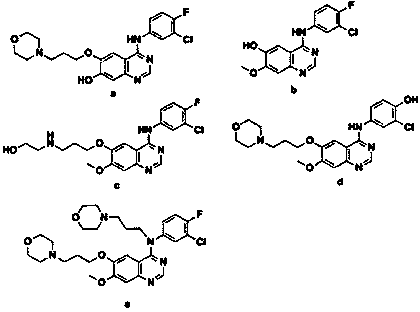
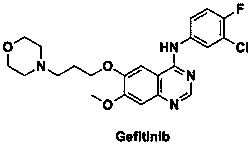
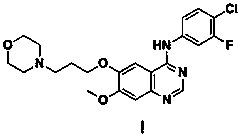
A kind of preparation method of known impurity of gefitinib
A technology of gefitinib and impurities, applied in the field of medicinal chemistry, can solve the problems of no CAS registration number and no impurities, etc., and achieve the effects of short reaction time, high product purity, and easy synthesis and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 2-amino-4-methoxy-5-(3-morpholine propoxy)benzonitrile (30g, 0.103mol), 3-fluoro-4-chloroaniline (15g, 0.103mol), triethyl orthoformate Ester (23g, 0.155mol), and 135mL of acetic acid solvent, mix well, heat up to 80°C, stir for 1.5h, TLC monitors the reaction progress; after the reaction is completed, slowly lower the internal temperature to 50°C, add 140mL of saturated saline , stir evenly, continue to cool down to an internal temperature of 5°C, stir and crystallize for 2 hours, and obtain an off-white precipitated solid by suction filtration, suspend the above solid in aqueous sodium bicarbonate solution (pH 8-9) and stir for 1 hour, and suction filter to dryness, that is 28g of impurity crude product must be changed. The crude impurity was heated and dissolved in 180 mL of ethanol / ethyl acetate (2:1, v / v) mixed solvent; under the condition of heat preservation, 0.9 g of activated carbon was added, and stirred for 1 h to decolorize; ℃ stirring and crystallizing, su...
Embodiment 2
[0039]2-Amino-4-methoxy-5-(3-morpholinepropoxy)benzonitrile (27g, 0.093mol), 3-fluoro-4-chloroaniline (14.9g, 0.101mol), orthoformic acid tris Methyl ester (16g, 0.148mol), and 148mL of acetic acid solvent, mix well, raise the temperature to 105°C, stir the reaction for 2h, monitor the reaction progress by TLC; after the reaction is completed, slowly lower the internal temperature to 60°C, add 152mL of saturated saline , stir evenly, continue to cool down to an internal temperature of 10°C, stir and crystallize for 3 hours, and obtain an off-white precipitated solid by suction filtration, suspend the above solid in aqueous sodium bicarbonate solution (pH 8-9) and stir for 1 hour, and suction filter until dry, that is 25g of impurity crude product must be changed. The crude impurity was dissolved by heating with 150mL of methanol / acetone (1:1, v / v) mixed solvent; under the condition of heat preservation, 0.9g of activated carbon was added, and stirred for 1h to decolorize; suct...
Embodiment 3
[0041] 2-Amino-4-methoxy-5-(3-morpholinepropoxy)benzonitrile (14g, 0.048mol), 3-fluoro-4-chloroaniline (7.7g, 0.0528mol), orthoformic acid tris Ethyl ester (12g, 0.0816mol), and 70mL of acetic acid solvent, mix well, raise the temperature to 90°C, stir for 2h, monitor the reaction progress by TLC; after the reaction is completed, slowly lower the internal temperature to 52°C, add 80mL of saturated saline , stir evenly, continue to cool down to an internal temperature of 7°C, stir and crystallize for 2 hours, and obtain an off-white precipitated solid by suction filtration, suspend the above solid in aqueous sodium bicarbonate solution (pH 8-9) and stir for 1 hour, and suction filter until dry, that is 14g of impurity crude product must be changed. The crude impurity was heated and dissolved with 70 mL of ethanol / ethyl acetate (4:1, v / v) mixed solvent; under the condition of heat preservation, 0.5 g of activated carbon was added, and stirred for 1 h to decolorize; ℃, stirring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com