagent bottle is used to reversely transcribemiRNA into cDNA; a real-time fluorescent quantitative PCR reagent provided by the reagent bottle is used to conduct real-time PCR amplification; various data provided by an analytical instrument are integrated together, and reasonable threshold and reference line are set for result analysis.
Owner:ANHUI IPROCOM BIOTECH CO LTD
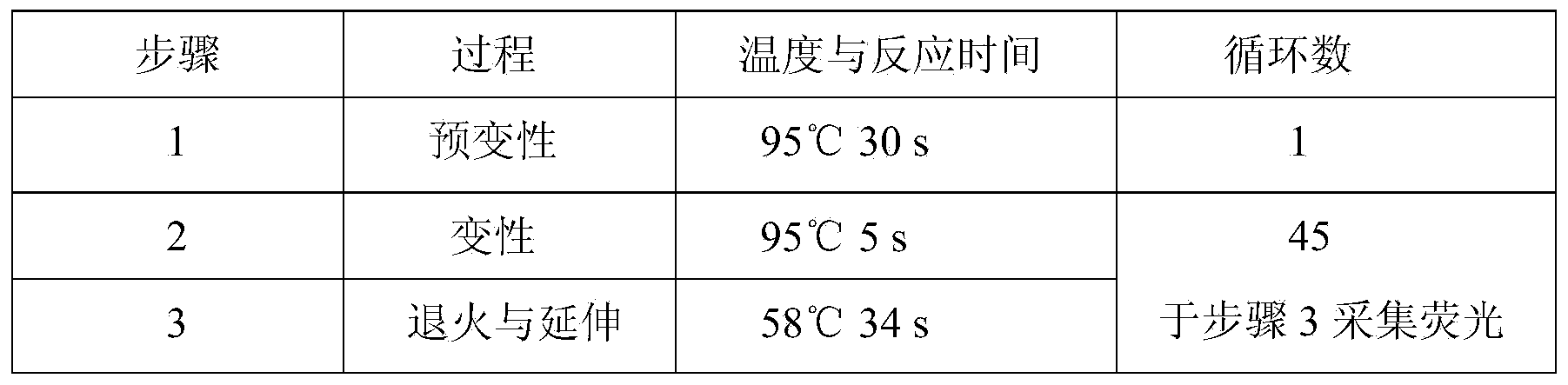
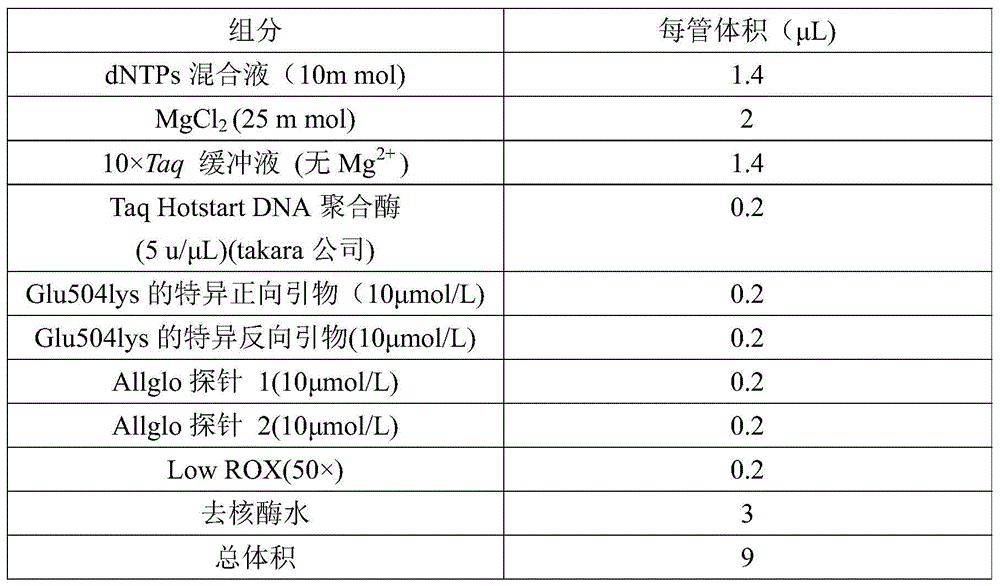
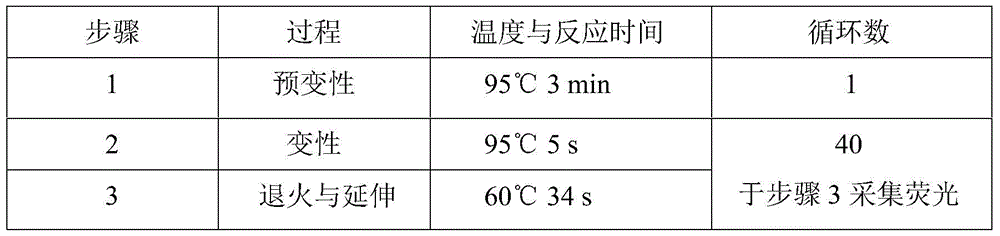
Glu504lys detection genotyping kit based on AllGlo probe and genotyping method thereof
InactiveCN105969842AAccurate typingImprove featuresMicrobiological testing/measurementPositive controlAnti freezing
The invention relates to single nucleotide polymorphism, in particular to a Glu504lys detection genotyping kit based on an AllGlo probe and a genotyping method thereof. The kit comprises a real-time fluorescence quantification PCR (polymerase chain reaction) reagent, positive control and negative control, Glu504lys is an SNP locus in an ALDH2 gene of human chromosome 12 provided by NCBI. The genotyping method comprises the steps that firstly, DNA in an EDTA anti-freezing whole blood sample is extracted through a conventional method; secondly, the real-time fluorescence quantification PCR reagent provided by the Glu504lys detection genotyping kit based on the probe AllGlo is used for conducting real-time fluorescence quantification PCR amplification on the DNA; and thirdly, genotyping is conducted on the Glu504lys locus of the human gene ALDH2 according to detected fluorescence signals. On the premise of keeping high specificity and sensitivity of the probe AllGlo, the detecting price is lower than that of a direct sequencing method, the process is simpler, and consumed time is shorter.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Mir-19a detection kit based on allglo probe fluorescence quantitative PCR and detection method thereof
InactiveCN105671038AIncrease the Tm valueHigh hybridization specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceBiology
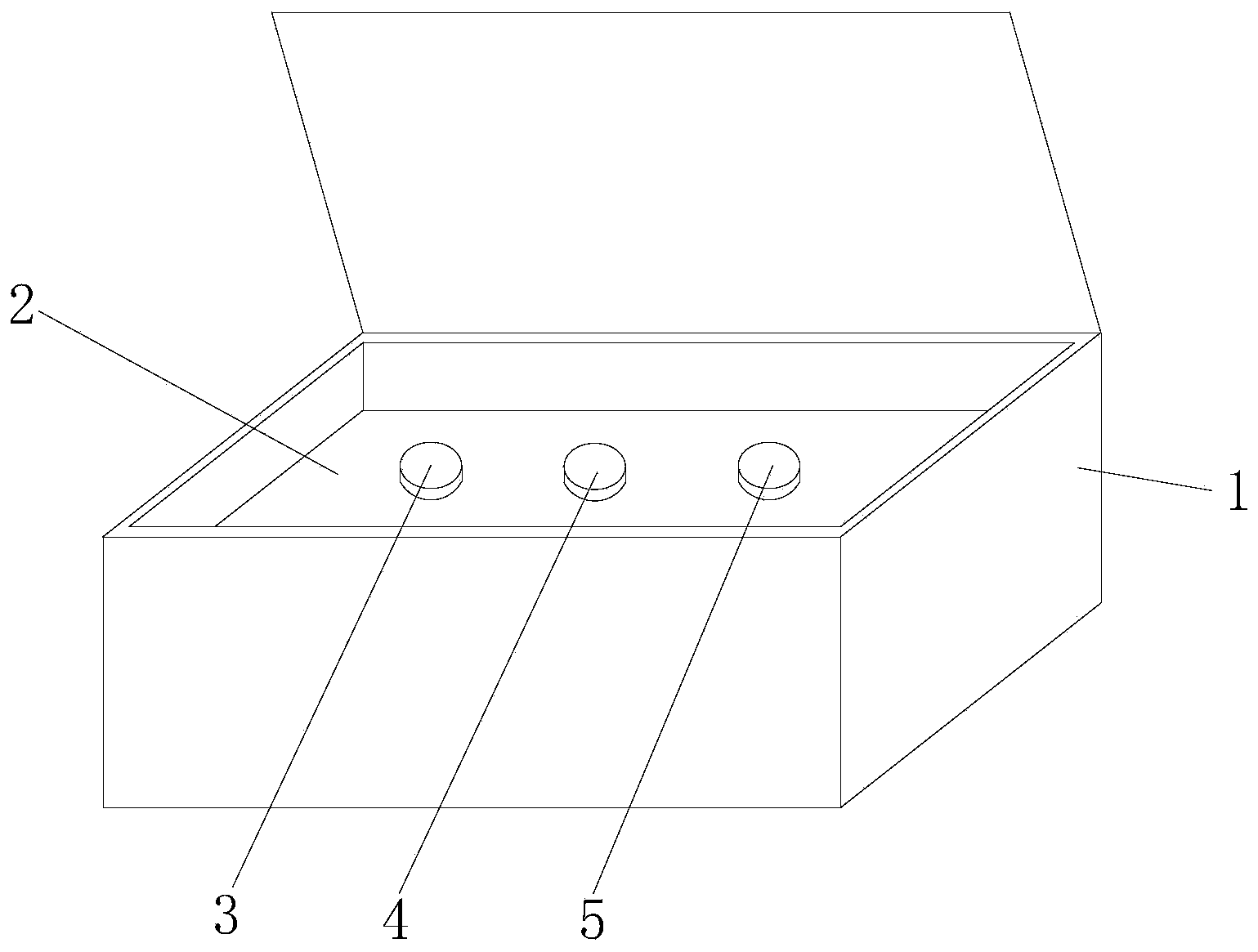
miR-19a detection kit and detection method based on AllGlo probe fluorescent quantitative PCR. The detection kit is equipped with a box body, a partition, an exogenous reference bottle, a neck ring reverse transcription reagent bottle, and a real-time fluorescence quantitative PCR reagent bottle; the partition is set in the box body, and the exogenous reference bottle, neck ring reverse transcription reagent bottle , The real-time fluorescent quantitative PCR reagent bottle is inserted on the partition, the exogenous reference bottle is equipped with an exogenous reference, the neck ring reverse transcription reagent bottle is equipped with a neck ring reverse transcription reagent, and the real-time fluorescent quantitative PCR reagent bottle is equipped with a real-time fluorescent quantitative PCR reagents. Detection method: extract miRNA in the sample; use the stem-loop reverse transcription reagent provided by the detection kit to reverse miRNA into cDNA; use the real-time fluorescent quantitative PCR reagent provided by the detection kit to perform real-time fluorescent quantitative PCR amplification of cDNA; comprehensive analysis instrument Given the various data, set the threshold and baseline, and analyze the results.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Magnetic bead sealer for magnetic particle chemiluminescence diagnosis system
InactiveCN108254548AGuaranteed long-term stabilityNo change in high signal strengthChemiluminescene/bioluminescenceCross-linkHigh signal intensity
The invention discloses a magnetic bead sealer for a magnetic particle chemiluminescence diagnosis system. The magnetic bead sealer comprises an aqueous solution of BSA, Gly, horse serum and triethanolamine. The final concentration of the BSA in the aqueous solution is 5-15 mg / mL, the final concentration of the Gly in the aqueous solution is 10-20 mg / mL, the final concentration of the horse serumin the aqueous solution is 100-300 ul / mL, and the final concentration of the triethanolamine in the aqueous solution is 50-150 ul / mL. The magnetic bead sealer of the magnetic particle chemiluminescence diagnostic system combines commonly used blocking agents for the first time, and has an unexpected technical effect. On the basis of ensuring already cross-linked proteins, the magnetic bead sealercan seal activated groups and non-specific adsorption sursurfaces on magnetic beads, not only does not change the high signal intensity of reaction in the prior art, but also ensures long-term continuous stability of a magnetic particle reagent, meanwhile has a lower background signal value, and can greatly improve test sensitivity and linear range.
Owner:JIANGSU ZECEN BIOTECH CO LTD
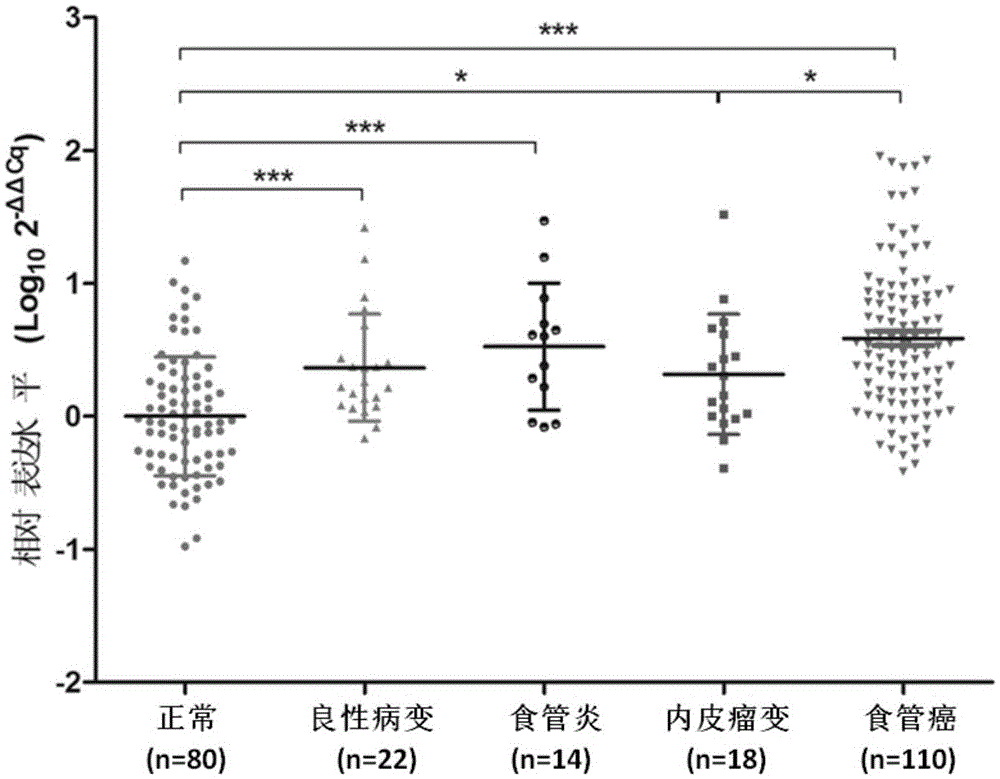
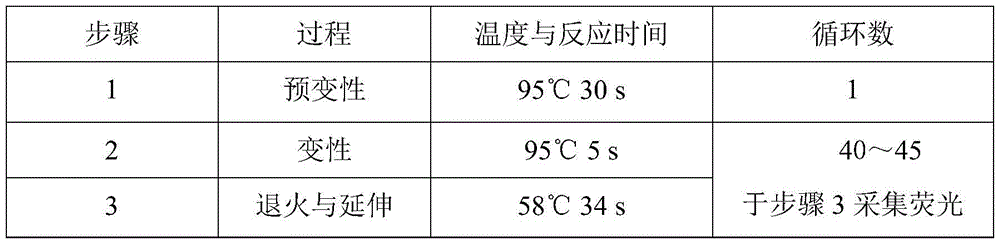
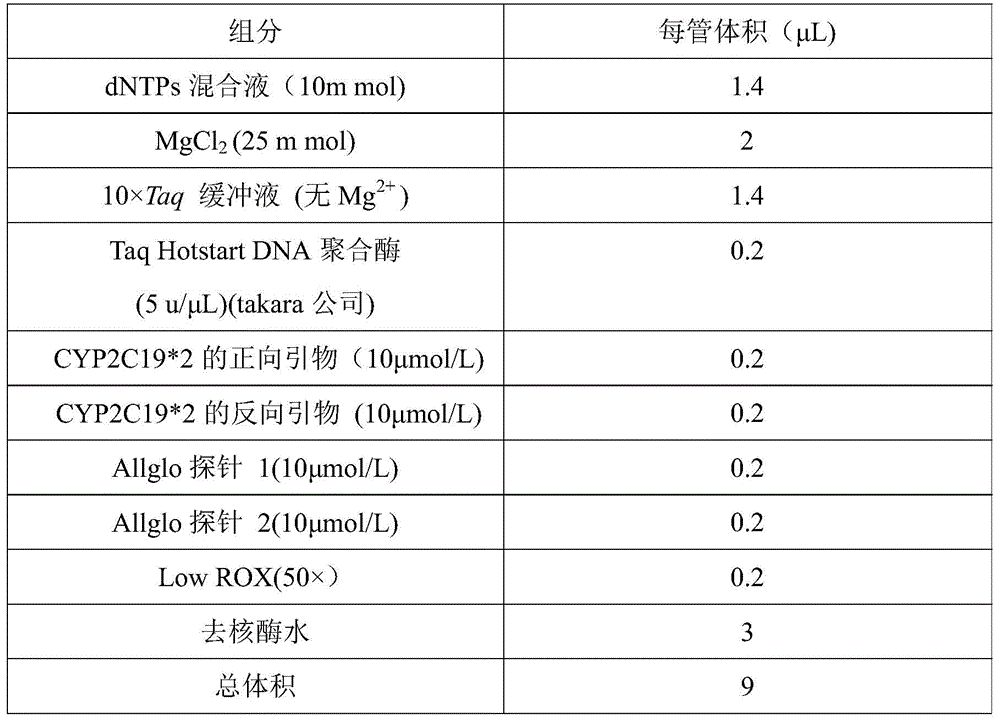
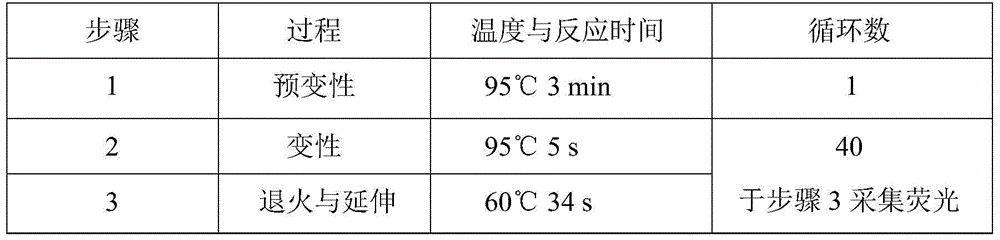
CYP2C19*2 detection parting kit based on probe AllGlo and parting method of CYP2C19*2 detection parting kit
InactiveCN105671151AAccurate typingImprove featuresMicrobiological testing/measurementPositive controlAnti freezing
The invention discloses a CYP2C19*2 detection parting kit based on a probe AllGlo and a parting method of the CYP2C19*2 detection parting kit and relates to single nucleotide polymorphism (SNP). The kit comprises a real-time fluorescence quantification PCR reagent, positive control and negative control. CYP2C19*2 is an SNP locus in a CYP2C19 gene of human chromosome 10 provided by NCBI. The detection parting method comprises the steps that firstly, DNA in an EDTA anti-freezing whole blood sample is extracted through a conventional method; secondly, the real-time fluorescence quantification PCR reagent provided by the detection parting kit is used for conducting real-time fluorescence quantification PCR amplification on the DNA; thirdly, parting is conducted on the SNP CYP2C19*2 of the human gene CYP2C19 according to detected fluorescence signals. On the premise of keeping high specificity and sensitivity of the probe AllGlo, the detecting price is lower than that of a direct sequencing method, the process is simpler, and consumed time is shorter.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Hsa-miR-137 (Human Serum Albumin-Micro Ribonucleic Acid-137) detection kit and hsa-miR-137 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction)
InactiveCN103740852AReduce sensitivityImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsLysisPlasma samples
The invention discloses an hsa-miR-137 (Human Serum Albumin-Micro Ribonucleic Acid-137) detection kit and an hsa-miR-137 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
A kind of modified fluorescent probe and its application
ActiveCN111961667BImprove target site recognition specificityImprove bindingMicrobiological testing/measurementDNA/RNA fragmentationNucleotideGenomic clone
The present invention provides an oligonucleotide probe, which comprises an oligonucleotide molecule and a label bound to the oligonucleotide molecule, wherein the oligonucleotide molecule comprises: a hairpin capable of forming a hairpin structure A self-complementary region, a target nucleic acid recognition region, and a target nucleic acid recognition-like region, the label (a) providing a detectable signal when the probe is in a non-hybridizing form but having a reduced or substantially detectable signal when the probe hybridizes to a complementary nucleic acid Provides no detectable signal, or (b) provides a detectable signal when the probe hybridizes to a complementary nucleic acid, but provides a reduced or substantially no detectable signal when the probe is in non-hybridized form.
Owner:SINGLERA HEALTH TECH SHANGHAI LTD
Hsa-mir-191-5p detection kit and detection method based on allglo probe fluorescent quantitative PCR
InactiveCN103740851BAccurate detectionImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsPlasma samplesLysis
The invention discloses an hsa-miR-191-5p (Human Serum Albumin-Micro Ribonucleic Acid-191-5p) detection kit and an hsa-miR-191-5p detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
hsa-miR-708 detection kit based on AllGlo probe fluorescence quantitative PCR (polymerase chain reaction) and detection method thereof
InactiveCN103773879AImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceBlood plasma
The invention relates to microRNA, particularly an hsa-miR-708 detection kit based on AllGlo probe fluorescence quantitative PCR (polymerase chain reaction) and a detection method thereof. The kit is provided with a box body, partitions, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescence quantitative PCR reagent bottle. The method comprises the following steps: extracting miRNA (microribonucleic acid) in a sample, wherein if the sample is serum / plasma or any other body fluid, after the sample is sufficiently cracked, 5 mu L of 5n mol exogenous reference cel-miR-39 provided by the kit is added and vortex oscillation is performed, and if the sample is a cell or tissue sample, no exogenous reference cel-miR-39 is needed; carrying out real-time PCR amplification on the stem-loop reverse transcription reagent reverse transcription miRNA provided by the kit as cDNA (omplementary deoxyribonucleic acid) by using the real-time fluorescence quantitative PCR reagent provided by the kit; and setting reasonable thresholds and base lines according to data given by a comprehensive analysis instrument, and carrying out result analysis.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-mir-9 detection kit and detection method based on allglo probe fluorescent quantitative PCR
InactiveCN103740850BImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsComplementary deoxyribonucleic acidBlood plasma
The invention discloses an hsa-miR-9 (Human Serum Albumin-Micro Ribonucleic Acid-9) detection kit and an hsa-miR-9 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-miR-132 (Human Serum Albumin-Micro Ribonucleic Acid-132) detection kit and hsa-miR-132 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction)
InactiveCN103740844AImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igePlasma samples
The invention discloses an hsa-miR-132 (Human Serum Albumin-Micro Ribonucleic Acid-132) detection kit and an hsa-miR-132 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
hsa-mir-363 detection kit and detection method based on allglo probe fluorescence quantitative PCR
InactiveCN103773874BImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceTissue sample
The invention relates to microRNA, particularly an hsa-miR-363 detection kit based on AllGlo probe fluorescence quantitative PCR (polymerase chain reaction) and a detection method thereof. The kit is provided with a box body, partitions, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescence quantitative PCR reagent bottle. The method comprises the following steps: extracting miRNA (microribonucleic acid) in a sample, wherein if the sample is serum / plasma or any other body fluid, after the sample is sufficiently cracked, 5 mu L of 5n mol exogenous reference cel-miR-39 provided by the kit is added and vortex oscillation is performed, and if the sample is a cell or tissue sample, no exogenous reference cel-miR-39 is needed; carrying out real-time PCR amplification on the stem-loop reverse transcription reagent reverse transcription miRNA provided by the kit as cDNA (omplementary deoxyribonucleic acid) by using the real-time fluorescence quantitative PCR reagent provided by the kit; and setting reasonable thresholds and base lines according to data given by a comprehensive analysis instrument, and carrying out result analysis.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
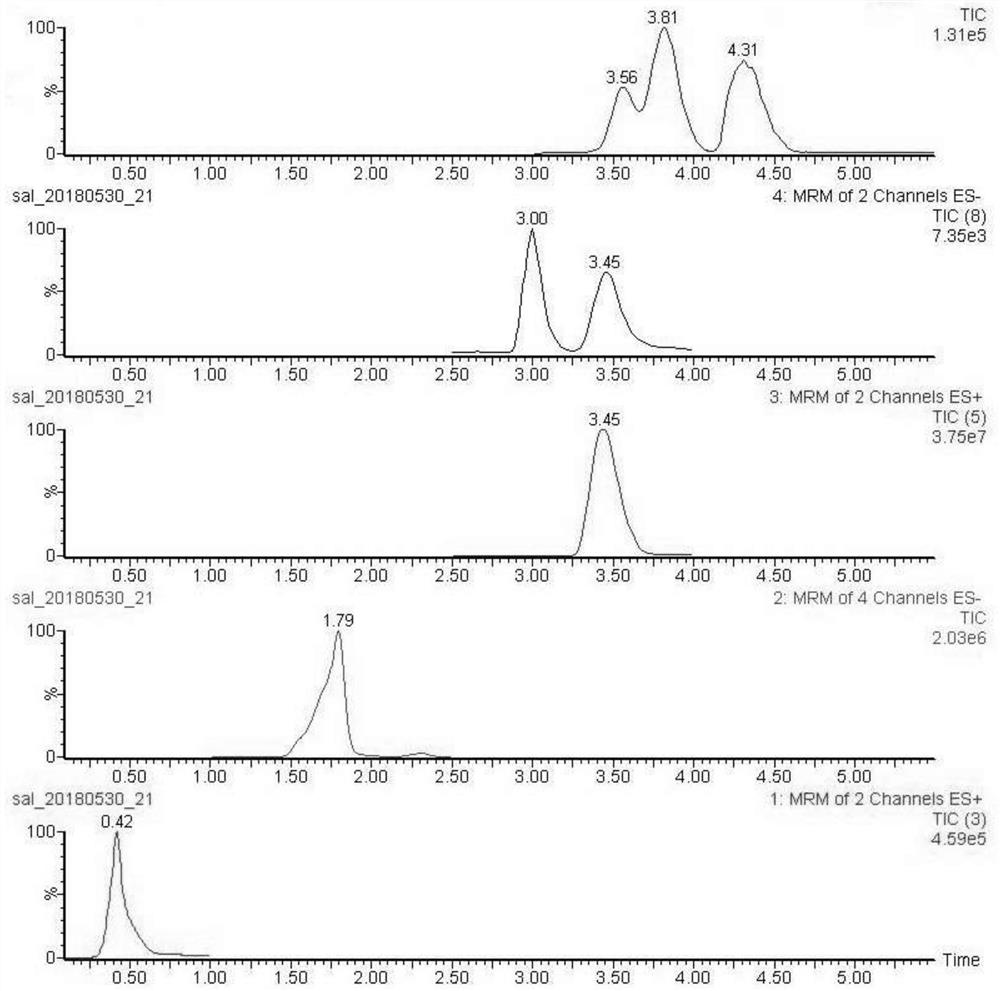
Liquid-mass spectrometry detection method of salicylate anti-ultraviolet finishing agents for textiles
ActiveCN109212105BQualitatively accurateSpirit length is highComponent separationSalicylic acid esterTest sample
The invention discloses a liquid mass spectrometry detection method for textile salicylate anti-ultraviolet finishing agents, which comprises the following steps: preparing a sample to be tested into a sample solution to be tested; The gradient standard working solution is prepared into a gradient standard working solution; the gradient standard working solution is injected into the liquid chromatography-tandem mass spectrometer, the positive and negative ion multiple reaction monitoring mode is measured, and the standard curve equation is made; the sample solution to be tested is detected according to the above method, so as to obtain the Contents of 7 kinds of salicylate anti-ultraviolet finishing agents in samples. The liquid chromatography-tandem mass spectrometry method is qualitative, quantitative, and highly sensitive, and is suitable for the detection of salicylate UV-resistant finishing agents in textiles.
Owner:吴刚
Hsa-mir-520a-3p detection kit and detection method based on allglo probe fluorescent quantitative PCR
InactiveCN103773876BAccurate detectionImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceTissue sample
The invention relates to microRNA, particularly an hsa-miR-520a-3p detection kit based on AllGlo probe fluorescence quantitative PCR (polymerase chain reaction) and a detection method thereof. The kit is provided with a box body, partitions, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescence quantitative PCR reagent bottle. The method comprises the following steps: extracting miRNA (microribonucleic acid) in a sample, wherein if the sample is serum / plasma or any other body fluid, after the sample is sufficiently cracked, 5 mu L of 5n mol exogenous reference cel-miR-39 provided by the kit is added and vortex oscillation is performed, and if the sample is a cell or tissue sample, no exogenous reference cel-miR-39 is needed; carrying out real-time PCR amplification on the stem-loop reverse transcription reagent reverse transcription miRNA provided by the kit as cDNA (omplementary deoxyribonucleic acid) by using the real-time fluorescence quantitative PCR reagent provided by the kit; and setting reasonable thresholds and base lines according to data given by a comprehensive analysis instrument, and carrying out result analysis.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-miR-26a-2 detection kit based on fluorescent quantitative PCR of AllGlo probe and detection method thereof
InactiveCN103773873AImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sampleMicroRNA
The invention provides an hsa-miR-26a-2 detection kit based on fluorescent quantitative PCR (Polymerase Chain Reaction) of an AllGlo probe and a detection method thereof, and relates to a MicroRNA (Micro Ribose Nucleic Acid). The kit comprises a box body, a separator, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time quantitative PCR reagent bottle; the miRNA in a sample is extracted; if the sample is serum / plasma or one of other body fluid samples, 5 microliters of exogenous reference cel-miR-39 having the concentration of 5nmol provided by the kit is added after the sample is sufficiently crack, and then shaken in vortexes; if the sample is a cell or tissue sample, the exogenous reference cel-miR-39 does not need to be added; a stem-loop reverse transcription reagent provided by the kit is used for reversely transcribing the miRNA or a cDNA (complementary Desoxvribose Nucleic Acid); a real-time quantitative PCR reagent provided by the kit is used for real-time PCR amplification on the cDNA; various pieces of data provided by an instrument are analyzed comprehensively, rational thresholds and base lines are set rationally, and the results are analyzed.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-mir-146 detection kit and detection method based on allglo probe fluorescent quantitative PCR
InactiveCN103740848BImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsComplementary deoxyribonucleic acidBlood plasma
The invention discloses an hsa-miR-146 (Human Serum Albumin-Micro Ribonucleic Acid-146) detection kit and an hsa-miR-146 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-mir-629-5p detection kit and detection method based on allglo probe fluorescent quantitative PCR
ActiveCN103757124BAccurate detectionImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsPlasma samplesTissue sample
A hsa-miR-629-5pdetection kit based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and a detection method of the hsa-miR-629-5pdetection kit relate to MicroRNA. The detection kit is provided with a kit body, a clapboard, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle, and a real-time fluorescent quantitative PCR reagent bottle. The miRNA in a sample is extracted, and if the sample is a serum / plasma sample or other liquid samples, then 5 mu L exogenous reference cel-miR-39 with the concentration of 5nmol, provided by the reagent bottle, is added after the sample is fully split, and the mixture is oscillated in vortex; but if the sample is a cell or tissue sample, then exogenous reference cel-miR-39 is not added; a stem-loop reverse transcription reagent provided by the reagent bottle is used to reversely transcribemiRNA into cDNA; a real-time fluorescent quantitative PCR reagent provided by the reagent bottle is used to conduct real-time PCR amplification; various data provided by an analytical instrument are integrated together, and reasonable threshold and reference line are set for result analysis.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Simultaneous detection method for 9 kinds of n-nitrosamines in food contact rubber products
InactiveCN104076106BQualitatively accurateQuantitatively accurateComponent separationNitrosoN-nitrosamine
The invention discloses a method suitable for simultaneously detecting 9 N-nitrosamines in food contact rubber products. The method comprises the following steps: utilizing a sample to be tested to establish a migration system to obtain a migration extract; utilizing the migration extract to prepare a sample solution to be tested to obtain a supernatant / filtrate; preparing 9 N-nitrosamines into a standard solution to further prepare a standard working solution with the gradient of 0.01-0.40 [mu]g / mL; injecting the gradient standard solution and the supernatant / filtrate into a liquid Chromatogram-tandem mass spectrograph, and testing the cation multi-reaction monitoring model to finally obtain the respective contents of 9 N-nitrosamines in the sample to be tested. The 9 N-nitrosamines are N-Nitrosodimethylamine, N-Nitroso-ethyl methylamine, N-Nitrosomorpholine, N-Nitrosopiperidine, N-Nitrosopyrrolidine, N-Nitroso-diethylamine, N-Nitrosodibutylamine, N-Nitrosodi-n-propylamine and N-Nitroso-diphenylamine.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
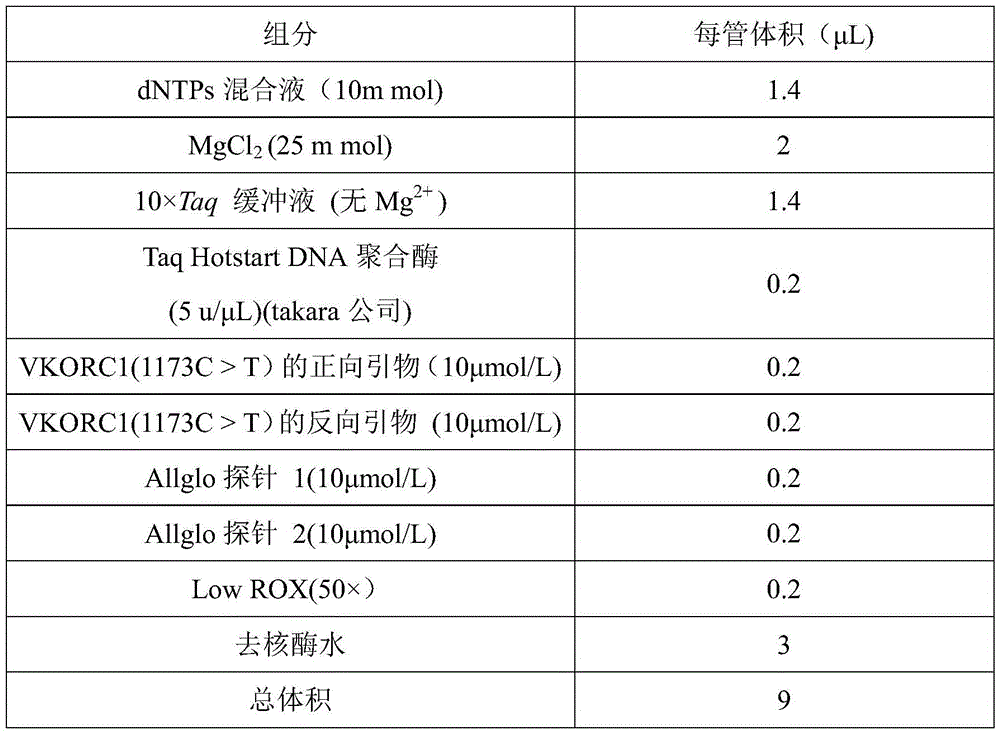
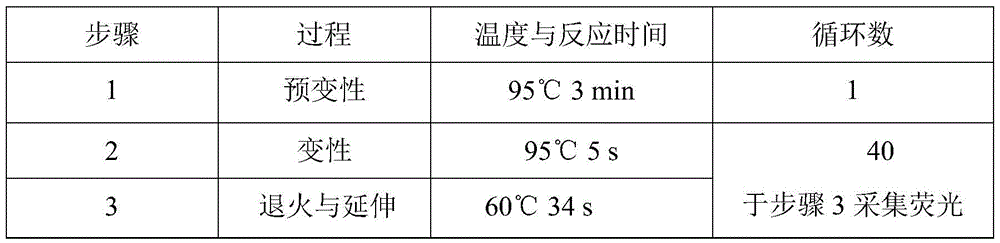
VKORC1 gene polymorphism detection genotyping kit based on AllGlo probe and genotyping method thereof
InactiveCN105483280ALow costAccurate typingMicrobiological testing/measurementVKORC1Positive control
The invention relates to single nucleotide polymorphism, in particular to a VKORC1 gene polymorphism detection genotyping kit based on an AllGlo probe and a genotyping method thereof. The kit comprises a real-time fluorescence quantification PCR (polymerase chain reaction) reagent, positive control and negative control, and VKORC1(1173C>T) is the SNP (single nucleotide polymorphism) locus, provided by NCBI(National Center of Biotechnology Information), of human chromosome 16 in the VKORC1 gene. The genotyping method includes the steps that DNA in an EDTA anticoagulant whole blood sample is extracted by adopting a conventional method; the real-time fluorescence quantification PCR reagent provided by the VKORC1 gene polymorphism detection genotyping kit based on the AllGlo probe is used for conducting real-time fluorescence quantification PCR amplification on DNA; the SNP locus VKORC1(1173C>T) of the human VKORC1 gene is genotyped according to detected fluorescence signals.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Hsa-mir-513b detection kit and detection method based on allglo probe fluorescence quantitative PCR
InactiveCN103740845BAccurate detectionImprove featuresBioreactor/fermenter combinationsBiological substance pretreatmentsComplementary deoxyribonucleic acidBlood plasma
The invention discloses an hsa-miR-513b (Human Serum Albumin-Micro Ribonucleic Acid-513b) detection kit and an hsa-miR-513b detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-mir-137 detection kit and detection method based on allglo probe fluorescent quantitative PCR
InactiveCN103740852BImprove featuresIncreased sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsComplementary deoxyribonucleic acidBlood plasma
The invention discloses an hsa-miR-137 (Human Serum Albumin-Micro Ribonucleic Acid-137) detection kit and an hsa-miR-137 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Hsa-miR-1321 (Human Serum Albumin-Micro Ribonucleic Acid-1321) detection kit and hsa-miR-1321 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction)
InactiveCN103740846AReduce sensitivityAccurate detectionBioreactor/fermenter combinationsBiological substance pretreatmentsLysisPlasma samples
The invention discloses an hsa-miR-1321 (Human Serum Albumin-Micro Ribonucleic Acid-1321) detection kit and an hsa-miR-1321 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction) and relates to MicroRNA (Micro Ribonucleic Acid). The kit is provided with a kit body, a partition, an exogenous reference bottle, a stem-loop reverse transcription reagent bottle and a real-time fluorescent quantitative PCR reagent bottle. The detection method comprises the steps of extracting miRNA from a sample, adding 5 microliters of exogenous reference cel-miR-39 (Caenorhabditis Elegans-Micro Ribonucleic Acid-39) at a concentration of 5n mol provided by the kit after full lysis of the sample, and swirling and shaking in case of a sample of serum / plasma sample or other body fluids, adding no exogenous reference cel-miR-39 in case of a sample of a cell or a tissue, performing reverse transcription on miRNA to form cDNA (Complementary Deoxyribonucleic Acid) with a stem-loop reverse transcription reagent provided by the kit, performing real-time PCR amplification on cDNA with a real-time fluorescent quantitative PCR reagent provided by the kit, and setting a reasonable threshold and a baseline for result analysis by synthesizing various data given by an analytical instrument.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com