Hsa-miR-1321 (Human Serum Albumin-Micro Ribonucleic Acid-1321) detection kit and hsa-miR-1321 detection method based on AllGlo probe fluorescent quantitative PCR (Polymerase Chain Reaction)
A technology of hsa-mir-1321 and cel-mir-39, which is applied in the field of MicroRNA, can solve the problems of expensive synthesis and unfavorable promotion, and achieve fast and accurate detection, low background signal, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
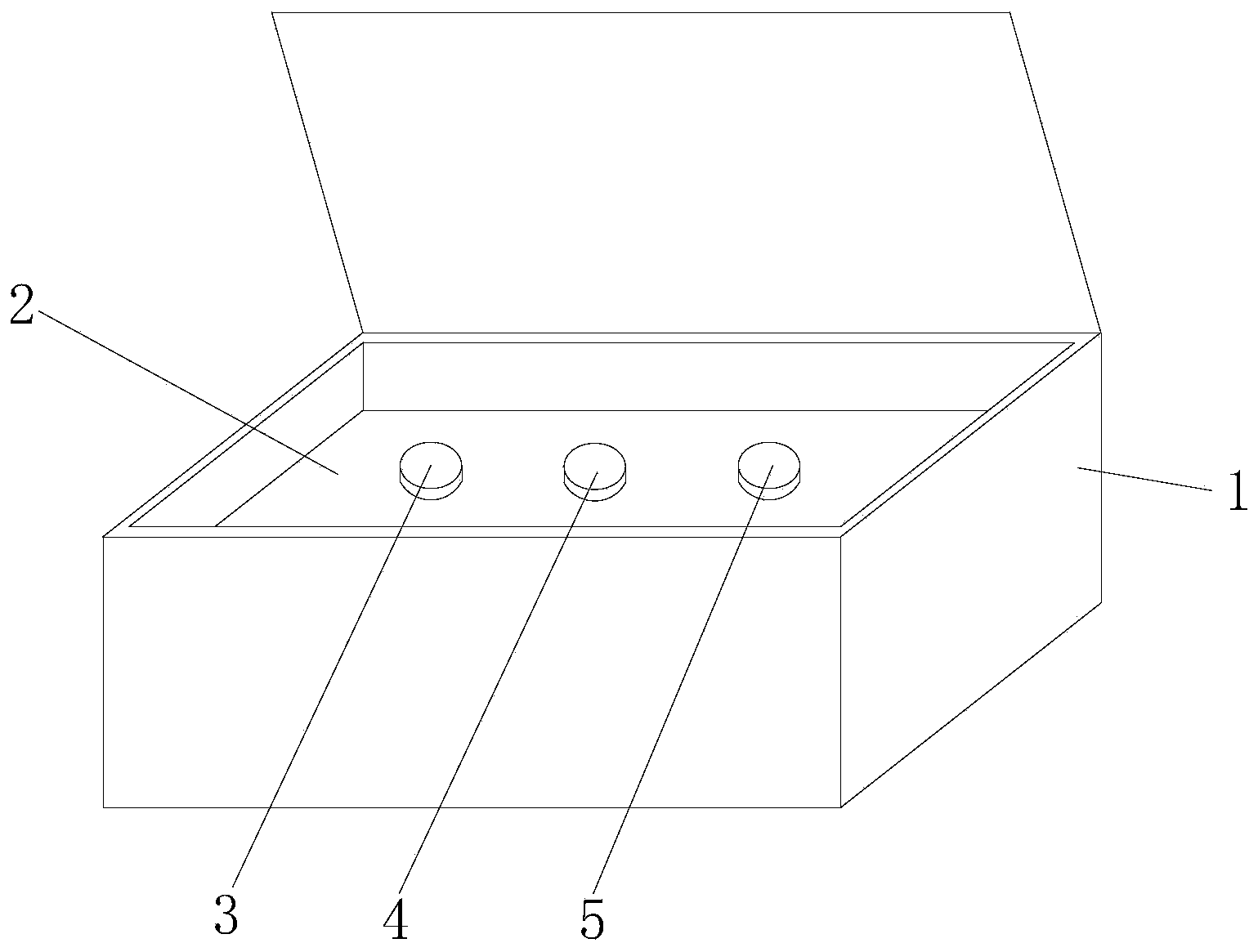
[0051] see figure 1 , the embodiment of the hsa-miR-1321 detection kit based on AllGlo probe fluorescent quantitative PCR of the present invention is provided with a box body 1, a partition 2, an exogenous reference bottle 3, a stem-loop reverse transcription reagent bottle 4, and a real-time Fluorescent quantitative PCR reagent bottle 5; partition 2 is set in the box body 1, exogenous reference bottle 3, stem-loop reverse transcription reagent bottle 4, real-time fluorescent quantitative PCR reagent bottle 5 are inserted on the partition 2, exogenous reference Bottle 3 is equipped with exogenous reference, stem-loop reverse transcription reagent bottle 4 is equipped with stem-loop reverse transcription reagent, and real-time fluorescent quantitative PCR reagent bottle 5 is equipped with real-time fluorescent quantitative PCR reagent.
[0052] ① The exogenous reference can be cel-miR-39, etc., the cel-miR-39 is a kind of nematode miRNA, its working concentration can be 5nmol,...
Embodiment 2
[0056] The detection of serum or plasma hsa-miR-1321 includes the following steps:
[0057] 1. Collect plasma or serum:
[0058] Take 2mL of fresh blood samples and put them in EDTA anticoagulant tubes or anticoagulant-free tubes. Immediately invert the above test tubes and mix them 6-8 times, then place the above test tubes in a centrifuge at 3000rr for 10min, take them out and place them in a test tube rack First, carefully draw the supernatant and put it into a new 1.5mL RNase-free centrifuge tube, place the 1.5mL centrifuge tube containing the supernatant in a centrifuge at 13000r for 10min, and transfer the upper plasma or serum to In a new 1.5mL RNase-free centrifuge tube (be careful not to absorb the cell pellet in the lower layer during this step), aliquot 400-500μL for each tube, take 200μL for the next step of extraction, and freeze the remaining plasma or serum at -80°C live.
[0059] 2. Extract microRNA
[0060] Use the miRNA extraction kit (DP501) produced by T...
Embodiment 3
[0081] Example 3. Tissue or cell miRNA detection
[0082] 1. Sample handling:
[0083] a. Tissue: Triturate the tissue in liquid nitrogen. For every 30-50 mg of animal tissue or 100 mg of plant tissue, add 1 mL of lysate MZ (manufactured by Tiangen Biotechnology Co., Ltd.), and use a homogenizer for homogenization. The sample volume should not exceed 10% of the MZ volume of the lysate.
[0084] b. Monolayer culture cells: directly add lysate MZ to the culture plate to lyse the cells, every 10cm 2 Add 1mL LMZ to the area. Swipe several times with a sampler.
[0085] c. Cell suspension: centrifuge for 800r5min to take the cells, and discard the supernatant. Add 1mL Lysis Buffer MZ, oscillate or pipette several times to mix. Do not wash the cells before adding Lysis Buffer MZ to avoid RNA degradation.
[0086] 2. Tissue, cell miRNA extraction and enrichment
[0087] Use the miRNA extraction kit (DP501) produced by Tiangen Biotechnology Co., Ltd. to extract tissue or cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com