Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "4-Chloroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Chloroaniline is an organochlorine compound with the formula ClC₆H₄NH₂. This pale yellow solid is one of the three isomers of chloroaniline.
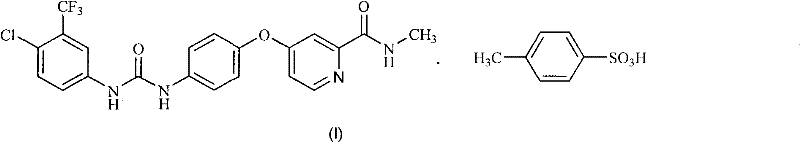
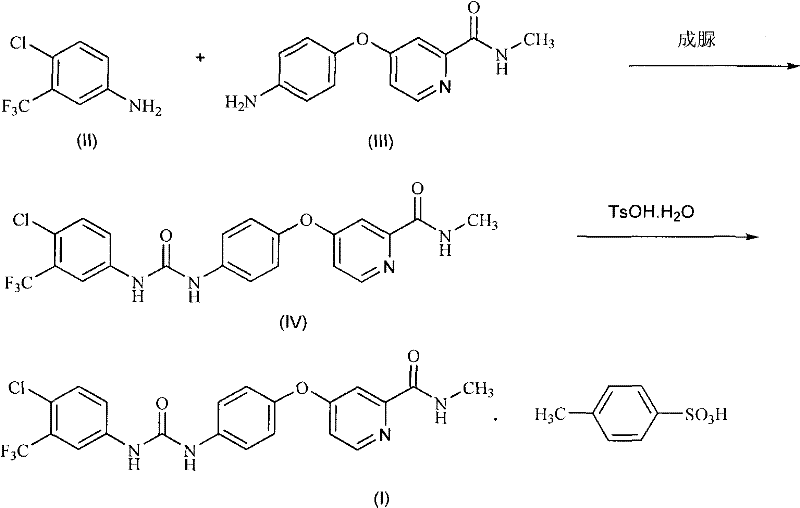
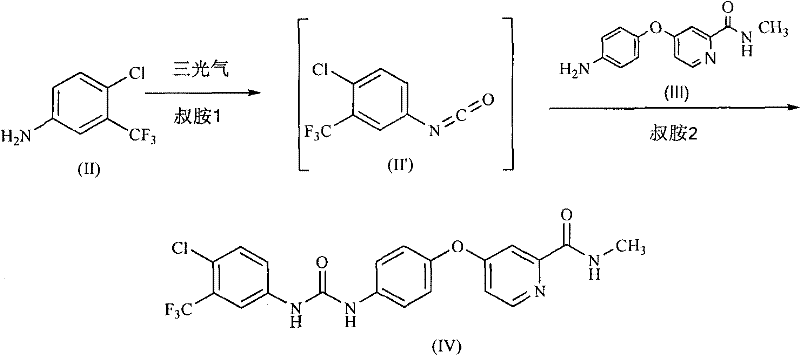
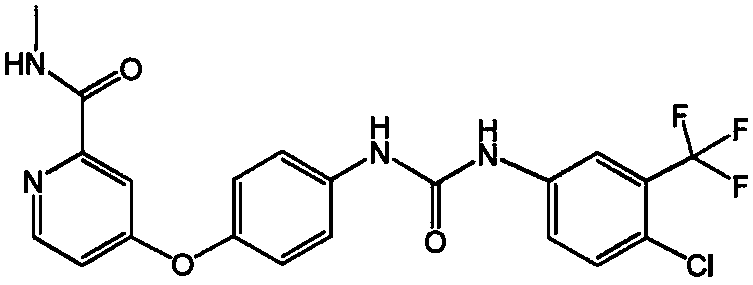
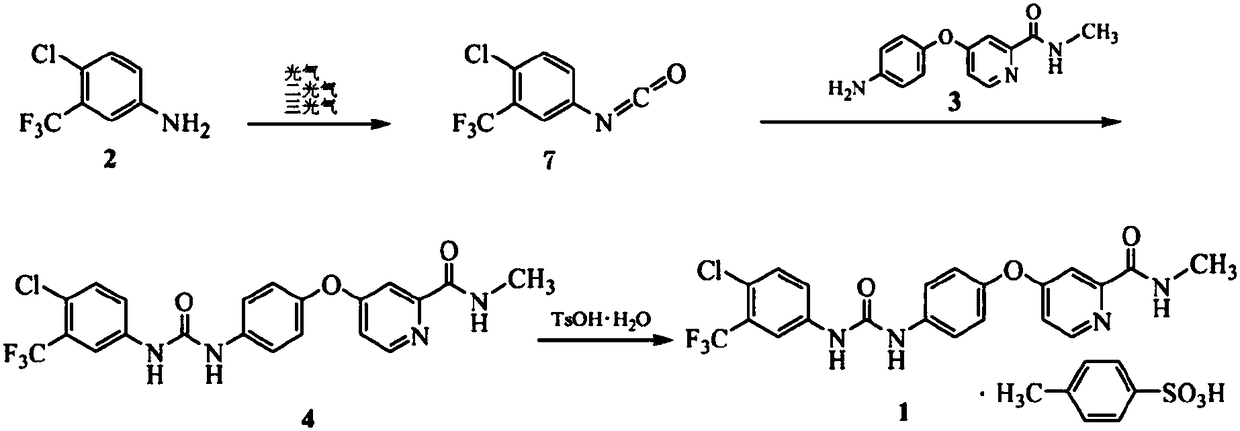
Method for preparing sorafenib
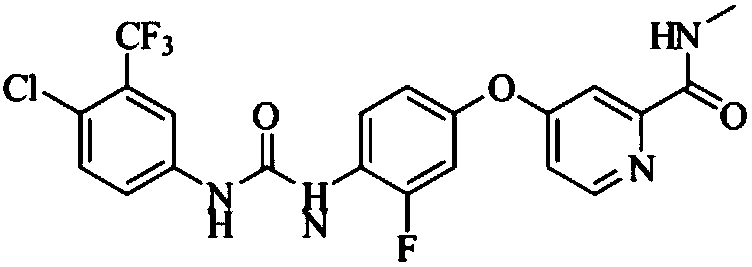
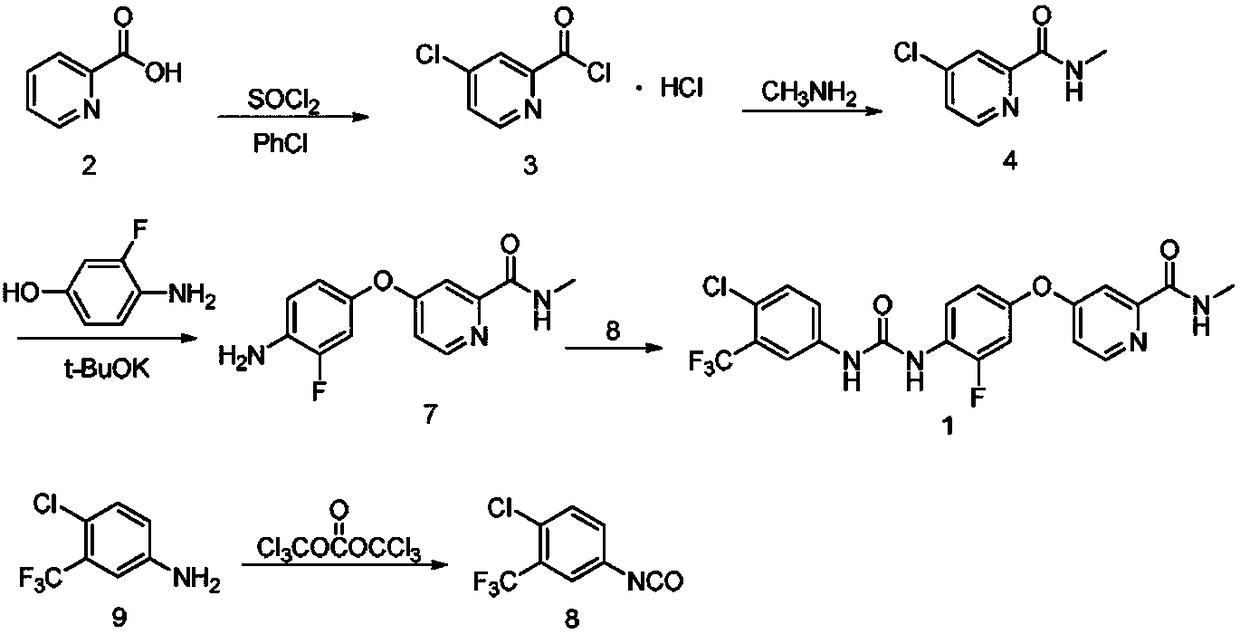
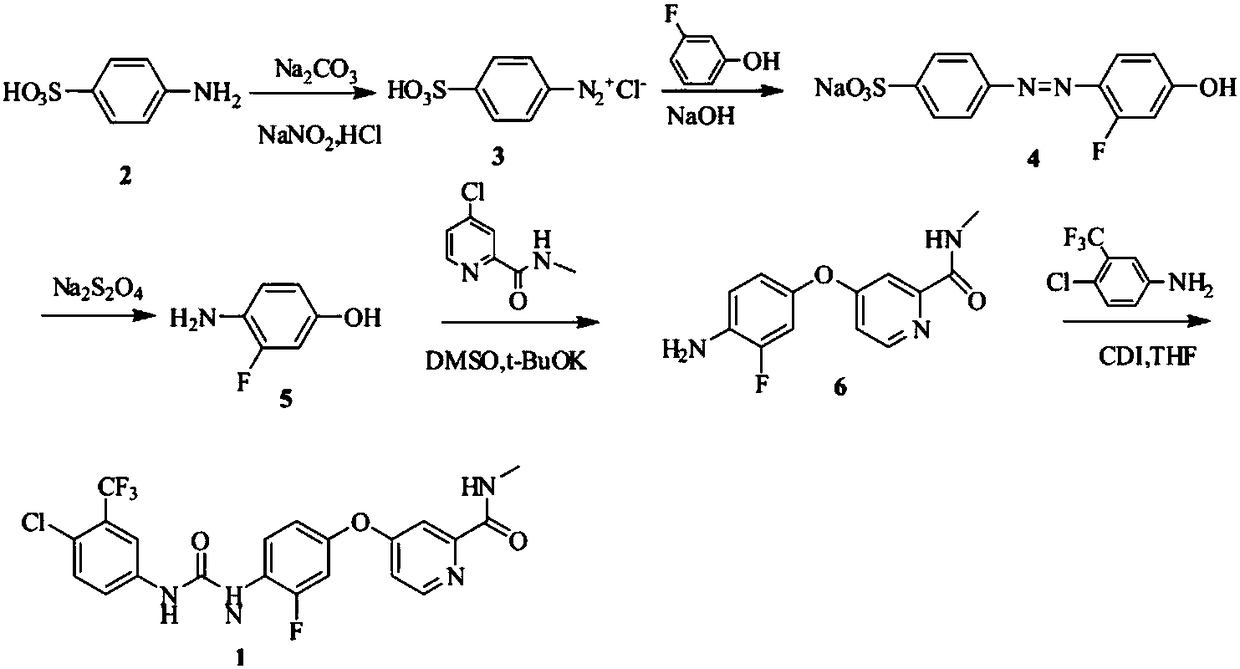
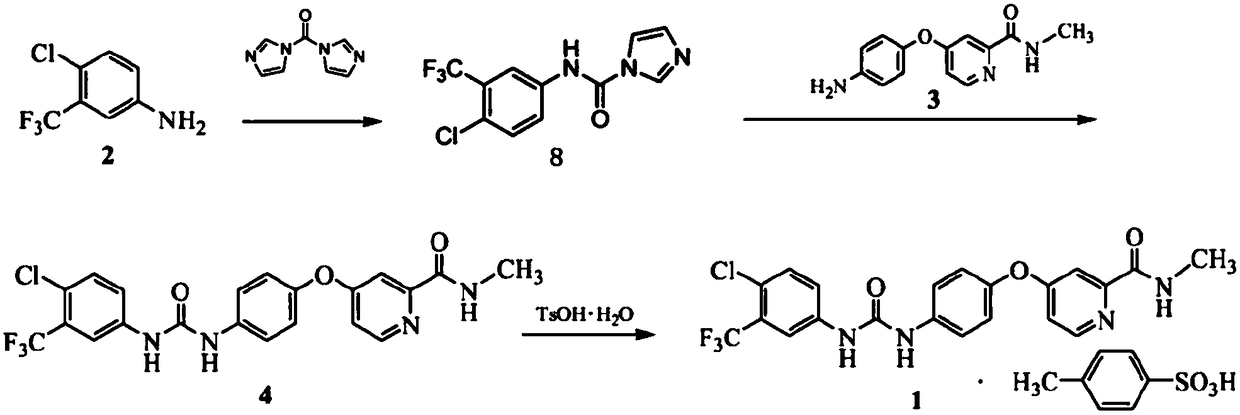
The invention discloses a method for preparing sorafenib, which comprises the steps of: 1) reacting triphosgene with 3-trimethyl fluoride-4-chloroaniline (II) in an inert solvent 1 under the existence of tertiary amine 1, thus obtaining a solution containing 3-trimethyl fluoride-4-chlorphenyl isocyanate (II'), wherein isolation and purification are not needed; and 2) reacting the 3-trimethyl fluoride-4-chlorphenyl isocyanate (II') with 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) in an inert solvent 2 under the existence of tertiary amine 2, thus obtaining sorafenib (IV). The tertiary amine 1 and the tertiary amine 2 are the same or different, the inert solvent 1 and the inert solvent 2 are the same or different, and the feeding sequence of the 3-trimethyl fluoride-4-chloroaniline (II) and the 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) can be interchangeable. The method is simple and convenient to operate, short for reaction time free from separation of intermediates with high reactivity, as well as is free from special equipment and conditions, and high in yield.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Specific antibody against herbicide anilofos
InactiveCN103073634ARetain chemical structureRetain molecular structureSerum albuminPeptide preparation methodsAcetic acidBovine serum albumin
The invention provides preparation of a semi-antigen and an artificial antigen and an antibody against herbicide anilofos, which relates to the semi-antigen, the artificial antigen and the antibody which have a basic structure of the herbicide anilofos. According to the invention, the antibody with good specificity to the herbicide anilofos is prepared by using an easy and convenient method. The antibody is prepared through the following steps: synthesizing the semi-antigen from N-chloracetyl-N-isopropyl-4-chloroaniline and mercaptoacetic acid; respectively connecting the semi-antigen with bovine serum albumin (BSA) and horseradish peroxidase (HRP) to synthesize the artificial antigen and an enzyme-labeled antigen; and subjecting the artificial antigen to animal immunization, blood drawing, separation of antiserum and purification so as to prepare the antibody. The antibody is stable and has good specificity and sensitivity; the synthetic method for the antibody is simple; and the antibody can be used for rapid immunodetection of residual herbicide anilofos in the environment, agricultural products and foodstuffs and has a good application prospect.
Owner:TIANJIN UNIV OF SCI & TECH
Preparation method for 4-chlorophenylhydrazine hydrochloride
InactiveCN103910650AEliminates the problem of easy agglomeration and difficult operation of feedingEasy dischargeHydrazine preparationSolubilityHydrogen Sulfate
A provided preparation method for 4-chlorophenylhydrazine hydrochloride comprises the following steps: 1) under an acidic condition, at 5-10 DEG C, dropwise adding a sodium nitrite aqueous solution with a concentration of 20% into 4-chloroaniline to perform a diazo reaction and to generate a diazo salt; 2) under a room temperature condition, dropwise adding an ammonium sulfite aqueous solution into a solution of the diazo salt obtained in the step 1, heating to 50-60 DEG C and keeping the temperature for 3-4 h; and 3) at 50-70 DEG C, dropwise adding a hydrochloric acid solution with a concentration of 20% into the solution obtained in the step 2, keeping the temperature for 1-2 h, reducing the temperature, filtering, washing, discharging the material, and drying to obtain a finished product. The beneficial effects comprise that by using ammonium sulfite aqueous solution as a reducing agent in the reduction reaction, the problems are solved that sodium sulfite solid is easy to cake during material charging in a conventional technology and material charging is not easy to operate; ammonium chloride and ammonium hydrogen sulfate which are generated in the acidification reaction have high solubility in water, and crystallization precipitation is late, thereby facilitating performing of the acidification reaction in the solution and reducing side reactions; and the product crystallizes loosely, is good in fluidity, easy for material discharging and washing, and product quality and yield are both better than conventional production levels.
Owner:TIANJIN QIDONG CHEM PLANT
Large steric hindrance ligand Pd complex catalyst as well as preparation method and application thereof
InactiveCN102659622AImprove stabilityHigh catalytic activityOrganic compound preparationGroup 8/9/10/18 element organic compoundsMethyl groupCoordination complex
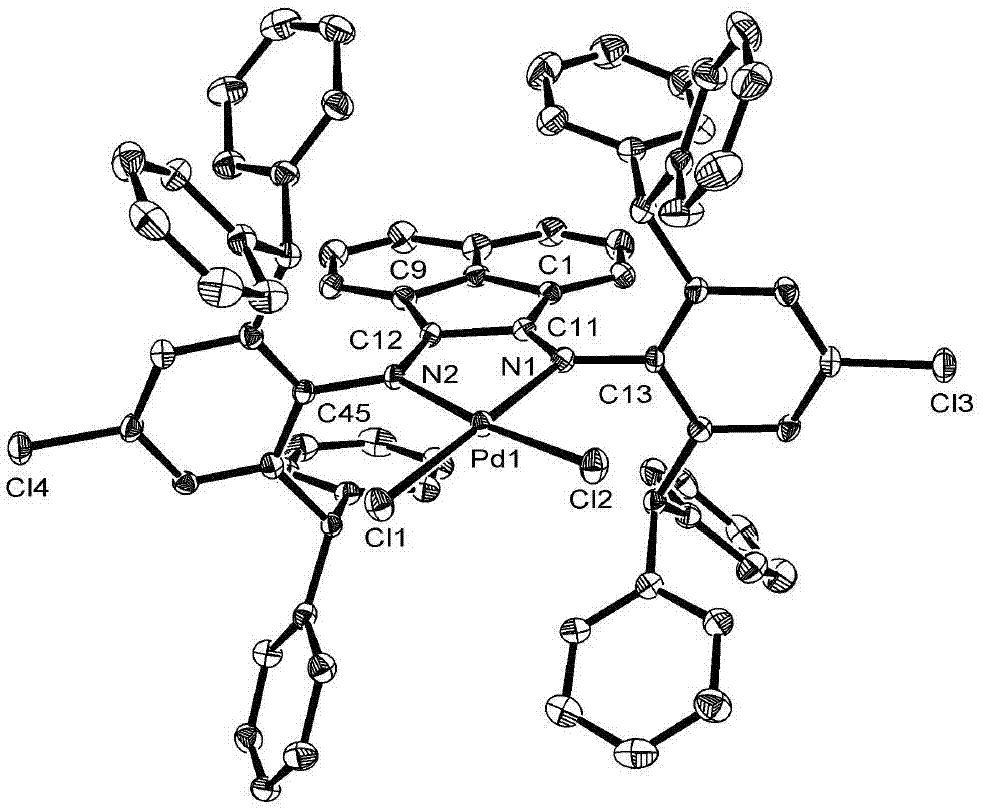
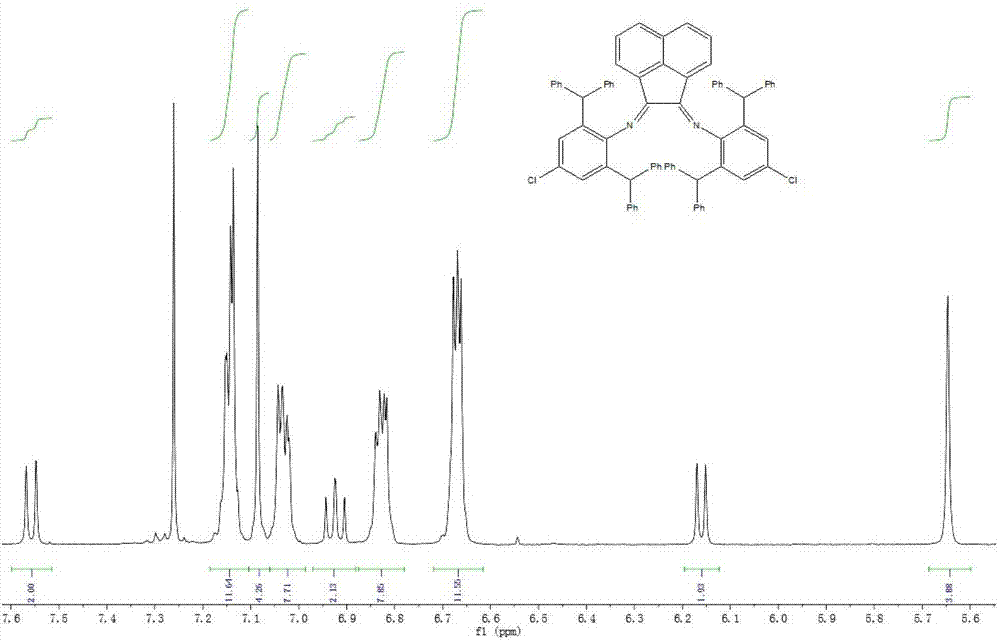
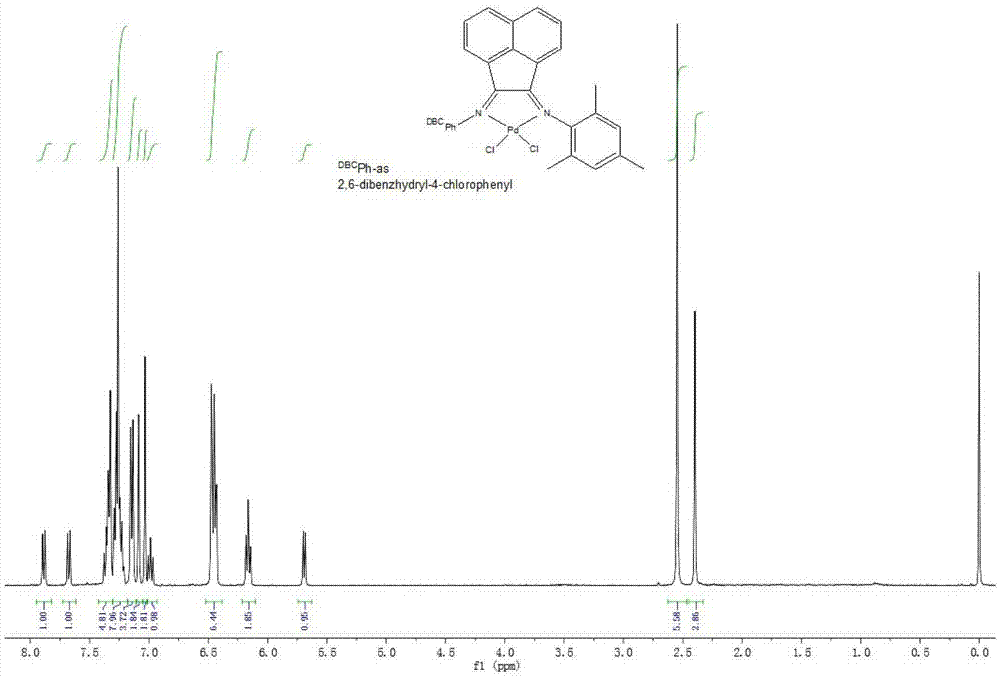
The invention provides a Pd complex catalyst as well as a preparation method and application thereof. The Pd metal complex catalyst provided by the invention has the structure shown by a formula (I), wherein R1 and R2 are alkyls or halogens; preferably, R1 is alkyl with 1-15 carbons, and R2 is alkyl with 1-5 carbons, or halogen; more preferably, R1 is methyl, ethyl, propyl or benzhydryl, and R2 is methyl or chlorine. DBCPh-NH2 is 2,6-bi (diphenylmethyl)-4-chloroaniline. According to the Pd metal complex catalyst, Heck reaction can be promoted when less Pd metal complex catalyst is used; and furthermore, the Pd metal complex catalyst has good catalytic performance and can be used for realizing the catalysis for the Heck reaction.
Owner:QILU UNIV OF TECH
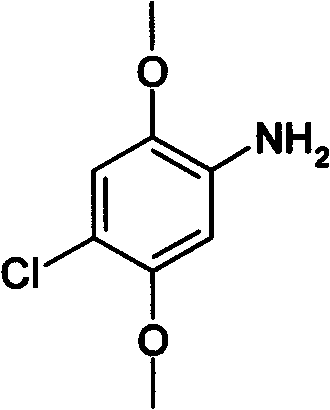
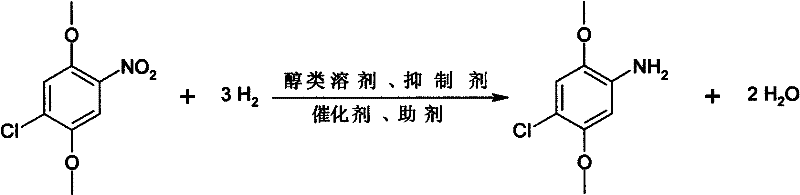
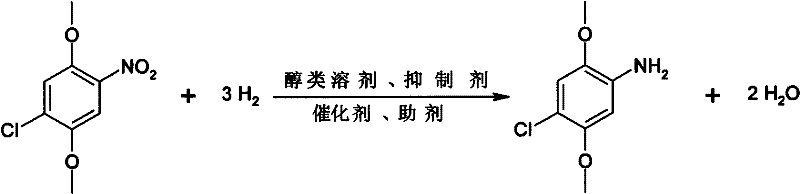
Method for preparing 2,5-dimethoxy-4-chloroaniline by using liquid-phase catalytic hydrogenation method
InactiveCN102344380AReact cleanReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationSolventHigh pressure
The invention relates to a method for preparing 2,5-dimethoxy-4-chloroaniline by using a liquid-phase catalytic hydrogenation method, which comprises the following steps: (a) under the solvent condition, performing a catalytic hydrogenation reaction by taking raneys nickel as a catalyst, dimethyl sulfoxide as an auxiliary agent, organic amine as a dehalogenation inhibitor and 2,5-dimethoxy-4-chloroaniline as a raw material in a high pressure reaction vessel; (b) carrying out decolorizing by using active carbon and filtering to a reaction solution obtained in a step (a), and then adding a protective agent into the reaction solution, cooling, crystallizing and filtering; (c) adding the protective agent into mother liquor filtered in a step (b), concentrating, cooling, crystallizing and filtering, then merging the obtained products filtered in the step (b) and step (c) to obtain the white 2,5-dimethoxy-4-chloroaniline. The invention has the advantages of clean reaction, less pollution, simple process, easy operation, less reaction energy consumption and low cost.
Owner:江苏力达宁化工有限公司
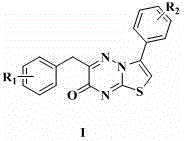
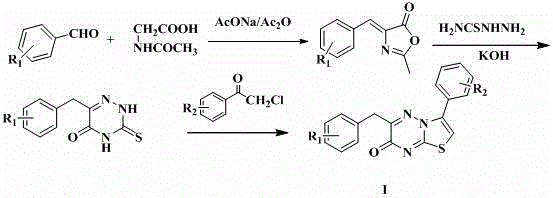
Antibacterial compound as well as preparation method and application thereof
The invention discloses application of a 3-aryl-6-aryl-7H-thiazolo [3,2-b]-1,2,4-triazine-7-ketone derivative with a general formula I shown in the description to an antibacterial drug. In the general formula I, R1 and R2 are methyl, halogen, hydroxyl, methoxyl, acetyl, propionyl, formylphenyl, carbamoyl-methoxy-benzyl, 4-methylanilino formyl-methoxyl or chloroaniline formyl-methoxyl respectively and independently. The 3-aryl-6-aryl-7H-thiazolo [3,2-b]-1,2,4-triazine-7-ketone derivative, namely an antibacterial compound provided by the invention, has obvious inhibiting effects on methicillin-resistant staphylococcus aureus, escherichia coli, pseudomonas aeruginosa and a variety of bacteria, and can be used for the preparation of the antibacterial drug.
Owner:SHIJIAZHUANG UNIVERSITY
Method for preparing 2,5-dimethoxy-4-chloroaniline by hydrogenation reduction
InactiveCN102344382AHighly selective reductionInhibit sheddingOrganic compound preparationAmino-hyroxy compound preparationIron powderFiltration
The invention provides a method for preparing 2,5-dimethoxy-4-chloroaniline by hydrogenation reduction, which comprises the following steps: (1) beating and dissolving a raw material of 2,5-dimethoxy-4-chloro nitrobenzene; (2) adding a catalyst and the material into a hydrogenation reactor, performing a relatively high temperature high pressure hydrogenation reaction in the early stage of the reaction; (3) transferring the material in the reaction of step (2) into a relatively low temperature low pressure reaction state; (4) after the reaction is complete, filtering the catalyst; (5) cooling and precipitating the material; (6) performing pressure filtration and precipitating the material to obtain the product. In the invention, the reduction process of 2,5-dimethoxy-4-chloroaniline is controlled to have two stages of a high temperature high pressure stage and a low temperature low pressure stage, which effectively controlled the dechlorination reaction, and realizes the selective reduction of nitro groups. Hydrogen reduction is adopted instead of iron powder reduction, which avoids the generation of a lot of iron mud, and reduces environment pollution.
Owner:河北彩客新材料科技股份有限公司
Synthetic method of hexamethylene flusulfamide
InactiveCN102838514AEliminate generationLow costSulfonic acid amide preparationAmmonium salt fertilisersCyclohexanoneAniline
The invention provides a synthetic method of hexamethylene flusulfamide. The synthetic method comprises the following steps: reacting to obtain 2-oxocyclohexyl ammonium sulphonate and ammonium sulfate by adopting cyclohexanone, 1, 4-dioxane, sulfur trioxide and ammonia as the raw materials; washing via reaction product through absolute methanol, and leaching to separate ammonium sulfate; and then carrying out reaction among 2-oxocyclohexyl ammonium sulphonate, oxalyl chloride and 2-trifluoromethyl-4-chloroaniline to obtain hexamethylene flusulfamide. According to the synthetic method provided by the invention, KOH solution is replaced via the ammonia, thus, the reaction endpoint is easily controlled; the byproduct ammonium sulfate can be used as chemical fertilizer after being separated, and the generation of three wastes such as waste barium sulfate can be removed; the reaction is easy to operate, the post-processing is simple to carry out, the product is high in purity and high in yield, and the method is suitable for large-scale industrial application.
Owner:CHINA AGRI UNIV +1
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzoazepine-2,5-diketone
The invention relates to a method for preparing a benzoazepine compound, in particular to a novel method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzoazepine-2,5-diketone. The compound can be used as an intermediate for preparing pitressin antagonist medicament Tolvaptan. By using 4-chloroaniline as a raw material, the method comprises the following steps of: (1) reacting 4-chloroaniline with succinic anhydride in a solvent environment to obtain 4-(4- chlorphenyl amino)-4-oxo-butyric acid; (2) protecting the amino of the compound 4-(4- chlorphenyl amino)-4-oxo-butyric acid by paratoluensulfonyl chloride under the action of alkali in the solvent environment to obtain 4-(N-(4-chlorphenyl)-4-(4-methyl benzolsulfonamido)-4-oxo-butyric acid; and (3) carrying out intramolecular cyclization on the 4-(N-(4-chlorphenyl)-4-(4-methyl benzolsulfonamido)-4-oxo-butyric acid under the action of a condensing agent and removing paratoluensulfonyl to obtain a target product. The process route disclosed by the invention has the advantages of low cost and easiness in acquisition of raw materials, few steps, simple process and convenience for obtaining the target product at high yield and high purity at an industrial production scale.
Owner:宁波人健药业集团股份有限公司
Method for converting arylamine polyhalide
ActiveCN101462967BReduce pollutionHigh yieldOrganic compound preparationAmino compound preparationActive componentSolvent
Owner:SHANDONG QINGYUAN GROUP CO LTD
Efavirenz preparation method
The invention relates to an efavirenz preparation method. The method concretely comprises the following steps: 1, washing a separated chloroform layer by using brine, drying, and concentrating; 2, separating out an organic layer, washing the organic layer by using brine, drying, and concentrating to obtain 2-trifluoroacetyl-4-chloroaniline; and 3, adding 1mol / L of hydrochloric acid into n-butanol, reacting at 60DEG C for 72h, and splitting to obtain optically active efavirenz. The efavirenz preparation method has the advantages of simple preparation, high output and low cost.
Owner:储海燕
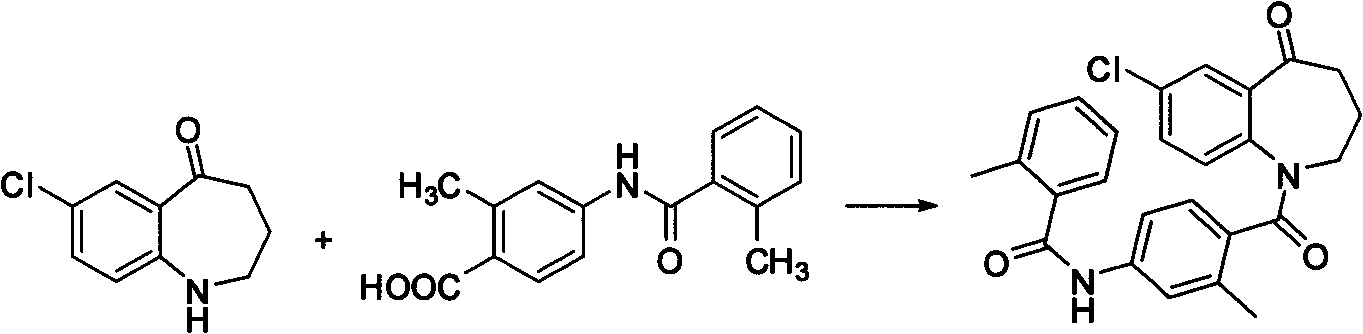
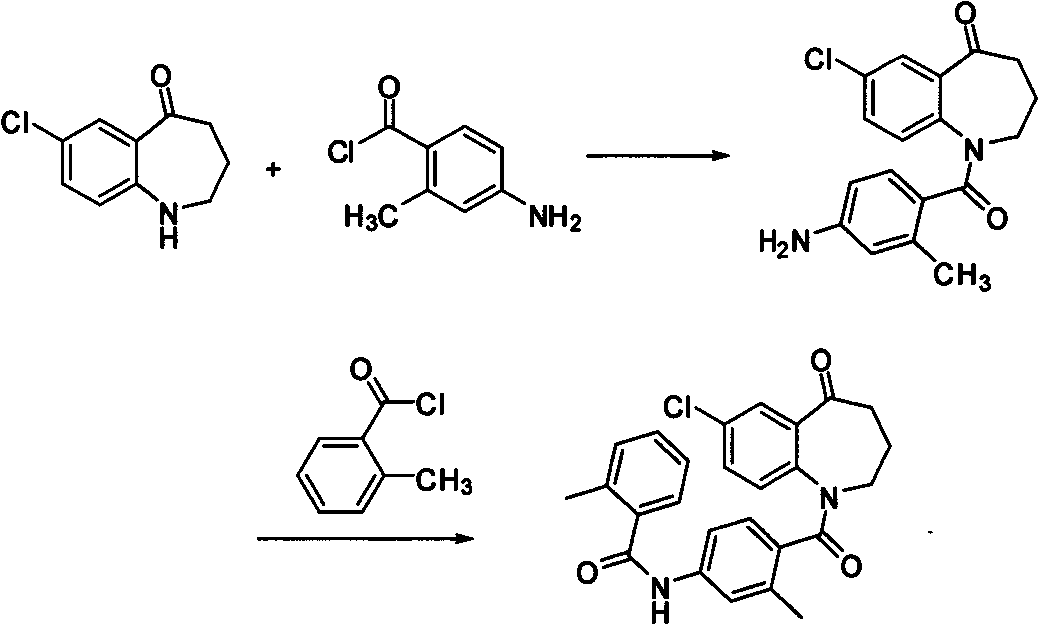
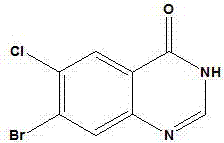
Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one
The invention belongs to the organic synthesis field, and specifically relates to a synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one. According to the invention, the technical problems to be solved are low yield, high cost and difficult after-treatment purification of the existing synthetic method; a technical scheme for solving the technical problems is to provide a synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one; the synthetic method comprises the following steps of: carrying out an acylation reaction between 4-chloroaniline and succinic anhydride to obtain 4-(4-chloroaniline)-4-oxobutyric acid; carrying out an intramolecular Friedel-Craft reaction on 4-(4-chloroaniline)-4-oxobutyric acid to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one; reacting 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one with ethylene glycol to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal; reducing 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal, and carrying out de-ketalation under an acidic condition to obtain 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one. The invention provides a new method which is short in steps and high in total yield.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Synthetic method for 4-chlorophenylhydrazine hydrochloride
The invention discloses a synthetic method for 4-chlorophenylhydrazine hydrochloride. The synthetic method comprises the following steps: 4-bromochlorobenzene and hydrazine hydrate are taken as reaction raw materials, and a phase transfer catalyst, a solvent and a catalyst are added for a reaction; after the reaction, solids are filtered and separated, and a solvent is removed from a filtrate through distillation; hydrochloric acid is added to residues obtained after distillation, and the mixture is stirred and filtered; filter residues are washed with water and dried, and 4-chlorophenylhydrazine hydrochloride is obtained. The synthetic method aims to solve the problem of generation of a large amount of wastewater due to adoption of a current commonly used method for reduction acidification after diazotization of 4-chloroaniline, 4-bromochlorobenzene and hydrazine hydrate are taken as the reaction raw materials, the phase transfer catalyst, the solvent and the catalyst are added for the reaction, the reaction steps are simple, the wastewater produced in a 4-chlorophenylhydrazine synthetic method is reduced remarkably, sulfur dioxide is prevented from being produced through reduction of sodium sulfite, excessive hydrazine hydrate and solvent can be recovered and reused after reaction, and the method is environment-friendly.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
Preparation method of regorafenib
InactiveCN108558747AAvoid breakingReduce consumption costsOrganic chemistryHydrazine compoundFormamide
The invention relates to a preparation method of regorafenib. The method comprises the following steps: heating and melting 3-fluoro-4-nitrophenol and reacting with 4-chloro-N-methylpyridine-2-formamide under the action of KOH; reducing through hydrazine hydrate to obtain an intermediate I; finally enabling the intermediate I and 3-trifluoromethyl-4-chloroaniline, and ethylene carbonate to be subjected to one-pot reaction under the catalysis of a catalyst; carrying out post-treatment to obtain a regorafenib pure product. The method provided by the invention has the advantages of high conversion rate, safety and no harms, no pollution, moderate reaction conditions, high yield and high product purity, and is applicable to industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Method for synthesizing 2,5-dimethoxy-4-chloroaniline
ActiveCN105601523ALess investmentEasy to operateOrganic compound preparationAmino-hyroxy compound preparationOrganic solventNitrobenzene
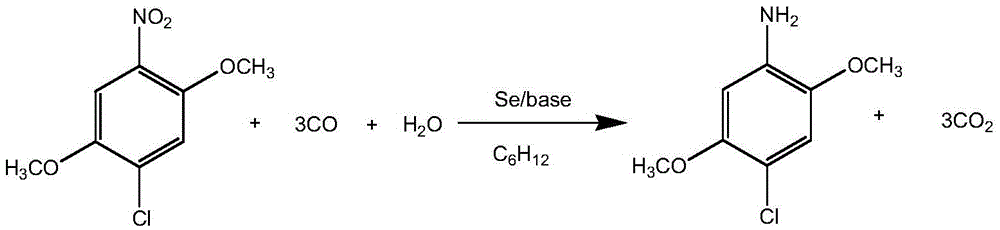
The invention relates to a method for synthesizing 2,5-dimethoxy-4-chloroaniline. The method comprises the steps: in the presence of carbon oxide and water, performing the reaction in an organic solvent under a high temperature and high pressure by adopting 2,5-dimethoxy-4-chloro-nitrobenzene as a raw material, selenium as a catalyst and organic alkali or inorganic alkali as an assistant catalyst, and reducing the nitro to amino to synthesize the 2,5-dimethoxy-4-chloroaniline. The method adopts high-pressure reaction and one-pot reaction, so that less equipment investment is needed, the operation is simple and convenient, and a product is relatively easy to separate and purify.
Owner:LIAONING UNIVERSITY
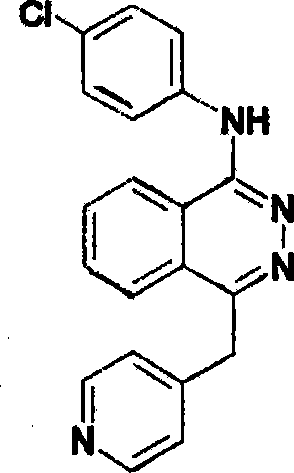
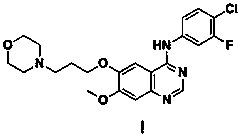
Application of compound 1-( 4-chloroaniline )-4-( 4-picoline )-2, 3-naphthyridine
ActiveCN101116664AAvoid formingImprove the quality of lifeOrganic active ingredientsAntineoplastic agentsDiseaseChlorobenzene
The invention relates to the use of compound 1-(4-chlorobenzene amidocyanogen)-4-(4-methylpyridine)-2, 3-phthalazine in preparing medicine used for preventing tumor diseases, wherein the medicine contains 1-(4-chlorobenzene amidocyanogen)-4-(4-methylpyridine)-2, 3-phthalazine and is used through administration method via or outside intestines and stomach. The compound can at least prolong or avoid occurrence or formation of tumor; moreover, tumor patient adopting medicine containing the compound for prevention obtains higher survival rate as compared with the patient who does not adopt the prevention method.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
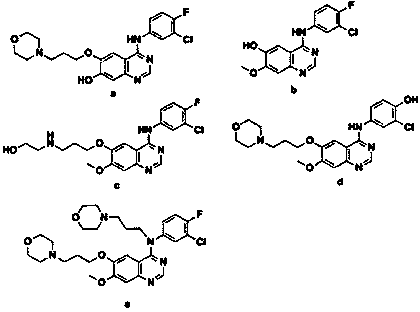
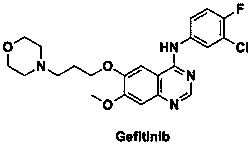
A kind of preparation method of known impurity of gefitinib
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof
ActiveCN110407835AAvoid interferenceReduce distractionsOrganic chemistryColor/spectral properties measurementsEscherichia coliSodium bicarbonate
The invention discloses an imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe, a preparation method of the probe and application of the probe in measuring the extremely-acid pH change in living cells and escherichia coli. Under inert gas protection, 4-dimethylamino-cinnamaldehyde or a derivative thereof, 4-chloroaniline and sodium bicarbonate are dissolved in methyl alcohol, andafter a heating reflux reaction is conducted, a reaction product and 2-(4-methoxyphenyl)-7-methyl imidazo[1,2-a]pyridine are dissolved in N,N-dimethylformamide; potassium tert-butoxide is taken as analkaline reagent, and a heating reflux reaction is conducted; after concentration, silicagel column separation is conducted. Under the extremely acid condition, a transmitted wave of the probe is longer than 600 nanometers, the transmitted wave is located in a near infrared region, a ratio transmission fluorescence characteristic is presented, and the probe has the advantages of high sensitivityand good selectivity for H+, large Stokes displacement and the like.
Owner:SHANGHAI UNIV OF MEDICINE & HEALTH SCI
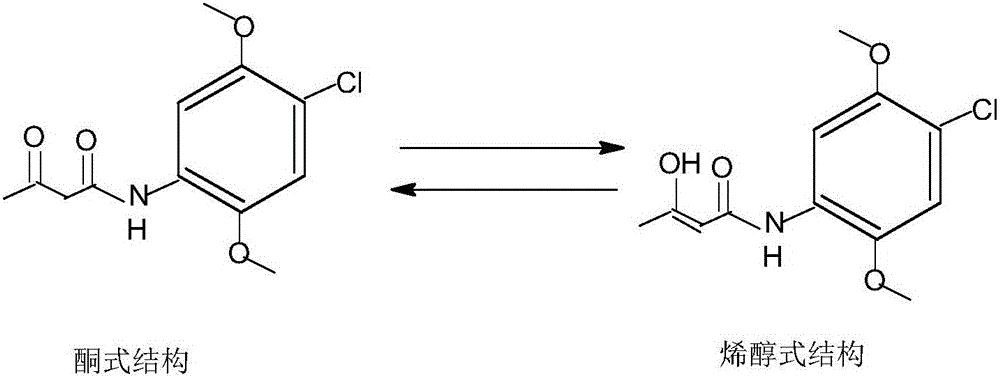
Preparation method of 4-Chloro-2,5-dimethoxyacetoace tanilide
ActiveCN106588685AImproved heat fastnessOrganic compound preparationCarboxylic acid amides preparationAcetic acidAlcohol
The invention discloses a preparation method of 4-Chloro-2,5-dimethoxyacetoace tanilide. The preparation method particularly comprises the following steps: adding an alcohols solvent or an acetic acid solvent into a reactor, meanwhile, adding 2,5-dimethoxy-4-chloroaniline, controlling the temperature to be 10-100 DEG C, dripping acetyl ketene, and finishing adding after 0.5-4 h, wherein the molar ratio of 2,5-dimethoxy-4-chloroaniline to acetyl ketene is 1:1-1:1.2; and after finish of dripping, preserving the temperature for 0.5-4 h, keeping the material in a fully-dissolved state after finish of reaction, adjusting the pH to be 0.5-5.0, quickly cooling with a refrigerating fluid, quickly stirring, controlling the cooling speed to be 5-40 DEG C / min, enabling 4-Chloro-2,5-dimethoxyacetoace tanilide to be quickly separated out as powder, cooling to -10 DEG C to 20 DEG C, filtering and drying obtain 4-Chloro-2,5-dimethoxyacetoace tanilide. The preparation method is simple, and the enolic isomer content in obtained 4-Chloro-2,5-dimethoxyacetoace tanilide can be controlled to be 50 ppm or below.
Owner:NANTONG ACETIC ACID CHEM
A kind of synthetic method of p-chlorophenylhydrazine hydrochloride
The invention discloses a synthetic method for 4-chlorophenylhydrazine hydrochloride. The synthetic method comprises the following steps: 4-bromochlorobenzene and hydrazine hydrate are taken as reaction raw materials, and a phase transfer catalyst, a solvent and a catalyst are added for a reaction; after the reaction, solids are filtered and separated, and a solvent is removed from a filtrate through distillation; hydrochloric acid is added to residues obtained after distillation, and the mixture is stirred and filtered; filter residues are washed with water and dried, and 4-chlorophenylhydrazine hydrochloride is obtained. The synthetic method aims to solve the problem of generation of a large amount of wastewater due to adoption of a current commonly used method for reduction acidification after diazotization of 4-chloroaniline, 4-bromochlorobenzene and hydrazine hydrate are taken as the reaction raw materials, the phase transfer catalyst, the solvent and the catalyst are added for the reaction, the reaction steps are simple, the wastewater produced in a 4-chlorophenylhydrazine synthetic method is reduced remarkably, sulfur dioxide is prevented from being produced through reduction of sodium sulfite, excessive hydrazine hydrate and solvent can be recovered and reused after reaction, and the method is environment-friendly.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
Preparation method of sorafenib
InactiveCN108276328AReduce usageImprove conversion rateOrganic chemistryPolyethylene glycolPotassium carbonate
The invention relates to a preparation method of sorafenib. The method comprises the following steps of allowing p-nitrophenol and 4-chlorine-N-picoline-2-formamide to react under the action of anhydrous potassium carbonate and PEG (polyethylene glycol)-400, generating a compound I via hydrazine hydrate reduction, allowing the compound I, 3-trifluoromethyl-4-chloroaniline and diphenyl carbonate orN,N-disuccinimidyl carbonate to give a 'one-pot' reaction by the action of a catalyst, performing post-treatment to form a sorafenib crude product, and performing further purification to form pure sorafenib. The method is high in conversion rate, safe, free from harm and pollution, mild in reaction condition, high in yield and applicable to industrial production, and can ensure high product purity.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
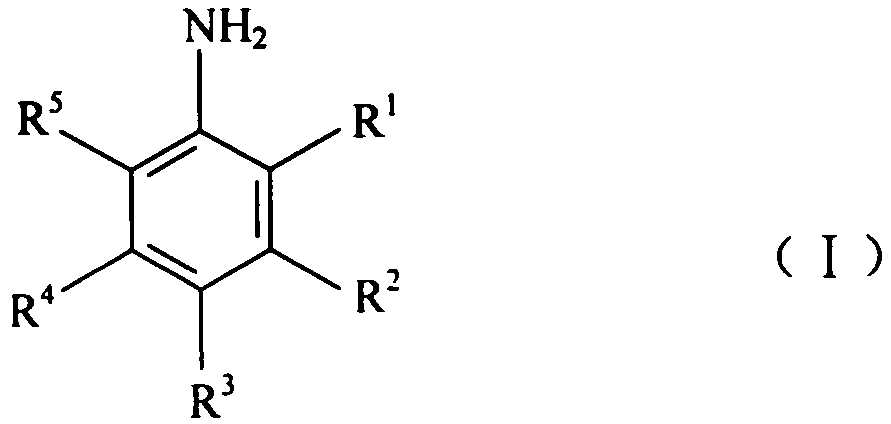
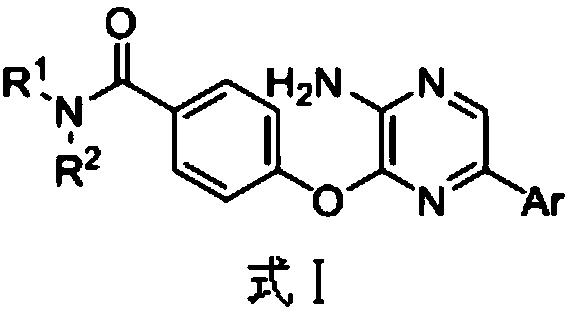
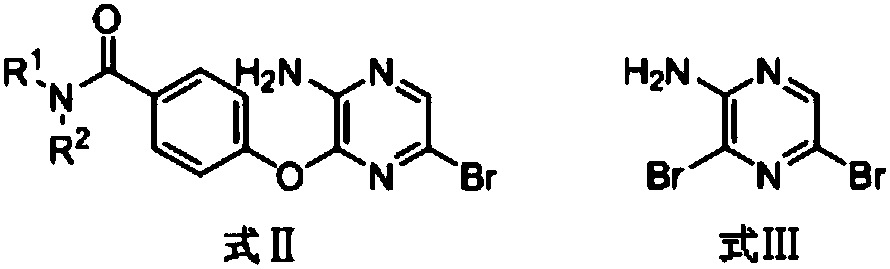
3,5-disubsitutued 2-amino-pyrazine compound and preparation technology and application thereof
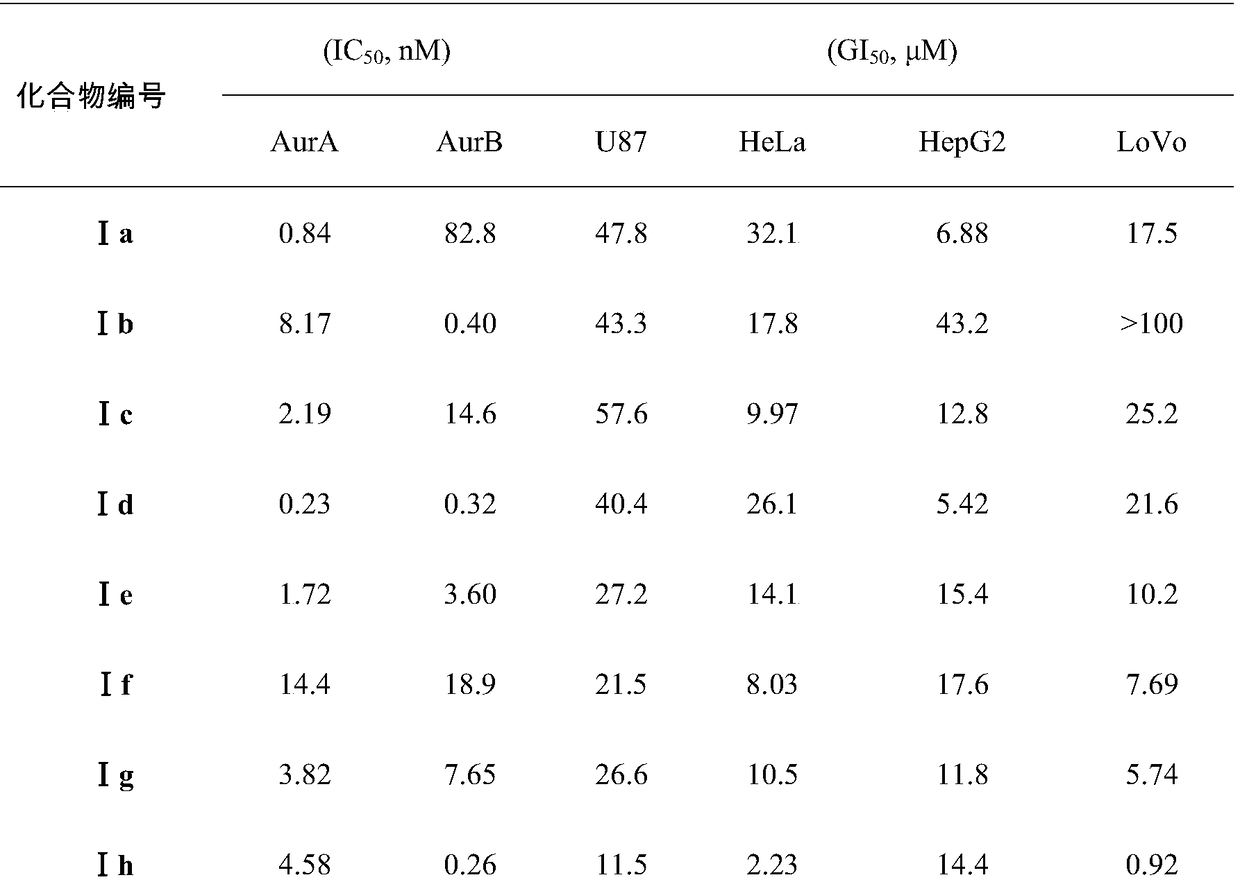
ActiveCN108250191AEnhanced inhibitory effectHigh activityOrganic chemistryAntineoplastic agentsPositive controlPyrazine
The invention discloses a 3,5-disubsitutued 2-amino-pyrazine compound. The compound has the structure shown in the formula I; in the formula I, NR1R2 is n-propylamino, cyclopropylamino, cyclopentylamino, cyclohexylamino, morpholinyl, 4-methyl piperazine, anilino, 4-chloroaniline or 3-chloroaniline; Ar is 3,5-dimethyl-isoxazolyl-4 or 1-methyl-1H-pyrazolyl-4; and the invention further provides the preparation technology and application thereof. The compound has the outstanding inhibiting effect on growth of four kinds of cancer cells including a human cervical cancer cell HeLa, a human brain glioma cell U87, a human hepatoma cancer cell HepG2 and a human colon cancer cell LoVo, wherein a large part of the compound has an obviously higher inhibiting effect on proliferation of the cancer cellsthan positive control VX-680, particularly the compound Ih and Ii have relatively high activity on the four kinds of cancer cells, and have prominent activity inhibiting effect on aurora kinase A andaurora kinase B, and therefore the compound can be applied to preparation of anti-cancer drugs.
Owner:LANZHOU UNIVERSITY
Large steric hindrance ligand Pd complex catalyst as well as preparation method and application thereof
InactiveCN102659622BImprove stabilityHigh catalytic activityOrganic compound preparationOrganic chemistry methodsMethyl groupCoordination complex
The invention provides a Pd complex catalyst as well as a preparation method and application thereof. The Pd metal complex catalyst provided by the invention has the structure shown by a formula (I), wherein R1 and R2 are alkyls or halogens; preferably, R1 is alkyl with 1-15 carbons, and R2 is alkyl with 1-5 carbons, or halogen; more preferably, R1 is methyl, ethyl, propyl or benzhydryl, and R2 is methyl or chlorine. DBCPh-NH2 is 2,6-bi (diphenylmethyl)-4-chloroaniline. According to the Pd metal complex catalyst, Heck reaction can be promoted when less Pd metal complex catalyst is used; and furthermore, the Pd metal complex catalyst has good catalytic performance and can be used for realizing the catalysis for the Heck reaction.
Owner:QILU UNIV OF TECH
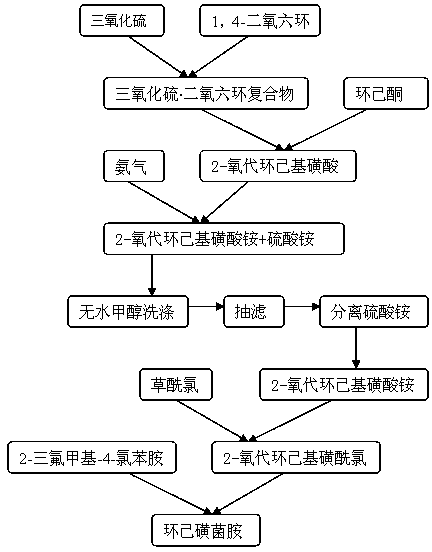
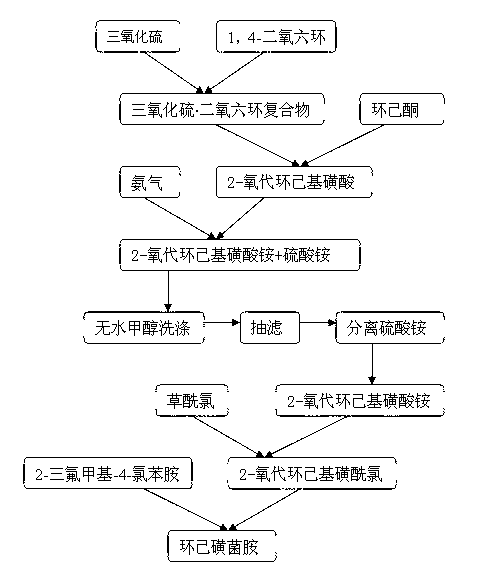
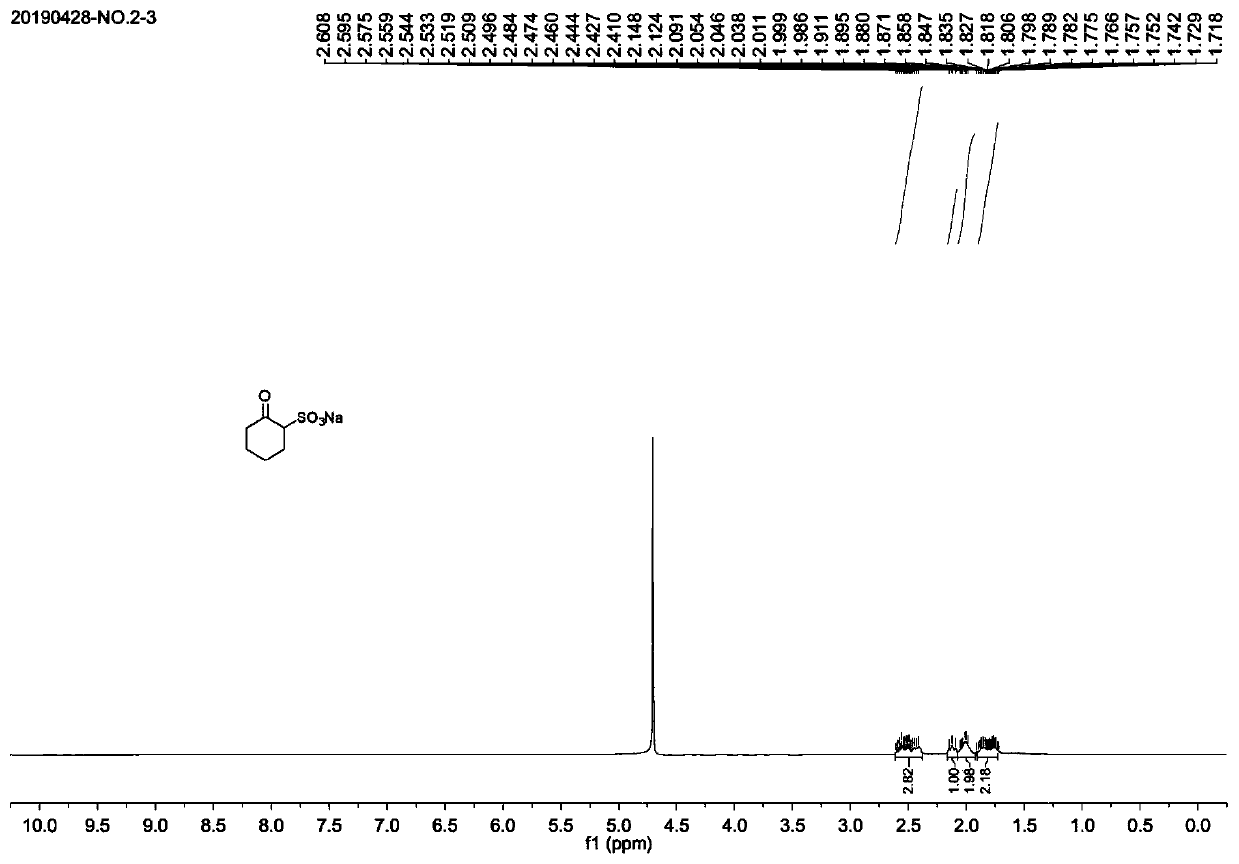
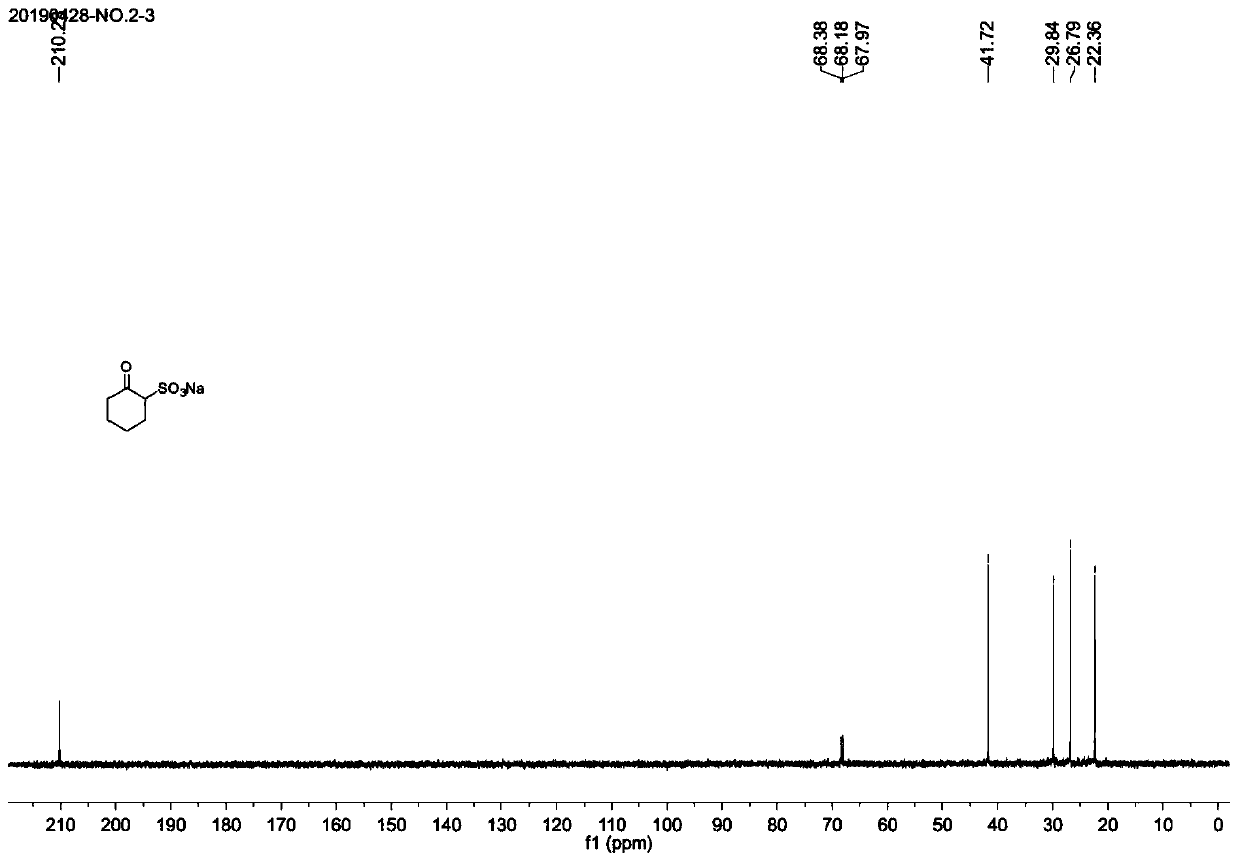
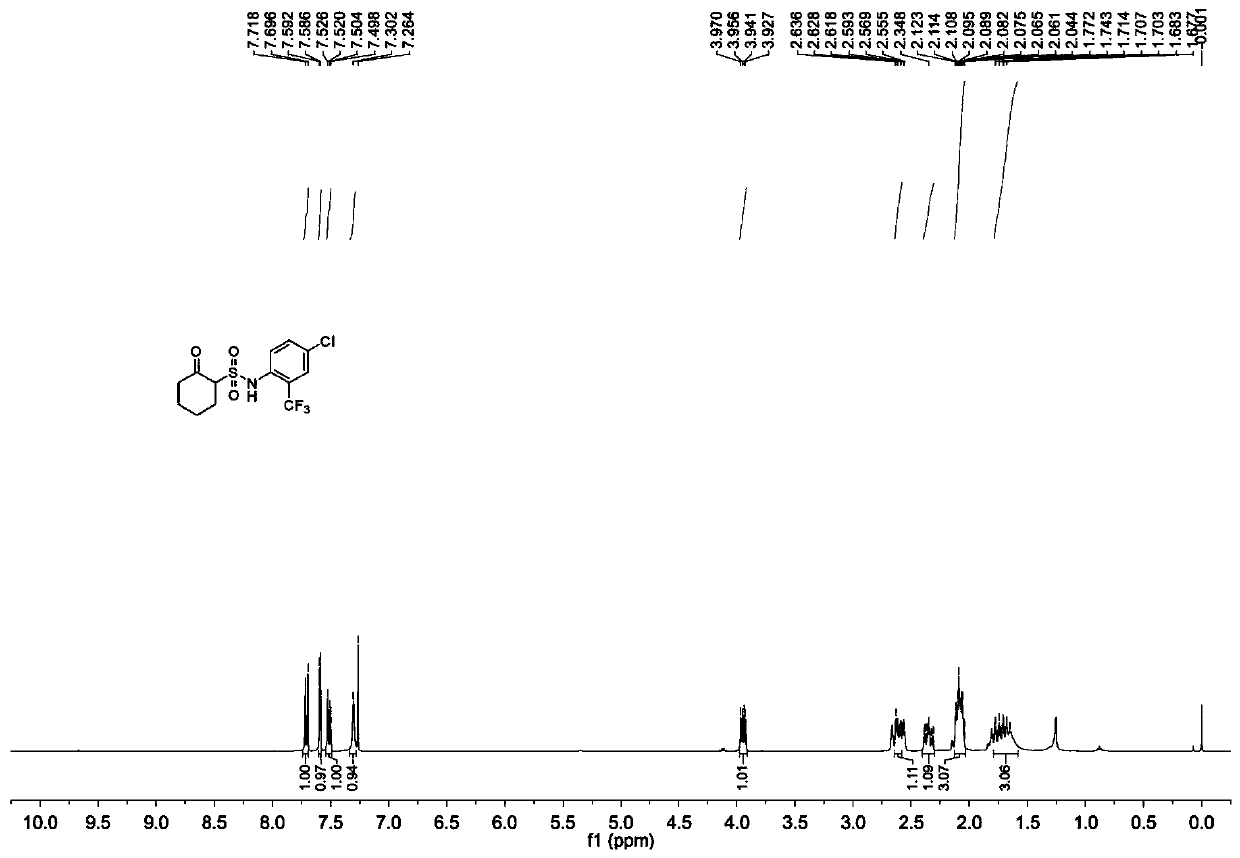
Synthesis method of chesulfamide
ActiveCN110343057AThe preparation process conditions are mildEasy to operateSulfonic acids salts preparationSulfonic acid amide preparationCyclohexanoneChemical reaction
The invention provides a synthesis method of chesulfamide. The method includes the steps of: reacting the halogenation product 2-halogenated cyclohexanone of cyclohexanone with sulfite to obtain 2-cyclohexylsulfonate; then carrying out reaction with oxalyl chloride, 2-trifluoromethyl-4-chloroaniline to obtain chesulfamide. In the invention, 2-halogenated cyclohexanone and sulfite are employed to substitute cyclohexanone and sulfur trioxide.1, 4-dioxane compound, thus avoiding the problems that the end point of chemical reaction for removal of SO4<2-> with precipitant BaCl2 or Ba(OH)2 is hardlycontrollable and the generated BaSO4 precipitate cannot be utilized and become three wastes, and overcoming the disadvantages of corrosion, difficult storage, difficult operation, strong irritating odor, easy cause of poisoning and the like of ammonia gas. The method provided by the invention has the advantages of mild reaction conditions, simple operation, high product purity and high yield, andis suitable for large-scale industrial application.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Synthetic method of drug intermediate of hemosanone, parent nucleus of hemosanone
The invention discloses a drug intermediate of halofuginone and a synthesis method of a halofuginone parent nucleus. The structural formula of the intermediate is disclosed in the specification. The synthesis method of the halofuginone parent nucleus is characterized by comprising the following steps: 1) in the presence of a catalyst, heating 3-bromo-4-chloroaniline and trimethyl orthoformate to sufficiently react, cooling, sufficiently reacting with NH3, separating, and carrying out primary purification to obtain a solid crude product; and 2) in the presence of a catalyst, sufficiently heating the obtained solid, acetic acid, CO and O2 to react, separating and purifying to obtain the end product. The synthesis method is a two-step process, and has the advantages of simple technique, environment friendliness, high yield and very low cost since the raw materials are accessible 3-bromo-4-chloroaniline and trimethyl orthoformate.
Owner:广州朗启生物科技有限公司
Efficient synthesis method of 5-chlorobenzotriazole
InactiveCN108329278ATo achieve the purpose of clean and pollution-freeHigh reaction yieldOrganic chemistrySulfate/bisulfate preparationDistillationSynthesis methods
The invention relates to an efficient synthesis method of 5-chlorobenzotriazole. The method comprises the following steps of (1) adding 2-nitro-4-chloroaniline, water and catalysts of raney nickel into a reaction kettle for reaction; performing climbing plate detection to obtain completely reacted materials; (2) performing filtering and reduced pressure dewatering on the completely reacted materials to obtain 4-chloro-1,2-diaminobenzene materials and waste water A; (3) adding the 4-chloro-1,2-diaminobenzene materials and sodium nitrite into a medium pressure reaction kettle; adding water; performing heat release reaction to obtain a 5-chlorobenzotriazole sodium salt water solution; (5) dripping sulphuric acid into the 5-chlorobenzotriazole sodium salt water solution; performing filtering and spin drying to obtain a 5-chlorobenzotriazole coarse product and waste water B; (5) washing and drying the 5-chlorobenzotriazole coarse product to obtain washing water and a finished product of 5-chlorobenzotriazole with the content being higher than or equal to 99 percent; (6) mixing the waste water A, the waste water B and washing water; then, performing negative pressure distillation to obtain distilled water and waste slag; returning the distilled water into the step (1); dewatering the waste slag to obtain a byproduct of sodium sulfate with the content being higher than or equal to 96percent. The method has the advantages of low cost, low energy consumption and high earnings.
Owner:肖志才
Triflumizole original drug, and preparation method thereof
ActiveCN110105289AMild reaction conditionsEasy to operateOrganic chemistryDrug contentChloroacetic acids
The invention provides a triflumizole original drug, and a preparation method thereof. The triflumizole original drug comprises, by weight, 370 parts to 400 parts of chloroacetic acid, 5 parts to 10 parts of diazabicyclo, 110 parts to 120 parts of n-propanol, 800 parts to 820 parts of sodium n-propoxide, 650 parts to 700 parts of diethyl ether, and 30 parts to 50 parts of concentrated sulfuric acid, 20 parts to 30 parts of isoamyl nitrite, 400 parts to 450 parts of toluene, 600 parts to 700 parts of 2-trifluoromethyl-4-chloroaniline, 410 parts to 440 parts of imidazole, 1000 parts to 1200 parts of cyclohexane, 450 parts to 470 parts of triphosgene, 1400 parts to 1700 parts of chloroform, and 180 parts to 200 parts of triethylamine. The total yield of the triflumizole original drug is higher than 85%, the original drug content is higher than 95%, the water content is less than 0.5, the drug effect is remarkable, and the effect is better.
Owner:JIANGSU HEBEN BIOCHEM
Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one
The invention belongs to the organic synthesis field, and specifically relates to a synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one. According to the invention, the technical problems to be solved are low yield, high cost and difficult after-treatment purification of the existing synthetic method; a technical scheme for solving the technical problems is to provide a synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one; the synthetic method comprises the following steps of: carrying out an acylation reaction between 4-chloroaniline and succinic anhydride to obtain 4-(4-chloroaniline)-4-oxobutyric acid; carrying out an intramolecular Friedel-Craft reaction on 4-(4-chloroaniline)-4-oxobutyric acid to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one; reacting 7-chloro-3,4-tetrahydrobenzo[b]azepine-2,5-one with ethylene glycol to obtain 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal; reducing 7-chloro-3,4-tetrahydrobenzo[b]azepine-2-one-5-glycol ketal, and carrying out de-ketalation under an acidic condition to obtain 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one. The invention provides a new method which is short in steps and high in total yield.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Synthetic method of hexamethylene flusulfamide
InactiveCN102838514BEliminate generationLow costSulfonic acid amide preparationAmmonium salt fertilisersCyclohexanoneAniline
The invention provides a synthetic method of hexamethylene flusulfamide. The synthetic method comprises the following steps: reacting to obtain 2-oxocyclohexyl ammonium sulphonate and ammonium sulfate by adopting cyclohexanone, 1, 4-dioxane, sulfur trioxide and ammonia as the raw materials; washing via reaction product through absolute methanol, and leaching to separate ammonium sulfate; and then carrying out reaction among 2-oxocyclohexyl ammonium sulphonate, oxalyl chloride and 2-trifluoromethyl-4-chloroaniline to obtain hexamethylene flusulfamide. According to the synthetic method provided by the invention, KOH solution is replaced via the ammonia, thus, the reaction endpoint is easily controlled; the byproduct ammonium sulfate can be used as chemical fertilizer after being separated, and the generation of three wastes such as waste barium sulfate can be removed; the reaction is easy to operate, the post-processing is simple to carry out, the product is high in purity and high in yield, and the method is suitable for large-scale industrial application.
Owner:CHINA AGRI UNIV +1
Medical aluminum foil packaging film
InactiveCN106273892AGood flexibilityEasy to useNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesEtherBoric acid
The invention discloses a medical aluminum foil packaging film. The medical aluminum foil packaging film comprises a printing layer, an outer film layer, a first adhesive layer, an aluminum foil layer, a second adhesive layer and a heat sealing layer which are arranged in sequence from outside to inside. The first adhesive layer and the second adhesive layer are both prepared from, by weight, 5-10 parts of acetic ether, 2-5 parts of a formaldehyde solution, 2-4 parts of polypropylene fibers, 2-6 parts of butyraldehyde anilin-econdersate, 1-3 parts of a vulcanizing agent, 2-5 parts of nano-silica, 30-45 parts of polyurethane resin, 1-3 parts of spice, 2-6 parts of nano-scale europium oxide, 2-6 parts of 2-amino-4-chloroaniline, 1-3 parts of boric acid, 2-5 parts of dodecyl diacyl polyethylenimine and 5-10 parts of water. In this way, the adhesive layers of the medical aluminum foil packaging film are good in flexibility, and the overall usability is improved.
Owner:无锡市华泰医药包装有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com













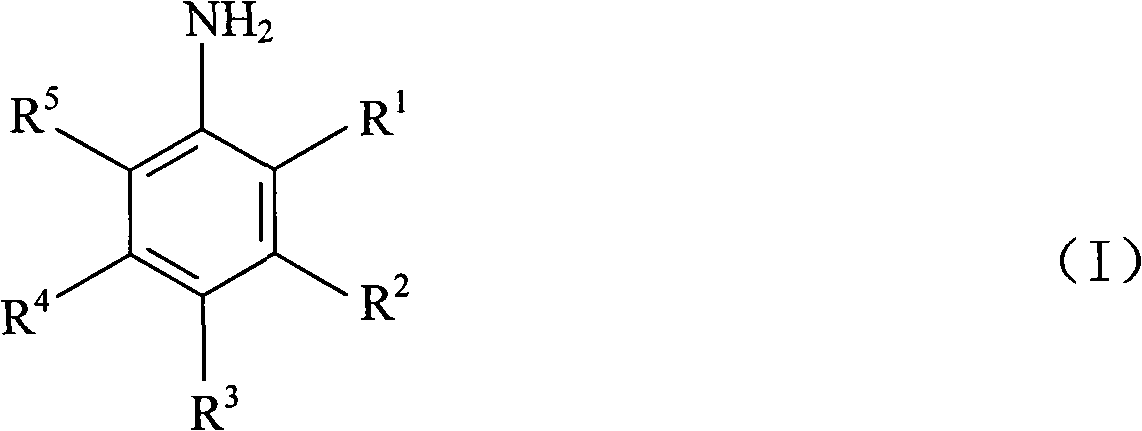
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec6ebb74-632a-4bbc-bb8b-d374bdfc5f25/BDA0000418988450000011.PNG)
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec6ebb74-632a-4bbc-bb8b-d374bdfc5f25/BDA0000418988450000021.PNG)
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec6ebb74-632a-4bbc-bb8b-d374bdfc5f25/BDA0000418988450000022.PNG)



![Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/070ca414-fe7a-4781-9704-8832edddeda0/HDA0002184527220000011.png)
![Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/070ca414-fe7a-4781-9704-8832edddeda0/HDA0002184527220000012.png)
![Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof Imidazo[1,2-a]pyridine near-infrared ratio type pH fluorescence probe and preparation and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/070ca414-fe7a-4781-9704-8832edddeda0/HDA0002184527220000021.png)
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/50cd28c9-0806-4ef3-af27-33ce5bf94faf/BDA0000418988450000011.PNG)
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/50cd28c9-0806-4ef3-af27-33ce5bf94faf/BDA0000418988450000021.PNG)
![Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one Synthetic method of 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/50cd28c9-0806-4ef3-af27-33ce5bf94faf/BDA0000418988450000022.PNG)