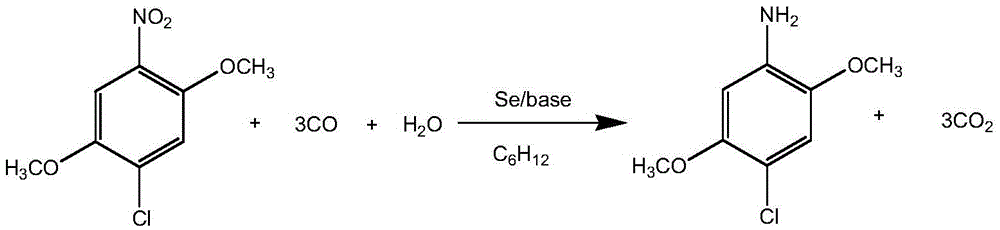
Method for synthesizing 2,5-dimethoxy-4-chloroaniline
A technology of dimethoxyl and chloroaniline, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve problems such as environmental pollution, complicated operation, reduction limitation, etc., and achieve low equipment investment and high reaction efficiency High selectivity and easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0020] The synthesis of embodiment 12,5-dimethoxy-4-chloroaniline
[0021] In a 100ml autoclave equipped with a stirring bar, add 2,5-dimethoxy-4-chloronitrobenzene (10mmol), selenium powder (0.0158g), H 2 O (2ml), C 6 h 12 (40ml), NaHCO 3 (0.42g), feed carbon monoxide to 6MPa, then heat to 150°C and stir to react for 8 hours, put the autoclave in the air to cool naturally, stir and oxidize the product in the air for a period of time, then suction filter, and wash with distilled water for 2 to 3 After suction filtration, a white solid was obtained, which was the target product, and dried in the dark.
[0022] The white solid was detected: mp117~118℃.
[0023] 1 HNMR (300MHz, CDCl 3 )δ7.26(s,2H,NH 2 ), 6.79 (s, 1H, ArH skeleton), 6.37 (s, 1H, ArH skeleton), 3.80 (s, J=3.5Hz, 6H, CH 3 ).
[0024] The purity of the product detected by high performance liquid chromatography was 98.38%.
Embodiment 22
[0025] The synthesis of embodiment 22,5-dimethoxy-4-chloroaniline
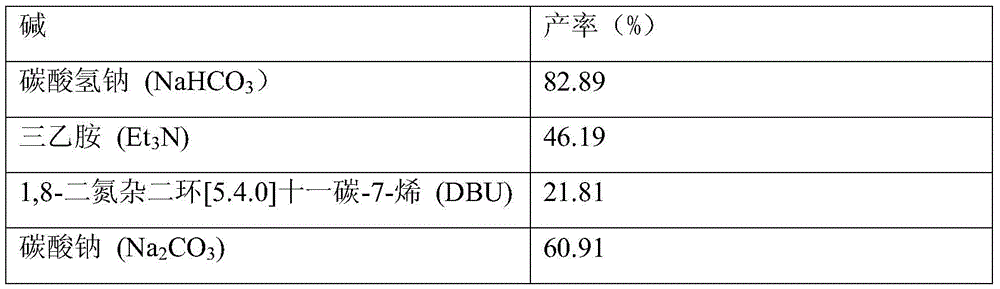
[0026] Other experimental methods and conditions are the same as in Example 1, only the reaction temperature is changed, and the yield of 2,5-dimethoxy-4-chloroaniline is shown in Table 1.
[0027] Table 1
[0028] temperature(°C)
Embodiment 32
[0029] Synthesis of embodiment 32,5-dimethoxy-4-chloroaniline
[0030] Other experimental methods and conditions are the same as in Example 1, only the CO pressure is changed, and the yield of 2,5-dimethoxy-4-chloroaniline is shown in Table 2.
[0031] Table 2
[0032] Pressure (MPa)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com